Abstract
In order to remain stable, dispersed social groups have to solve two fundamental problems: the coordination of movement and cohesiveness within a group and the spacing between the groups. Here, we investigate mechanisms involved in intra-group coordination and inter-group spacing using the golden brown mouse lemur, Microcebus ravelobensis, as a model for a nocturnal, solitary foraging mammal with a dispersed social system. By means of radiotelemetry and bioacoustics we studied the olfactory and vocal behaviour during nocturnal dispersal and reunion of five sleeping groups.
All groups used 3–17 sleeping sites exclusively, suggesting a sleeping site-related territoriality and competition for them. The occurrence of olfactory and vocal behaviour showed an asymmetrical temporal distribution. Whereas marking behaviour was observed exclusively during dispersal, a particular call type, the trill, was used by all groups during reunions. Interestingly, these trills carried group-specific signatures.
Our findings provide the first empirical evidence for nocturnal primates in a natural environment that olfactory signals represent an important mechanism to regulate the distribution of different groups in space, whereas acoustic signals control intra-group cohesion and coordination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How members of dispersed social groups regulate their distribution in time and space and how they coordinate group movement and maintain group cohesiveness are fundamental questions in socio-ecology (e.g. Boinski and Garber 2000; Couzin and Krause 2003; de Waal and Tyack 2003). Anthropoid primates, with the exception of the orang-utan, as well as diurnal lemurs share a common organisation pattern, i.e. permanent social groups in which adult individuals live constantly together and interact in foraging, predator detection and defence, offspring rearing or defence of resources (e.g. van Schaik and van Hooff 1983; Wrangham 1987; Janson 2000; Kappeler and van Schaik 2002). The individuals use rich repertoires of visual, auditory, tactile and olfactory signals for social communication (Zimmermann 1992; Hauser 1996; Fleagle et al. 1999). In contrast, the social structure of the nocturnal Malagasy lemurs is highly diverse. Adults of either sex may sleep and forage solitarily and come together primarily for mating, e.g. in the aye-aye (Sterling and Richard 1995). Alternatively, one male and one female of solitary foraging species may form a dispersed pair which sleeps permanently together such as in fat-tailed dwarf (Fietz 1999; Müller 1999), fork-marked (Müller and Thalmann 2002; Schülke and Kappeler 2003) or sportive lemurs (Rasoloharijaona et al. 2003; Zinner et al. 2003). In other species (e.g. mouse lemurs) several individuals form dispersed groups in which animals forage alone but reunite in fairly permanent groups to sleep (Barre et al. 1988; Radespiel 2000; Weidt et al. 2004). Finally, there exist nocturnal lemurs living in permanent pairs which forage and sleep together, for example woolly lemurs (Harcourt 1991). This high adaptive diversity with regard to social structure (Müller and Thalmann 2000; Kappeler and van Schaik 2002) renders nocturnal Malagasy lemurs an ideal model to understand the evolution of communication signals for inter-group spacing and group coordination in primates. Yet, empirical studies addressing this question in nocturnal solitary foraging lemurs are totally lacking.
The golden brown mouse lemur (Microcebus ravelobensis) represents an excellent model to investigate inter- and intra-group communication of nocturnal primates. Discovered in 1994 in the National Park Ankarafantsika in northwest Madagascar (Zimmermann et al. 1998), this primate lives in dry deciduous forest, partly sympatric with its sibling species, the grey mouse lemur (Microcebus murinus). Both species weigh about 60 g, are omnivorous and show similar feeding habits (Radespiel et al. unpublished), but differ in morphology (Schmelting et al. 2000), genetics (Pastorini et al. 2001) and acoustic communication (Zietemann 2001; Braune et al. 2001). The social organisation of the golden brown mouse lemur was described as a dispersed multimale/multifemale system with a promiscuous mating pattern (Weidt et al. 2004). Individuals usually forage alone at night, but establish long-term, mixed sex sleeping groups of about five individuals during the day. Home ranges overlap within and between sexes and for individuals from the same or even from different sleeping groups. Groups occasionally change their sleeping sites, mainly leaf nests or tree holes. Nevertheless, the composition of sleeping groups remains stable over time.
The aim of our study was to investigate spacing and group coordination in a solitary foraging mammal forming individualised long-term sleeping groups, using the golden brown mouse lemur as a model. First, sleeping sites have been described as potentially limited resources for mouse lemurs (Radespiel et al. 1998). We hypothesised that restricted sleeping sites should lead to competition among groups. Therefore we expected direct or indirect competition at the sleeping sites, reflected in the spacing pattern of the groups’ sleeping sites. Second, we postulated that mouse lemurs should have evolved communication signals to gather at a common sleeping site. It is known that mouse lemurs show marking behaviours such as urine-marking, anogenital rubbing and mouth-wiping (Schilling 1979; Buesching et al. 1998) and display a high vocal activity (Zimmermann 1995). We expect that communication signals facilitate the reaggregation of the group members dispersed in space, and coordinate the search for a specific sleeping site. Olfactory and/or acoustic communication signals may contribute to these inter- and intra-group processes and were studied during dispersal and reunion of groups. Third, we hypothesised that vocal signals for group reunion carry long-term group-specific signatures which may provide a means for group recognition and discrimination.
Methods
Study site and data sampling
The study was conducted in the Reserve forestière d’Ampijoroa in the Ankarafantsika National Park (16°19′S, 46°48′E), about 110 km south-east of Mahajanga, north-west Madagascar. Data collection took place in the 5.1-ha research area Jardin Botanique B (JBB) in a dry deciduous forest. In JBB, the golden brown mouse lemur occurs without any other congeneric species. We worked in the field from September to October 2000 and from July to October 2001, covering a period before and during the mating season (Randrianambinina et al. 2003; Schmelting et al. 2000). Data on communication signals were collected in both the years, spacing data in 2001.
We studied five sleeping groups of the golden brown mouse lemur, three of them in both the observation periods (Table 1). We equipped 16 animals with a radio collar (TW-4 button cell tags; Biotrack, Wareham, UK). Six animals from three groups carried transmitters in both the years. In addition, we banded three individuals of two groups with a reflective collar in the second year. Each of the five groups consisted of three to six members (one to five collared and up to three non-collared animals). Sleeping site locations of radio-collared individuals were determined telemetrically during daytime once a day using a portable receiver (TR-4 with RA-14K antenna; Telonics, Inc., Impala, AZ). All detected sleeping sites of the radio-collared mouse lemurs were registered on a map. We defined a sleeping group as individual mouse lemurs that repeatedly slept together (cf. Weidt et al. 2004). Additional data concerning sleeping group composition were collected during observations of radio-collared individuals at dusk and dawn. All sleeping sites occupied by identified group members were counted for the respective group.
An overview of identified individuals and sleeping groups and the data obtained from them for analysis is given in Table 1.
Vocal and behavioural data were collected during sleeping group dispersal in the evening and reunion in the morning. In the evenings, we went to the sleeping sites while the mouse lemurs were still inactive and positioned ourselves about 8–12 m in front of the sleeping site for direct observation. Evening observation sessions referred to as dispersals (n=32; min=2, max=11, median=6 sessions per group) ended when all animals of the sleeping group had left the area visible from the observation position. For morning observation sessions referred to as reunions (n=23; min=2, max=8, median=3 sessions per group), we waited for the group at the previous sleeping site of that group at least 1 h before sunrise. These sessions came to an end after sunrise when the sleeping group members had entered the site and became inactive. Median duration of dispersal and reunion was determined as the time span between the first and the last animal leaving, respectively entering the sleeping site. In each session, we recorded the presence or absence of marking and vocal behaviour using all occurrence-sampling. The vocal behaviour was attributed post-hoc to six different contexts.
For analysis, we counted the number of dispersals and reunions in which the respective behaviour occurred, as well as the number of sleeping groups involved. The number of absolute frequencies of marking and vocal behaviour during dispersal and reunion was compared using the chi-square test. Small sample sizes were adjusted by the Yates method (Zöfel 1992).
Marking behaviour
We distinguished two types of marking behaviours (Schilling 1979; Glatson 1983): urine washing and mouth-wiping. In urine washing, urine is deposited on the hands and then rubbed along the feet. Afterwards, urine marks are placed by running over the substrate. During mouth-wiping, the corner of the mouth, the face and sometimes the head are rubbed along a branch.
Sound recording and analysis
The vocal repertoire of the golden brown mouse lemur extends into the ultrasonic range (Braune et al. 2001; Zietemann 2001). Consequently, a special device for ultrasound recording was necessary. We connected the high-frequency output of a bat detector (U30, Ultrasound Advice) via a filter/control unit (Pettersson) to a high-speed A/D-card (DAS 16/330, Computerboards, Inc.) in a laptop (Compaq Armada) equipped with the recording software BatSoundPro 3.0. The filter/control unit allowed us to ‘start’ and ‘stop’ the recordings which were made with a sampling frequency of 200 kHz (16 bit, mono). The use of a circular buffer function made it possible to record the last 10 or 15 s before the recording was stopped. All recorded vocalisations were analysed using BatSoundPro 3.0 (FFT size: 512; Hanning window).
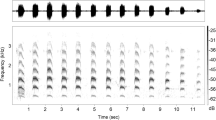
The calls were classified in three categories, i.e. trill, wide-band zip and whistle/tsak (Fig. 1), according to Zimmermann (1995) and Zietemann (2001) by visual inspection of the sonagrams. Between these categories there were no transitions.
Trills were subjected to a more detailed analysis. We analysed 53 trills produced by the three sleeping groups in the year 2000, and 81 trills from these and one additional group in the year 2001. Trills of the fifth sleeping group (group 5) were visually inspected but not of sufficient quality for a quantitative analysis, for example due to background noise, overlapping calls or echo clutter. For each group, calls from at least two individuals were considered by including non-overlapping trills from overlapping trill series of two different individuals. We measured 22 acoustic parameters for each trill (Table 2): temporal parameters were determined using the waveforms, frequency parameters from the power spectra (BatSoundPro 3.0).
The trills of the four 2001-groups formed the basis for a discriminant function analysis. The 22 acoustic parameters of the 81 trills were tested for correlation (Spearman-Rank-Correlation; Statistica 5.0, StatSoft, Inc.). From a pair of parameters with rs>0.75, only one was selected for the discriminant function analysis. Parameter pairs with rs<0.75 were defined as sufficiently non-related (SPSS 11.0, SPSS, Inc.). This method yielded 11 acoustic variables for our analysis (indicated in Table 2) for which medians were calculated. We used the stepwise forward method (statistic: Wilk’s-λ) with the criteria Fto enter=3.84 and Fto remove=2.71 and a tolerance level of ≤0.01 to calculate the discriminant function model.
The computed discriminant functions were used to classify cases with regard to their group membership. First, the 81 cases of the year 2001 were cross-validated by the “leave-one-out” method, where each case in the analysis was classified by the functions derived from all cases other than that case; for this classification a priori probabilities were dependent on group sizes (SPSS 11.0, SPSS, Inc.). Second, we assumed that groups containing identical individuals in 2000 and 2001 represent the same group. To test whether group signatures of trills remain constant over the years, all cases of the year 2000 were classified as new cases. Here, it was assumed that a case was equally likely to be a member of any group, so a priori probabilities were equal for each group.
The tests on number of sessions as well as the discriminant function analysis were based on pooled data for every group because we could not always determine the identity of a marking or calling group member. Therefore we cannot discard the possibility that some individuals, e.g. age-sex groups may have attributed more to the results than others (see Bart et al. 1998).
Results
Spacing
Sleeping groups used between 3 and 17 sleeping sites in 2001. The groups changed their sleeping site every 2–9 days (median=three days). We found the sleeping groups in 98% on average of all sleeping site localisations during daytime (cf. Table 1). Sleeping sites were occupied exclusively, i.e. there was no case in which a group slept at a sleeping site of another group (Fig. 2). Due to predation or transmitter problems, we lost several study animals, and in two cases (groups 1 and 2) the whole sleeping group after 41 and 7 days, respectively.
Behaviour during dispersal and reunion
During dispersal the group members left the vicinity of the sleeping site one after another and in the majority of cases they disappeared in different directions (median duration=3 min, nsessions=32). During reunion the individuals of a sleeping group arrived at the site in two different ways: they came one by one or as a whole group (median duration=4 min, nsessions=16). In the latter case, we could sometimes observe that group members met at a place near the sleeping site and then moved together towards it. Several times, groups came to the previous sleeping site but then decided to change to another. During dispersal and reunion, we recorded distinct communication signals.
Marking behaviour
The mouse lemurs used olfactory signals significantly more often during dispersal (31% of sessions, nsessions=32) than during reunion (0% of sessions, nsessions=23; χ2=6.494, p<0.05). No individual showed marking behaviour during reunions, but three individuals of the five groups displayed urine washing (ten times, three groups) or mouth-wiping (four times, two groups) near sleeping sites on 30% of observed dispersals. This olfactory behaviour occurred before and during the mating season.
Vocal behaviour
Vocal behaviour was produced by subjects during both dispersals and reunions. The vocal activity at reunions in the mornings, where calls were recorded in 96% of the sessions (nsessions=23), was significantly higher than during dispersal in the evenings, where vocalisations were recorded in only 38% of the sessions (nsessions=32; χ2=16.788, p<0.001). The three call categories could occur during a given session. Whistles/tsaks were recorded in about 30% of the observation sessions, but were equally likely produced during dispersals and reunions (χ2=0.000, p>0.05). In contrast, there were prominent differences in the occurrence of wide-band zips (χ2=5.248, p<0.05) and of trills (χ2=39.928, p<0.001) between dispersal and reunion. Zips were only produced during reunions and only in conjunction with trills. They were found in three groups in about 20% of the observation sessions. Trills were found in all five groups and were observed during all reunions besides one. In the remaining case, the whole group entered the sleeping site later in time than on other days without giving any calls. During dispersal, trills were only recorded from male strangers (i.e. males not belonging to the observed group) approaching a sleeping site in the mating season, not from members of the observed sleeping groups.
Context of acoustic signals
The behavioural context in which whistles/tsaks and wide-band zips occurred was not clear and is therefore not considered in this analysis. Trills occurred in one specific context during dispersal, and in five during reunion.
During dispersal, trills were uttered in only 2 of 32 sessions by male strangers while inspecting the sleeping site of the observed group. In one session, the caller passed the site quickly while the group members were still at the sleeping site, watching him. In the second session, trills occurred while the group was leaving the sleeping site. We observed chasing and fighting as well as other vocalisations in addition to trills.
In contrast, during reunion, trills occurred in 22 of 23 sessions. We excluded one session from this analysis because the situation was complicated by the presence of a stranger. For trills uttered during the remaining 21 reunions in which only the group members were in the vicinity of the sleeping site, we classified five different contexts, namely ‘vocal response’ (trills were responded to by uttering trills and approaching the caller, nsessions=1), ‘phonotactic approach’ (trills caused an approach to the caller, nsessions=5), ‘phonotactic aggregation’ (trills resulted in an aggregation of group members, the caller could not be identified, nsessions=6), ‘group movement’ (trills were recorded while the whole group or a part of it was moving towards the sleeping site, the caller could not be identified, nsessions=15) and ‘no responding animal present’ (single individuals called but no other group members were visible, nsessions=3).
Trill structure
Trills consisted of two to six harmonically structured syllables or elements (Fig. 1). In general, elements were upward frequency modulated. The initial and final element started with a steep upward frequency modulation followed by a nearly constant frequency component and terminated with a second steep frequency modulated component. In the centre elements, the nearly constant frequency component was often missing. Sometimes the elements ended with a constant frequency or downward frequency modulated hook. The duration of trills was between 120 and 400 ms. Minimum frequencies of the fundamental ranged from 9 to 18 kHz, maximum frequencies of the fundamental from 28 to 50 kHz. For the 11 acoustic parameters used for a detailed analysis (see ‘group-specific signatures of trills’) we present medians in Table 3.
Group specific signatures of trills
The stepwise forward discriminant function analysis used 6 of the 11 variables for model calculation, namely start frequency, call duration, bandwidth of element 1, duration of element 1, relative position of minimum frequency and end frequency. Three functions were computed explaining a significant part of the acoustic variability between the four groups (Wilks’ λ=0.037; F(18,204)=24.9; p<0.001; Table 4).
In the year 2001, 92.6% of cross-validated cases were classified correctly and 73.6% of the trills from the year 2000 were allocated to their respective group of 2001 (Table 5). A chi-square test revealed that this distribution is significantly different from chance in each group (group 1: χ2=46.67, p<0.001; group 2: χ2=9.0, p<0.029; group 3: χ2=19.89, p<0.001). Thus, trills provided sufficient information to discriminate between neighbouring groups in our study area.
Discussion
Our study revealed an exclusive use of several sleeping sites by the observed sleeping groups of the golden brown mouse lemur. Communication signals used by group members during dispersal and reunion differed markedly. Marking behaviour occurred exclusively in the evenings during dispersal. In vocal behaviour, the distribution of trills showed a reversed asymmetry: they were recorded regularly during reunion in the morning, whereas, during dispersal, we recorded them only twice in the mating season and only when strangers were present. The trills of the different groups carried specific signatures.
Spacing
Safe sleeping sites protect individuals and groups against predators and adverse climatic conditions. If those sites represent limited resources like the tree holes or nests used by mouse lemurs (Radespiel et al. 1998, 2003), competition for them should be expected. Indeed, the exclusive sleeping site usage in the golden brown mouse lemur may reflect an indirect competition. A similar pattern is characteristic for a variety of animals which sleep in nests or tree holes, for example other nocturnal lemurs such as sportive lemurs (Rasoloharijaona et al. 2003), fork-crowned lemurs (Charles-Dominique and Petter 1980), fat-tailed dwarf lemurs (Müller 1999), and other mammals such as bats (Kerth et al. 2002).
The ownership of several safe sleeping sites may be indispensable for survival and reproductive success. The use of several sleeping sites scattered in space, however, raises three problems for a solitary ranging but communal nesting species: how to advertise the ownership of a given site, how to relocate it, and how to gather at a particular site and a distinct time on each day.
Marking behaviour
Marking behaviour at sleeping sites, predominantly urine-washing, occurred during dispersal but never during reunion. A similar pattern was found in female sleeping groups of the grey mouse lemur (Glatson 1983; Peters 1999).
Marks could on the one hand facilitate the relocation of the animals’ own sleeping sites (e.g. Seitz 1969) and could on the other hand serve to establish the group ownership of a sleeping site (e.g. Wyatt 2003) in order to reduce conflict between groups for a limited resource (e.g. Charles-Dominique 1977; Mertl-Millhollen 1988; Swaisgood et al. 2000). These relocation- and conflict avoidance hypotheses are supported by our data: if marking serves to relocate the sleeping sites there is no need for marking after relocation. Likewise, if marks indicate ownership and act as a signal to monopolise sites and to deter members of other groups, marks should be refreshed at the beginning of the active period in the evenings.
Vocal behaviour
Olfactory signals are not sufficient to attract and to guide group members at a particular time to a specific sleeping site. As groups change their sleeping sites from time to time (see this study and Weidt et al. 2004) the group members need signals which are not only attributable to the own group but also are indicators for a specific location at a particular moment. In dense forest, at night, acoustic signals are adequate communication signals to achieve these tasks. Observations in African galagos and pottos summarised in Bearder et al. (2003) suggest that vocalisations are important for group cohesion. Indeed, we found a specific call type, the trill, which occurred regularly during the reunions of sleeping groups. The trill may serve different functions: mate attraction/mate defence (Buesching et al. 1998; Zimmermann et al. 2000), resource defence and group coordination. According to the mate attraction- / mate defence-hypothesis, males and females of the golden brown mouse lemur should use trills during the mating season for courtship and/or to deter competitors. Similar vocal behaviours in the mating context are known for the grey mouse lemur (Zimmermann and Lerch 1993; Hafen 1998) and the coquerel’s dwarf lemur (Stanger 1995) as well as for other nocturnal strepsirrhines: bushbabies (Bearder and Doyle 1974; Zimmermann 1985a), slender loris (Radhakrishna and Singh 2002), slow loris (Zimmermann 1985b) and pottos (Charles-Dominique 1977). Moreover, trills used in the reproductive context were found in captive golden brown mouse lemurs (Polenz 2000; Zietemann 2001). Thus, the mate attraction- / mate defence-hypothesis may account for the trills recorded during dispersals. In the two dispersal cases where we heard trills, male strangers were in the area and presumably searching for oestrous females, and in one of these cases fights broke out.
However, the mate attraction- / mate defence-hypothesis is not sufficient to explain the occurrence of all trills: during reunions we recorded trills even 1 month before the beginning of the mating season (for reproduction cycle see Randrianambinina et al. 2003). In addition, this hypothesis cannot explain the temporal asymmetry in the occurrence of trills in our study, in which trills were uttered mainly during reunions.
Both, the resource defence- and the group coordination hypothesis are supported by the above temporal asymmetry. For resource defence, however, the group members are expected to use trills regularly at the resource, i.e. the sleeping site. In our study, trills occurred only occasionally at the sleeping site, whereas, in most cases, the individuals uttered trills before they reached the respective site: trills were predominantly uttered while members of a group aggregated in the vicinity of the sleeping site or while the whole group was moving towards the site. This renders it unlikely that the main function of trills is resource defence.
Three lines of evidence support the group coordination hypothesis. First, during reunions, trills of a group member never attracted collared members of other groups. Similarly, Weidt et al. (2004) which had fully collared groups never found strangers joining a sleeping group. Second, during four reunions, group members already present at the sleeping site left it to meet arriving individuals. Afterwards they returned together to the sleeping site. In this situation, trills were uttered. Finally, members of a group uttered trills during group movement towards the sleeping sites.
Group-specific acoustic signatures
A prerequisite for vocalisations regulating group coordination is their inter-group acoustic distinctiveness. Group differences may be based on individual differences or on group signatures. Individual call signatures have been reported for a number of primate species (e.g. Marler and Hobbett 1975; Zimmermann and Lerch 1993; Hammerschmidt and Todt 1995) and may have a perceptual relevance for conspecifics (e.g. Snowdon and Cleveland 1980; Cheney and Seyfarth 1982; Rendall et al. 1996). In our study, we could not always attribute the trills to the respective caller due to observational constraints at night. Overlapping series of trills from different individuals were found in all sleeping groups indicating that at least two individuals of the same group were calling and contributed to our sample. Thus, the characteristic differences in the trills between groups represent group signatures rather than those of single individuals. The signatures of the groups tested both in 2000 and 2001 showed a high degree of similarity. Group-specific signatures have been found in a variety of birds (Nowicki 1989; Hopp et al. 2001) and mammals (e.g. dolphins: Watwood et al. 2004; bats: Boughman and Wilkinson 1998; Dörrie et al. 2001).
Our study is the first account of group-specific signatures in group coordination calls of a nocturnal primate. The signatures may be explained by two different factors, inheritance (Winter et al. 1973; Scherrer and Wilkinson 1993), or acoustic convergence, especially within non-kin groups (e.g. Mundinger 1982; Zimmermann and Hafen 2001; Boughman 1997). Generally, the vocal system of anthropoid non-human primates is considered to be relatively unaffected by learning (e.g. Seyfarth and Cheney 1997). However, several studies suggest that the social environment may influence social call structure (e.g. Egnor and Hauser 2004) .
Conclusion
Our study presents the first context-related and quantitative evidence for mechanisms regulating inter-group spacing and intra-group cohesion in a nocturnal primate species. Most interestingly, we revealed that a call with group-specific signatures, the trill, is used during group coordination. So far, group coordination calls have only been shown for a number of diurnal permanently group-living primates (e.g. Boinski and Garber 2000) but not for nocturnal primates. Moreover, we have shown in the present study that trills of comparable structure may be used for mate attraction and/or mate defence. This suggests that group coordination calls might originate from mate attraction and/or mate defence calls, thus providing insight into the mechanisms driving the evolution of vocal communication.
References
Barre V, Lebec A, Petter J-J, Albignac R (1988) Étude du Microcèbe par radiotracking dans la forêt de l’Ankarafantsika. Deuxième Séance Technique 61–71
Bart J, Fligner MA, Notz WI (1998) Sampling and statistical methods for behavioural ecologists. Cambridge University Press, Cambridge, pp 177–187
Bearder SK, Doyle GA (1974) Field and laboratory studies of social organization in bushbabies (Galago senegalensis). J Hum Evol 3:37–50
Bearder SK, Ambrose L, Harcourt C, Honess P, Perkin A, Pimley E, Pullen S, Svoboda N (2003) Species-typical patterns of infant contact, sleeping site use and social cohesion among nocturnal primates in Africa. Folia Primatol 74:337–354
Boinski S, Garber PA (2000) On the move: how and why animals travel in groups. The University of Chicago Press, Chicago
Boughman JW (1997) Greater spear-nosed bats give group-distinctive calls. Behav Ecol Sociobiol 40:61–70
Boughman JW, Wilkinson GS (1998) Greater spear-nosed bats discriminate group mates by vocalizations. Anim Behav 55:1717–1732
Braune P, Polenz S, Zietemann V, Zimmermann E (2001) Species-specific signaling in two sympatrically living nocturnal primates, the grey and the golden brown mouse lemur (Microcebus murinus and Microcebus ravelobensis), in Northwestern Madagsacar. Adv Ethol 36:126
Buesching CD, Heistermann M, Hodges JK, Zimmermann E (1998) Multimodal oestrus advertisement in a small nocturnal prosimian, Microcebus murinus. Folia Primatol 69:295–308
Charles-Dominique P (1977) Ecology and behaviour of nocturnal primates. Columbia University Press, New York
Charles-Dominique P, Petter JJ (1980) Ecology and social life of Phaner furcifer. In: Charles-Dominique P, Cooper HM, Hladik A, Hladik CM, Pages E, Pariente GF, Petter-Rousseaux A, Schilling A, Petter JJ (eds) Nocturnal Malagasy primates. Ecology, physiology, and behavior. Academic Press, New York, pp 75–95
Cheney DL, Seyfarth RM (1982) Recognition of individuals within and between groups of free-ranging vervet monkeys. Am Zool 22:519–529
Couzin I, Krause J (2003) Self-organization and collective behavior in vertebrates. Adv Study Behav 32:1–75
de Waal FBM, Tyack PL (2003) Animal social complexity: intelligence, culture, and individualized societies. Harvard University Press, Cambridge, MA
Dörrie M, Schmidt S, Suba M, Sripathi K (2001) Contact calls of the bat, Megaderma lyra: a comparison between an Indian and a Sri Lankan population. Zoology 104:5
Egnor SER, Hauser MD (2004) A paradox in the evolution of primate vocal learning. Trends Neurosci 27:649–654
Fietz J (1999) Monogamy as a rule rather than exception in nocturnal lemurs: the case of the fat-tailed dwarf lemur, Cheirogaleus medius. Ethology 105:259–272
Fleagle JG, Janson C, Reed KE (1999) Primate communities. Cambridge University Press, Cambridge
Glatson AR (1983) Olfactory communication in the Lesser Mouse Lemur (Microcebus murinus). In: Seth PK (ed) Perspectives in primate biology. Today & Tomorrow’s Printers and Publishers, New Delhi, pp 63–73
Hafen TG (1998) Dialekte bei Lemuren: bioakustische, morphometrische und molekulargenetische Untersuchungen zur intraspezifischen Variabilität beim grauen Mausmaki (Microcebus murinus). Cuvillier Verlag, Göttingen
Hammerschmidt K, Todt D (1995) Individual differences in vocalisations of young Barbary macaques (Macaca sylvanus): a multi-parametric analysis to identify critical cues in acoustic signalling. Behaviour 132:381–399
Harcourt C (1991) Diet and behaviour af a nocturnal lemur, Avahi laniger, in the wild. J Zool Lond 223:667–674
Hauser MD (1996) The evolution of communication. MIT Press, Cambridge, MA
Hopp SL, Jablonski P, Brow JL (2001) Recognition of group membership by voice in Mexican jays, Aphelocoma ultramarina. Anim Behav 62:297–303
Janson C (2000) Spatial movement strategies: theory, evidence, and challenges. In: Boinski S, Garber PA (eds) On the move: how and why animals travel in groups. The University of Chicago Press, Chicago, pp 165–203
Kappeler PM, van Schaik CP (2002) Evolution of primate social systems. Int J Primatol 23:707–740
Kerth G, Safi K, König B (2002) Mean colony relatedness is a poor predictor of colony structure and female philopatry in the communally breeding Bechstein’s bat (Myotis bechsteinii). Behav Ecol Sociobiol 52:203–210
Marler P, Hobbett L (1975) Individuality in a long-range vocalization of wild chimpanzees. Z Tierpsychol 38:97–109
Mertl-Millhollen AS (1988) Olfactory demarcation of territorial but not home range boundaries by Lemur catta. Folia Primatol 50:175–187
Mundinger PC (1982) Microgeographic and macrogeographic variation in the acquired vocalizations of birds. In: Kroodsma DE, Miller EH, Ouellet H (eds) Acoustic communication in birds, vol. 2. Song learning and its consequences. Academic Press, New York, pp 147–208
Müller AE (1999) Social organization of the fat-tailed dwarf lemur (Cheirogaleus medius) in Northwestern Madagascar. In: Rakotosamimanana B, Rasamimanana H, Ganzhorn JU, Goodman SM (eds) New directions in lemur studies. Kluwer /Plenum, New York, pp 139–157
Müller AE, Thalmann U (2000) Origin and evolution of primate social organisation: a reconstruction. Biol Rev 75:405–435
Müller AE, Thalmann U (2002) Biology of the fat-tailed dwarf lemur (Cheirogaleus medius E. Goeffroy 1812): new results from the field. Evol Anthropol 11(Suppl.1):79–82
Nowicki S (1989) Vocal plasticity in captive black-capped chickadees: the acoustic basis and rate of call convergence. Anim Behav 37:64–73
Pastorini J, Martin RD, Ehresmann P, Zimmermann E, Forstner MJR (2001) Molecular phylogeny of the lemur family cheirogaleidae (Primates) based on mitochondrial DNA sequences. Mol Phylogenet Evol 19:45–56
Peters C (1999) Intrasexuelle Konkurrenz bei grauen Mausmaki-Männchen (Microcebus murinus) in Nordwest-Madagaskar. Diploma-thesis, University of Hannover, Germany
Polenz S (2000) Akustisches und soziales Verhalten des goldbraunen Mausmakis (Microcebus ravelobensis) während der Paarungszeit. Diploma-thesis, University of Hannover, Germany
Radespiel U (2000) Sociality in the gray mouse lemur (Microcebus murinus) in northwestern Madagascar. Am J Primatol 51:21–40
Radespiel U, Cepok S, Zietemann V, Zimmermann E (1998) Sex-specific usage patterns of sleeping sites in grey mouse lemurs (Microcebus murinus) in Northwestern Madagascar. Am J Primatol 46:77–84
Radespiel U, Ehresmann P, Zimmermann E (2003) Species-specific usage of sleeping sites in two sympatric mouse lemur species (Microcebus murinus and Microcebus ravelobensis) in Northwestern Madagascar. Am J Primatol 59:139–151
Radhakrishna S, Singh M (2002) Social behaviour of the slender loris (Loris tardigradus lydekkerianus). Folia Primatol 73:181–196
Randrianambinina B, Rakotondravony D, Radespiel U, Zimmermann E (2003) Seasonal changes in general activity, body mass and reproduction of two small nocturnal primates: a comparison of the golden brown mouse lemur (Microcebus ravelobensis) in Northwestern Madagascar and the brown mouse lemur (Microcebus rufus) in Eastern Madagascar. Primates 44:321–331
Rasoloharijaona S, Rakotosamimanana B, Randrianambinina B, Zimmermann E (2003) Pair-specific usage of sleeping sites and their implications for social organization in a nocturnal malagasy primate, the Milne Edwards’ sportive lemur (Lepilemur edwardsi). Am J Phys Anthropol 122:251–258
Rendall D, Rodman P, Emond RE (1996) Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim Behav 51:1007–1015
Scherrer JA, Wilkinson GS (1993) Evening bat isolation calls provide evidence for heritable signatures. Anim Behav 46:847–860
Schilling A (1979) Olfactory communication in Prosimians. In: Doyle GA, Martin RD (eds) The Study of Prosimian Behavior. Academic Press, New York, San Francisco, London, pp 461–542
Schmelting B, Ehresmann P, Lutermann H, Randrianambinina B, Zimmermann E (2000) Reproduction of two sympatric mouse lemur species (Microcebus murinus and M. ravelobensis) in north-west Madagascar: first results of a long term study. In: Lorenzo WR, Goodman SM (eds) Diversité et Endémisme à Madagascar. Société de Biogéographie, Paris, pp 165–175
Schülke O, Kappeler PM (2003) So near and yet so far: territorial pairs but low cohesion between pair partners in a nocturnal lemur, Phaner furcifer. Anim Behav 65:331–343
Seitz E (1969) Die Bedeutung geruchlicher Orientierung beim Plumplori Nycticebus coucang Boddaert 1785 (Prosimii, Lorisidae). Z Tierpsychol 26:73–103
Seyfarth RM, Cheney DL (1997) Some general features of vocal development in nonhuman primates. In: Snowdon CT, Hausberger M (eds) Social influences on vocal development. Cambridge University Press, Cambridge, pp 249–273
Snowdon CT, Cleveland J (1980) Individual recognition of contact calls by pygmy marmosets. Anim Behav 28:717–727
Stanger KF (1995) Vocalizations of some cheirogaleid prosimians evaluated in a phylogenetic context. In: Alterman L, Doyle GA, Izard MK (eds) Creatures of the dark: the nocturnal prosimians. Plenum Press, New York, pp 353–376
Sterling EJ, Richard AF (1995) Social organization in the aye-aye (Daubentonia madagascariensis) and the perceived distinctiveness of nocturnal primates. In: Alterman L, Doyle GA, Izard MK (eds) Creatures of the dark: the nocturnal prosimians. Plenum Press, New York, pp 439–451
Swaisgood RR, Lindburg DG, Zhou X, Owen MA (2000) The effects of sex, reproductive condition and context on discrimination of conspecific odours by giant pandas. Anim Behav 60:227–237
van Schaik CP, van Hooff JARAM (1983) On the ultimate causes of primate social systems. Behaviour 85:91–117
Watwood SL, Tyack PL, Wells RS (2004) Whistle sharing in paired male bottlenose dolphins, Tursiops truncatus. Behav Ecol Sociobiol 55:531–543
Weidt A, Hagenah N, Randrianambinina B, Radespiel U, Zimmermann E (2004) Social organization of the golden brown mouse lemur (Microcebus ravelobensis). Am J Phys Anthropol 123:40–51
Winter P, Handley P, Ploog D, Schott D (1973) Ontogeny of squirrel monkey calls under normal conditions and under acoustic isolation. Behaviour 67:230–239
Wrangham RW (1987) Evolution of social structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate Societies. The University of Chicago Press, Chicago, London, pp 282–296
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press, Cambridge
Zietemann V (2001) Artendiversität bei Mausmakis: die Bedeutung der akustischen Kommunikation. PhD thesis, University of Hannover, Germany
Zimmermann E (1985a) The vocal repertoire of the adult Senegal bushbaby (Galago senegalensis senegalensis). Behaviour 94:212–233
Zimmermann E (1985b) Vocalizations and associated behaviors in adult slow loris (Nycticebus coucang). Folia Primatol 44:52–64
Zimmermann E (1992) Vocal communication by non-human primates. In: Jones S, Martin R, Pilbeam D (eds) The Cambridge encyclopedia of human evolution. Cambridge University Press, Cambridge, pp 124–127
Zimmermann E (1995) Acoustic communication in nocturnal prosimians. In: Altermann L, Doyle GA, Izard MK (eds) Creatures of the dark. Plenum Press, New York, pp 311–330
Zimmermann E, Hafen TG (2001) Colony specificity in a social call of mouse lemurs (Microcebus ssp.). Am J Primatol 54:129–141
Zimmermann E, Lerch C (1993) The complex acoustic design of an advertisement call in male mouse lemurs (Microcebus murinus, Prosimii, Primates) and sources of its variation. Ethology 93:211–224
Zimmermann E, Cepok S, Rakotoarison N, Zietemann V, Radespiel U (1998) Sympatric mouse lemurs in north-west Madagascar. A new rufous mouse lemur species (Microcebus ravelobensis). Folia Primatol 69:106–114
Zimmermann E, Vorobieva E, Wrogemann D, Hafen T (2000) Use of fingerprinting for species-discrimination of the gray (Microcebus murinus) and the rufous mouse lemur (Microcebus rufus). Int J Primatol 5:837–852
Zinner D, Hilgartner RD, Kappeler PM, Pietsch T, Ganzhorn JU (2003) Social organization of Lepilemur ruficaudatus. Int J Primatol 24:869–888
Zöfel P (1992) Statistik in der Praxis. Gustav Fischer Verlag, Stuttgart, Germany
Acknowledgements
We thank the Commission Tripartite of the Malagasy government, the Departement des Eaux et Forêts and the Association pour la Gestion des Aires for their permission to work in Ampijoroa and Prof. Berthe Rakotosamimanana and Dr. Daniel Rakotondravony, Faculté des Sciences, Université d’Antananarivo for logistic support. For field assistance, we thank Andrea Weidt, Katja Wallmeyer and Julia Schwarzer. The latter also contributed to the sound analysis. Dagmar Söndgerath gave helpful comments for statistical analysis. We thank the anonymous reviewers for helpful comments on earlier versions of the manuscript. The study complies with the current laws of Madagascar and was funded by the German Research Council (GK 289; Zi 345/12) and the Volkswagen Foundation (VW I/76968)
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Nunn
Rights and permissions
About this article
Cite this article
Braune, P., Schmidt, S. & Zimmermann, E. Spacing and group coordination in a nocturnal primate, the golden brown mouse lemur (Microcebus ravelobensis): the role of olfactory and acoustic signals. Behav Ecol Sociobiol 58, 587–596 (2005). https://doi.org/10.1007/s00265-005-0944-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0944-4