Abstract
Multilevel societies are unique in their ability to facilitate the maintenance of strong and consistent social bonds among some individuals while allowing separation among others, which may be especially important when social and sexual bonds carry significant and reliable benefits to individuals within social groups. Here we examine the importance of social and sexual bonds in the multilevel society of hamadryas baboons (Papio hamadryas) and apply these principles to social evolution in Plio-Pleistocene hominins. The behavior, adaptations, and socioecology of baboons (Papio spp.) have long been recognized as providing an important comparative sample to elucidate the processes of human evolution, and the social system of hamadryas baboons in particular shares even more similarities with humans than that of other baboons. Here we draw parallels between processes during the evolution of hamadryas social organization and those characterizing late Pliocene or early Pleistocene hominins, most likely Homo erectus. The higher costs of reproduction faced by female Homo erectus, exacerbated by an increased reliance on difficult to acquire, nutrient-dense foods, are commonly thought to have been alleviated by a strengthening of male–female bonds (via male provisioning and the evolution of monogamy) or by the assistance of older, postreproductive females (via grandmothering). We suggest that both of these social arrangements could have been present in Plio-Pleistocene hominins if we assume the development of a multilevel society such as that in hamadryas baboons. The evolution of a multilevel society thus underlies the adaptive potential for the complexity that we see in modern human social organization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multilevel societies, also known as hierarchical or modular societies, characterize a wide array of mammals, including some primates (Connor et al. 1998; Dunbar and Dunbar 1975; Rubenstein 2010; Wittemyer et al. 2005; Zhang et al. 2006). Unlike societies with classic or “atomistic” fission–fusion dynamics (cf. Grueter et al. 2012) —in which patterns of cleavage are variable and subunits not hierarchical (Mitani et al. 2002; Smolker et al. 1992; Strier 1992)— multilevel societies are “molecular” in that they cleave in highly predictable ways according to established membership in one or more subunits, often kin-based, within larger units (Grueter et al. 2012).
Multilevel societies thus allow the possibility of varying sized social units that are differentially expressed depending on ecological and social contingencies. Patterns of cleavage and coalescence may depend on the cost–benefit ratio of food and mate competition, which may favor smaller social groupings, versus social coordination to defend resources or guard against predation, which usually favors larger groupings (van Schaik 1983; Wrangham 1980). Such flexibility presumably benefits individuals within these societies, and fitness benefits to both male (dolphins, Tursiops spp.: Krützen et al. 2003) and female (elephants, Loxodonta africana: Archie et al. 2006) members of multilevel societies have been identified and measured.
In primates, multilevel societies often occur in extreme or marginal habitats in which resource availability drops sharply on a seasonal basis (Grüter and Zinner 2004). Snub-nosed monkeys (Rhinopithecus spp.), for example, occupy highly seasonal montane regions of China (Ren et al. 2012 and Zhang et al. 2012), geladas (Theropithecus gelada) the high altitude Simien plateau of Ethiopia (Dunbar and Dunbar 1975), and hamadryas baboons (Papio hamadryas) the arid, semidesert lowlands of the Horn of Africa and Arabia (Kummer 1968; Schreier and Swedell 2012a). A multilevel social system in such habitats allows groups to splinter for more efficient foraging and coalesce for protection against predation or defense of resources according to changes in food distribution and predator pressure, which may vary spatially and over both short, e.g., daily, and long, e.g., seasonally, time scales. Whereas nonmodular societies with fission–fusion dynamics, e.g., chimpanzees (Pan spp.) also allow flexibility, modular societies cleave along predictable lines and thus facilitate consistent social bonds among some individuals while allowing separation among others. This may be especially important when social and sexual bonds carry significant and reliable benefits to individuals.
Here we examine the importance of social and sexual bonds in the multilevel society of hamadryas baboons and apply these principles to social evolution in a Plio-Pleistocene hominin, tentatively identified as Homo erectus (sensu lato). Our aim is not to present a rigid reconstruction by analogy, but to point out the potentially significant implications of a modular social structure in the context of the discussion of pair bonding and food sharing in hominin evolution. As new fossils are unearthed and paleoanthropological interpretations revised, our hypothesized scenario may best apply to a taxon other than Homo erectus. Nevertheless, we hope that the general principles and processes that we describe here will be useful in improving our understanding of the evolution of sociality during the history of the hominin lineage.
A Baboon Multilevel Society as a Model for Human Evolution
Baboons are large-bodied, terrestrial, highly social, and behaviorally flexible primates that occupy a wide array of habitats similar to those of early hominins —all features that have made them one of the three most successful primate genera in the world today and exceptionally useful as analogues for hominin behavioral and biological evolution (Washburn and DeVore 1961a, b; DeVore and Washburn 1963; Elton 2006; Harvati et al. 2004; Jolly 1970, 2001; Rose 1976; Strum and Mitchell 1987; Swedell 2011; Codron et al. 2008; Zuckerman 1932). A key difference, however, between humans and the well studied savanna-dwelling or “savanna” baboons (cf. Jolly 1993) is that only humans live in groups that are “dependent on and affiliated with one another in a semiopen system” with “subgroups based on kinship” (Washburn and DeVore 1961a). One need only turn to the closest relative of savanna baboons, the hamadryas or sacred baboon (Papio hamadryas), to find a better analogue for humans in this respect.
Unlike other baboons, hamadryas display a combination of the male kin bonding thought to have characterized early hominins (Foley 1989, 1996), the male–female pair-bonding thought to have developed at some point during human evolution (Isaac 1978; Lovejoy 1981), and the female bonding underlying the grandmother hypothesis for the evolution of female postreproductive longevity (Hawkes et al. 2000). Like modern humans, hamadryas baboons (and Guinea baboons, from data collected thus far: Galat-Luong et al. 2006; Patzelt et al. 2011) are organized into hierarchical social networks in which individuals are connected at multiple levels; this hierarchical structure characterizes both Western industrialized societies and modern hunter-gatherers (Chapais 2008; Foley and Gamble 2009; Hamilton et al. 2007; Layton et al. 2012; Rodseth et al. 1991). Jolly (1970) proposed an explicit link between early hominin social organization and the multilevel society of geladas and hamadryas baboons, and Alexander and Noonan (1979) suggested that if early humans did live in “small harem groups” then they probably also formed multimale groupings for cooperative predator defense. Rodseth et al. (1991), in their expansion of Foley and Lee’s (1989) framework, noted that in only humans and hamadryas baboons do males remain with their kin and maintain exclusive sexual associations with females over time. Chapais (2008) elaborated on this comparison, noting that the hamadryas social system is unique among nonhuman primates in its combination of outbreeding, male kin groups, and exclusive cross-sex mating bonds. Parker (2004) also drew indirect parallels between humans and hamadryas baboons, using hamadryas as a point of comparison with chimpanzees to elucidate the implications of socialization within multiple levels of society for the development of human cognition. Most recently, Foley and Gamble (2009, p. 3268) listed several key derived behavioral traits of humans —“a fission-fusion social system”; “much greater substructuring within multimale, multifemale communities”; “strong and persistent male–female relationships”; “higher levels of paternal investment”; and “larger group sizes”— all of which characterize hamadryas baboons.
Following Jolly (1970), one might posit that geladas (Theropithecus gelada) are an equally valid analogue for a multilevel society during human evolution. We focus on hamadryas here, for several reasons. First, hamadryas society includes the “clan” layer of social structure, which is crucial to the retention of male kin bonding in our evolutionary scenario. In geladas, the structural equivalent to the hamadryas clan is the “team,” but teams appear to be merely the outcome of a fission of one large one-male unit (OMU; Kawai et al. 1983; Snyder-Mackler et al. 2012). Moreover, geladas do not exhibit the strong social bonds among males nor the apparent male kin network of hamadryas (Abegglen 1984; Schreier and Swedell 2009), even in gelada bachelor groups (Beehner pers. comm.). Second, the composition of gelada bands is variable, i.e., they may consist of different sets of OMUs over time (Snyder-Mackler et al. 2012), and experimental evidence suggests that gelada individuals may not even recognize one another within bands (Bergman 2010). Together this suggests that bands are not as socially meaningful entities to geladas as they are for hamadryas. Third, strong and persistent bonds between the sexes do not occur in geladas, which are instead characterized by a more strictly female-bonded social organization (le Roux et al. 2011; Matsuda et al. 2012). Finally, modern geladas rely primarily on an abundant and evenly dispersed resource, monocotyledon grasses (Iwamoto 1979; Dunbar 1992a), whereas the hamadryas diet includes more patchily distributed resources such as flowers, seeds, and leaves of Acacia (Schreier 2010; Swedell et al. 2008), a difference in resource use that would predict far greater food-related ecological pressures in hamadryas compared to geladas (Dunbar 1983; Kummer 1968; Schreier and Swedell 2012b). As hypothesized by Dunbar (1983), in hamadryas the ancestral Papio multimale group probably broke up into smaller units “to facilitate foraging under conditions of scarce food resources,” whereas in geladas isolated OMUs congregated into larger groupings “under conditions of better food availability in response to predation pressure.” Together the aforementioned differences, both empirical and hypothesized, render geladas an ecologically and socially less appropriate model for the scenario here. Possibly more appropriate than geladas would be Guinea baboons (Papio papio), as evidence to date suggests that their social structure bears many similarities to hamadryas (Galat-Luong et al. 2006; Patzelt et al. 2011); continuing research on the social behavior of this taxon should further elucidate its applicability to the discussions herein.
Hamadryas Baboon Social Organization
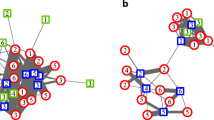
The hamadryas social system is characterized by four hierarchical layers: the troop, band, clan, and one-male unit (Abegglen 1984; Kummer 1968, 1971; Schreier and Swedell 2009; Swedell 2006). Troops emerge as aggregations at common resources but are not cohesive or consistent social entities. Bands, whose members share a common home range and generally coordinate their movements, are the likely homologue to a group or troop of other Papio and cercopithecine monkeys. Within bands are less spatially distinct subgroups called clans, defined via patterns of spatial association among males, which tend not to transfer out of their natal clan (Sigg et al. 1982; Schreier and Swedell 2009); members of clans resemble one another and may be close kin (Abegglen 1984; Pines et al. 2011). Finally, within bands and clans are a number of one-male units (OMUs), each comprising one adult leader male, one or more adult females, dependent offspring, and sometimes one or more follower males. The largest proportion (>30 %) of OMUs are male–female pairs, i.e., leader males that have accumulated only one female in their OMU (Swedell et al. 2011). Most social interactions occur within OMUs, and cohesion of OMUs is maintained via aggressive herding behavior of leader males (Swedell and Schreier 2009). As a result, leaders have nearly exclusive sexual access to females in their OMUs (Abegglen 1984; Kummer 1968; Swedell and Saunders 2006). Also within bands and clans are solitary males, which are neither leaders nor followers of OMUs but move throughout the band and maintain social bonds with other males and juveniles (Pines et al. 2011).
Hamadryas social organization adds complexity to the sex-biased dispersal of most cercopithecoids (Colmenares 2004; Swedell 2011). Unlike in other baboons (Alberts and Altmann 1995, 2006), females are the main agents of gene flow in hamadryas populations (Hammond et al. 2006; Hapke et al. 2001; Sigg et al. 1982; Swedell et al. 2011). Females do not disperse voluntarily nor via eviction, however, but are coerced by males to leave one OMU and join another (Kummer 1968; Swedell and Schreier 2009). Female transfer across bands occurs via abduction during encounters at sleeping cliffs and elsewhere (Kummer 1968; Pines and Swedell 2011; Sigg et al. 1982). Males may also occasionally disperse (usually temporarily) in search of reproductive opportunities (Phillips-Conroy et al. 1992; Sigg et al. 1982).
Thus, unlike other baboons in which philopatric females form the stable core of the social group, hamadryas are characterized by kin bonding among (mostly) philopatric males, which largely control female behavior (Abegglen 1984; Colmenares 2004; Kummer 1968; Pines et al. 2011; Sigg et al. 1982; Swedell and Schreier 2009). Ultimately, this system is an outcome of intrasexual competition among males, which compete to “possess” females (Kummer 1968).
Social Bonding in Hamadryas Baboons: Males
All evidence to date suggests that most hamadryas males remain within (or return to) their natal clans and that male kinship facilitates access to females (Abegglen 1984; Colmenares 1991a, b, 2004; Pines et al. 2011; Sigg et al. 1982). Males within clans form and maintain social bonds, which are characterized by grooming and formalized, stereotypical notifying behavior, whereby one male quickly approaches, looks at, presents to, and then leaves another male (Abegglen 1984; Kummer 1968). In contrast to other baboons, adult male hamadryas rarely interact aggressively over estrous females or attempt to copulate with females in other OMUs of the same band (Kummer 1968, 1971; Kummer et al. 1974). Female transfers also occur among members of the same clan more often than between clans (Swedell et al. 2011). While genetic data are still forthcoming, follower males of OMUs may be close relatives of leader males, and followers that help defend members of their OMUs may stand to gain via inclusive fitness.
Social Bonding in Hamadryas Baboons: Females
Hamadryas females have fewer opportunities to interact with kin owing to male-imposed dispersal (Swedell et al. 2011). Despite this, they do forge and maintain intrasexual bonds, albeit highly variable, and these bonds occasionally cross OMU boundaries (Swedell 2002, 2006). Because females are transferred one at a time, the occurrence of kin dyads within OMUs undoubtedly varies widely, and this highly variable potential for kin selection may thus underlie variation in patterns of affiliation among females (Colmenares 2004; Swedell 2002, 2006). Whether with kin or nonkin, bonds among females are likely beneficial through their fitness enhancing effects (cf. Silk 2007a, b), possibly via stress reduction (cf. Silk et al. 2009; Uchino 2006).
Social Bonding in Hamadryas Baboons: Cross-Sex Bonds
Arguably the strongest in hamadryas society, bonds between females and their leader males underlie cohesion of OMUs and associated variance in reproductive success among males (Colmenares 2004; Kummer 1968; Swedell 2002). Males use aggression to condition females in their OMUs to remain nearby, and consequently females maintain proximity to males more so than in other baboons (Kummer 1968; Swedell and Schreier 2009). Females and their leader males also exchange substantial amounts of grooming, though this varies widely across females and OMUs (Swedell 2002). To the extent that leaders can ward off copulation attempts by nonleaders, the number of bonds that a male can forge and maintain with females, combined with his tenure as leader, will predict his eventual reproductive success (Pines et al. 2011).
These strong cross-sex bonds also likely facilitate offspring survival. Infanticide can be a risk for baboon infants (Palombit 2003; Swedell and Tesfaye 2003), and hamadryas females may effectively mitigate this risk by focusing their social and reproductive efforts on a single male and thereby following a paternity concentration rather than paternity confusion anti-infanticide strategy (cf. Swedell and Saunders 2006; van Schaik et al. 1999). Infant survival is in fact higher in hamadryas compared to other baboons (Sigg et al. 1982), probably a consequence of the closed OMU structure and the protective nature of hamadryas leader males.
Social Bonds at Multiple Levels
Hamadryas social organization is thus multilayered, with affiliative bonds operating at multiple levels. Within OMUs, strong cross-sex bonds tie each female to her leader male. Bonds among females within OMUs vary in strength, perhaps according to degree of kinship (Colmenares 2004; Swedell 2002, 2006). Leader and follower males within OMUs rarely interact except via formalized “notifying” behavior, but males in general are linked via social bonds at the clan level (Abegglen 1984; Pines et al. 2011; Swedell and Schreier 2009). The hamadryas system thus includes female–female bonds within (and occasionally between) OMUs, male–female bonds within OMUs, and male–male bonds within clans and possibly bands (Abegglen 1984; Colmenares 2004; Kummer 1968; Pines et al. 2011; Swedell 2002, 2006).
The Evolution of Hamadryas Social Organization
The hamadryas social system most likely evolved in response to the aridity of the Horn of Africa and/or the Arabian Peninsula, where resources are (and were during hamadryas evolution) scarcer and more patchily distributed than baboon habitats elsewhere (Bedaso et al. 2010; Dunbar 1988; Jolly 1963, 1993; Kummer 1968, 1971; Schreier and Swedell 2012b). Given overall patterns in primate social evolution (cf. Shultz et al. 2012) and our understanding of the evolutionary history of Papio (Jolly 2009), the most likely evolutionary scenario would posit a transition from a multimale–multifemale system, such as that of most extant baboons (most notably Papio anubis and P. cynocephalus), to the substructured system characterizing hamadryas today (Bercovitch 1990; Dunbar 1988). The first step, cf. Jolly (2009), may have been the evolution of male philopatry during the northward expansion of ancestral baboons from southern Africa, where they likely originated, or during the subsequent northward dispersal and differentiation of hamadryas from olive baboons in East Africa at a later point in time (Zinner et al. 2009). This expansion into unoccupied territory would have occurred in three directions, increasing distance between troops and leaving only one main direction, i.e., backwards, in which dispersing males were guaranteed to find fertile females. Such a scenario would select for male philopatry (Jolly 2009).
Regardless of when male philopatry evolved, the patchier distribution of food resources across space and time in these drier northern African environments would have favored the temporary splintering of groups into smaller units during foraging. These may have been female kin groups, reflecting an evolutionary retention of the propensity of cercopithecine females to remain with kin. Alternatively, these units might have originated as multiple sets of cross-sex friendships (cf. Bercovitch 1990; Smuts 1985), as unrelated olive baboon females are known to fission into OMUs with a mutual male friend during times of limited resources (Bercovitch pers. comm.; Strum 1987). Either way, selection would have eventually favored males that were able to defend these groups from other males semipermanently so as to maximize both mating exclusivity and infant survival. This would have increased levels of male competition, as these polygynous groups would have left a surplus of unattached males and increased the risk of infanticide by these males, thereby intensifying selection for male defense. The ability to carve off extra females from existing groups —as well as the ability to defend females from these incursions— would have been favored, leading to the ritualized herding behavior of hamadryas males. It is this high level of male competition and coercion of females that differentiates hamadryas OMUs from those of other baboons: as noted by previous scholars in comparisons of baboon social behavior (Aldrich-Blake et al. 1971; Anderson 1983), hamadryas OMUs are not simply foraging parties but are social and reproductive units that are socially cohesive, consistent over time, and sustained by male behavior. This control of female behavior by males, by virtue of the fact that it substructures reproduction into subunits within larger social units, effectively allows both sexes to remain largely philopatric within bands, thereby avoiding the high costs of locational dispersal in arid habitats (cf. Isbell and Van Vuren 1996). It is thus likely that the ritualized male herding and associated substructuring of hamadryas society evolved in concert with male philopatry. During this process, the largest social units of hamadryas society would have been retained owing to their defense benefits, which probably led to the intergroup tolerance that allows formation of troops at sleeping cliffs.
During hamadryas evolution, each level of society was likely important in different ways: one-male units as the primary reproductive units and social units for females as well as ecological units during times of heightened food scarcity; clans as kin-based cooperative networks among males and ecological units when necessary; bands as social units for males and ecological units to reduce predation risk and defend resources, e.g., doum palm forest patches and sleeping sites; and troops as ephemeral units of convenience at shared sleeping sites, providing enhanced protection against nocturnal predation (Abegglen 1984; Kummer 1968, 1990; Schreier and Swedell 2012b). The large, striking manes, red faces, and red paracallosal skin of hamadryas males would have been favored by intrasexual selection, as signals to other males —and possibly also via intersexual selection, as the basis for female choice and cooperation, cf. Jolly (1963)— as such testosterone-driven features (Zuckerman and Parkes 1939) are good indicators of competitive ability.
Key Differences Between Hamadryas and Other Baboons
Hamadryas society differs from that of other baboons in several key respects. First, male–male bonds within clans (and possibly bands) are stronger and males appear to cooperate over access to females. Second, strong cross-sex bonds persist regardless of female reproductive state, an unusual pattern for baboons and primates as a whole, and males effectively control female behavior. Third, hamadryas society has multiple layers, varying in function. Fourth, both males and females are largely philopatric: neither sex is motivated to disperse from clans or bands to any large degree. Rather, males occasionally leave their natal clans (though more often remain in them) in search of available females, and females are forcibly transferred among OMUs by males.
Predictability in the cleavage points of hamadryas society reflects the importance of its underlying social bonds. Of these, male–female bonds are paramount and carry obvious benefits: males via exclusive access to mates, and both sexes via protection from infanticide (Kummer 1968; Swedell and Saunders 2006). Bonds among males are beneficial in increasing access to females (Kummer 1968; Pines et al. 2011), and bonds among females are likely beneficial via stress reduction effects on health and fitness (cf. Uchino 2006). We now turn to potentially similar patterns of bonding and associated reproductive strategies in Plio-Pleistocene hominins.
Parallels with Hominin Social Evolution
Pliocene Hominins: The Ancestral Condition
Patterns of dispersal and philopatry in Pliocene hominins are unknown yet fundamental in drawing inferences about kinship and patterns of social bonding. That australopiths were characterized by male philopatry is usually postulated based on the assumption that this pattern characterized the last common ancestor of chimpanzees and australopiths (Foley 1989; Maryanski 1996; McHenry 1996; Wrangham 1987). This characterization has recently found support from geochemical analysis of Australopithecus africanus and Paranthropus robustus teeth from South Africa, in which smaller (presumably female) individuals exhibited a nonlocal isotopic signature compared to the larger (presumably male) individuals (Copeland et al. 2011), suggesting that females were born elsewhere. We adopt a more conservative approach following Strier (2001) and suggest that the last common ancestor of humans and chimpanzees was in fact characterized by bisexual dispersal. We base this on the relatively conservative nature of sex-biased dispersal and the fact that bisexual dispersal is the ancestral primate condition (Shultz et al. 2012) and characterizes the majority of extant apes as well as a wide array of other primates, including most modern hunter-gatherer societies (Chapais 2008; Jack and Isbell 2009).
The Emergence of Homo erectus
Middle Pliocene Australopithecus fossil finds are associated with environments with moderate to substantial amounts of woodland (Reed 1997). Marine sediment sequences suggest that variations in Earth’s orbit influenced global climate, leading to oscillations between wetter and drier conditions in Africa in the late Pliocene and Pleistocene, with steplike increases in African climate variability and aridity around 2.8 million years ago (Ma), 1.7 Ma, and 1.0 Ma related to the onset and intensification of northern latitude glacial cycles (deMenocal 2004; Cerling et al. 2011; Kingston 2007). Whereas late Pliocene and early Pleistocene African environments continued to be complex, spatially heterogeneous mosaics of forest, woodland, bushland, and grassland, grasses and other arid-adapted vegetation often made up a greater proportion of the floral community than at middle Pliocene Australopithecus sites (Bobe and Behrensmeyer 2004; Cerling et al. 2011; Kingston 2007; Plummer 2004; Plummer et al. 2009; Vrba 1985, 1988). Events in the hominin evolutionary record between roughly 3.0 and 1.7 Ma, including the extinction of Australopithecus, the origins of Homo and Paranthropus, the appearance of the first stone tools, the first evidence for large mammal butchery, and the origin of Homo erectus have been viewed as evolutionary responses to shifts in resource availability reflecting the impact of global climate change on continental African flora and fauna (deMenocal 2004; Plummer 2004; Potts 1998).
The taxon Homo erectus (sensu lato) appeared in Africa ca. 1.9 Ma, and shortly thereafter in Eurasia, and with its modern limb proportions it signaled a clear commitment to obligate bipedality and terrestrial foraging (Antón 2003; Ruff 2008, 2009). Homo erectus had a larger average body size than previous hominin taxa (Antón 2008; Pontzer in press), though the traditional view that it was significantly less sexually dimorphic than earlier hominins has been recently questioned (Antón 2003; Baab 2008). Indirect evidence for adaptation to greater aridity includes limb proportions comparable to modern African populations adapted to arid climates today, as well as evidence for a projecting nose to facilitate water retention during respiration (Franciscus and Trinkaus 1988; McHenry and Coffing 2000; Ruff 2002; Wheeler 1984). Homo erectus made and used stone tools for processing animal carcasses, the bones of which are found at archeological sites, as well as for working wood (Dominguez-Rodrigo et al. 2001), and probably used tools to acquire and process plant foods (O’Connell et al. 1999; Schick and Toth 1993). Resource transport was an important part of its adaptation, both to bring stone tools to places with resources requiring processing, and also in the delayed consumption and transport of at least some classes of nutrient dense foods, e.g., animal tissue, possibly for sharing among individuals (Isaac 1978; Plummer 2004; Potts 1991).
The fragmentary fossil record for early Homo species makes it difficult to define them taxonomically and to infer the types and significance of adaptive shifts that took place during the early evolution of the genus (Antón 2008). What does seem clear is that members of the genus Homo were extracting resources from a range of environments by ca. 2.0 Ma (Plummer et al. 2009; van der Merwe et al. 2008). Evidence that Homo erectus exhibited an expansion in landscape use over previous hominins is found in the archeological records of the Lake Turkana basin in Kenya and Ethiopia, and the Olduvai basin in Tanzania (Leakey 1971; Potts 1998; Rogers et al. 1994). In both basins, comparisons of archeological sites likely to have been made by Homo erectus to those formed earlier in time indicate that H. erectus transported lithic raw materials for longer distances and deposited tools in more diverse settings, i.e., a broader array of habitats, compared to previous hominins. At Olorgesailie, Kenya, Homo erectus formed sites in open, grassland-dominated landscapes that included large predators (Potts 1994; Sikes et al. 1999), suggesting that their foraging was not significantly constrained by the presence of carnivores. This expansion in utilized habitats may be related to an increase in dietary breadth. Microwear texture complexity suggests that Homo erectus was exploiting a broader range of foods, at least in terms of hardness, than did Homo habilis, Australopithecus afarensis, A. africanus, and Paranthropus boisei (Ungar et al. 2011).
Adaptation to these changing landscapes was probably instrumental in favoring a suite of traits that we think may have characterized Homo erectus (Fig. 1). First, the increased frequency of drier, more open habitats and dispersion of food resources in space and time likely required groups of Homo erectus to range more widely than previous hominins, resulting in larger home range sizes and more variable travel patterns. This would have allowed them to better track and exploit a wide variety of resources, including nutritionally dense foods acquired through tool use such as animal carcasses and plant underground storage organs (USOs) (O’Connell et al. 1999; Plummer 2004). Increased ranging is concordant with the aforementioned archeological data, as well as with the observed increase in body mass in Homo erectus relative to earlier hominins, as body size correlates positively with home range size in nonhuman primates and may increase with climatic shifts to drier, more open habitats (Aiello and Key 2002; Antón 2003; Antón et al. 2002; McHenry 1994; Pontzer in press). The possibility that the locomotor repertoire of Homo erectus included bouts of endurance running (Bramble and Lieberman 2004) is also consistent with long-distance travel. In many mammalian lineages, increases in daily travel distances appear to be associated with the procurement of more food energy, as well as increases in total fertility and total offspring mass (Pontzer and Kamilar 2009). Pontzer (in press) suggests that the apparent increase in daily travel distance in Homo erectus relative to preceding hominin taxa is part of a foraging strategy yielding increased caloric returns and higher reproductive output.
Diagrammatic schema of the major lines of causality in the scenario proposed here. Black arrows represent selective forces shaping behavioral and morphological changes. White arrow represents incidental effect. Shaded arrows and boxes represent new elements proposed in this model. See text for details.
Second, with more dispersed resources, group cohesion would not always have been possible, resulting in periodic fissioning into foraging parties. These parties would not have split permanently, however, owing to the advantages of larger groups for resource defense, some types of resource acquisition (e.g., hunting and confrontational scavenging), and predator avoidance, especially at night (cf. van Schaik 1983; Wrangham 1980). These parties may have foraged in disparate locations during the day but coalesced at night, at safe or defensible points on the landscape. An additional benefit of group coalescence may have derived from the risk reduction benefits of sharing transported food among parties with different levels of foraging success.
Third, advantages provided by greater ecological intelligence in the form of mental maps of plant foods and their phenological variability —or behavioral strategies to reduce predation or more effectively capture prey— would have favored increased cognitive power and associated changes in brain size and structure. Although brain size relative to body weight of early Homo erectus may have been similar to earlier Homo, average brain size was greater in H. erectus than in Australopithecus, and absolute brain and body size were on average larger in H. erectus than in all previous taxa (Antón 2008).
The greater patchiness and dispersion of resources encountered by early Homo erectus, combined with an increase in brain size and its associated energetic costs, would have both necessitated and enabled a higher-quality diet and an increased reliance on technology to facilitate food acquisition (Aiello and Wheeler 1995; Antón 2003; Antón and Swisher 2004; Milton 1999; Plummer 2004; Wrangham and Conklin-Brittain 2003; Wrangham et al. 1999). Diets of Homo erectus likely included tissue from large mammal carcasses, possibly acquired through hunting (Egeland and Dominguez-Rodrigo 2008; Dominguez-Rodrigo and Pickering 2003; Monahan 1996; Pante 2010; Pobiner et al. 2008), and underground storage organs (USOs), both high-quality foods that would have helped compensate for the increased energy costs of larger body size, larger brain size, and wider ranging patterns (Antón 2003; Leonard et al. 2003; O’Connell et al. 1999; Plummer 2004; Wrangham et al. 1999). USOs in particular would have been more abundant in these open, drier habitats relative to more wooded or forested settings (Laden and Wrangham 2005; O’Connell et al. 1999; Wrangham et al. 2009) and could thus have been important seasonally or year-round. Overall, a reliance on high-quality, heterogeneously distributed resources that required tool use to acquire or process (e.g., USOs and meat) would have “fueled” the increase in body size and absolute brain size in Homo erectus (Fig. 1).
The Cost of Reproduction for Females
The larger body and brain size of Homo erectus compared to earlier hominins likely imposed a greater cost of reproduction on females, especially during gestation and lactation, due to their own larger bodies and brains and those of their infants (Aiello and Key 2002; Aiello and Wells 2002; Ellison 2008; Foley and Lee 1991; Leonard and Robertson 1997; Parker 1990). Assuming a similar degree of sexual dimorphism in Homo erectus as modern humans, models of the relationship between body size and energetics across nonhuman primates suggest a minimum increase in basal metabolic requirements of 40 % from Australopithecus to modern humans and 31 % from H. habilis to H. erectus, 16 % for males and 46 % for females (Leonard and Robertson 1997). A further cost may derive from the relative altriciality of Homo erectus infants: If adult brain size of H. erectus was larger than that of earlier hominins, then infants born with smaller brains —i.e., at an earlier stage of brain development— would have been more likely to survive. These relatively altricial infants would have posed an additional cost on females via postnatal care and provisioning (Smith and Tompkins 1995). Further, even juveniles, who are weaned and in theory capable of provisioning themselves (Pereira 1993), may have had difficulty meeting their own needs when faced with hard to extract resources requiring use of stone or wooden tools. If Homo erectus was also ranging over wider areas, adult energy demands would have been heightened by obligate carrying of infants and even juveniles if they were unable to withstand the lengthy travel distances postulated for this species (Antón et al. 2002; Zihlman 1978). Carrying infants imposes energetic costs on adults (Achenbach and Snowdon 2002), and the costs of carrying juveniles, even for short distances, would be even greater. Together, these increased energy demands may have been substantially greater for Homo erectus than for australopithecines (Leonard and Robertson 1997). Finally, if Homo erectus was indeed less sexually dimorphic than earlier hominins (but see Antón 2003; Baab 2008; Pontzer in press), then female costs of reproduction would have been even higher relative to those of males, as sexual dimorphism in body size is a key determinant of sex differences in these energetic costs (Key and Ross 1999). Taken together, these factors suggest it is possible, perhaps even likely, that female Homo erectus required assistance to successfully raise offspring (Aiello and Key 2002; Plummer 2004; Fig. 1).
Until recently, it was thought that Homo erectus was characterized by growth rates and patterns of development far closer to modern humans than to australopithecines, with strong secondary altriciality at birth and slow growth rates between weaning and puberty (Begun and Walker 1993; Walker and Ruff 1993). If so, then the costs of reproduction for females may have been partially offset by decreased periods of lactation and slow growth during childhood, with the energetically costly growth spurt being deferred until adolescence when offspring are self-sufficient (Key 2000). However, such a change would have also increased the need for assistance in child-rearing, as it would have increased the number of overlapping dependents.
Recent work has suggested that Homo erectus may not have been as close to modern humans in its growth rates and patterns of development as previously thought (Bromage and Dean 1985; Dean 2006; Dean and Smith 2009; Graves et al. 2010; Simpson et al. 2008). Dean et al. (2001), for example, estimate that M1 in Homo erectus emerged at an age of 4–4.4 yr and M2 at 7.6 yr, intermediate between that in chimpanzees and modern humans, and from this work it seems reasonable to conclude that growth rates had begun to slow down compared to those in earlier hominins but had not yet reached a rate of growth similar to that of modern humans. From their assessment of a fossil hominin pelvis they attributed to Homo erectus, Simpson et al. (2008) estimated that brain size at birth would be ca. 330 g, decreasing the likelihood of secondary altriciality (see also Coqueugniot et al. 2004; Graves et al. 2010). Even if growth rates were high in Homo erectus, females would not necessarily have been less burdened, as infants that grow faster at younger ages substantially increase the energetic burden on their mothers, requiring either a significant increase in subadult production or food subsidies from other adults (Gurven and Walker 2006). The potential increased burden on mothers from a combination of a larger adult body size, rapid development with increased energetic requirements of offspring early in their growth, a focus on nutritionally dense but hard to acquire foods, and greater travel distances would still have been greater compared to that of earlier hominins, regardless of differences in growth rate estimates.
Who Helps Homo erectus Females?
Numerous reconstructions of hominin social evolution have suggested that Plio-Pleistocene hominin females overcame their greater burden by relying on the assistance of pair-bonded males, via provisioning of food resources or increased reproductive effort (Darwin 1871; Fisher 1983; Foley and Lee 1989; Isaac 1978; Kaplan et al. 2000; Lovejoy 1981, 2009). In most accounts of such a scenario, the male is bonded to the female via a “sex contract” whereby high-quality resources (via hunting) are provided in return for sexual fidelity (Fisher 1983; Lovejoy 1981), with strong pair bonds between males and females (Deacon 1997; Lovejoy 1981).
An alternative model posits instead that female Homo erectus received assistance from older, nonreproductive females who benefited from provisioning their maternal grandchildren (Hawkes et al. 1997, 2000; Hrdy 2009; O’Connell et al. 1999, 2002). The fitness benefits of contributing to an increase in survival and reproduction of one’s grandchildren would have selected for females who were more active in older age, leading to a lengthening of the female lifespan beyond menopause. Called the “grandmother hypothesis,” this model suggests that it is not males but females that mitigate the costs of reproduction for female Homo erectus. In this scenario, male hunting and provisioning function as mate investment rather than parental investment, with paternal care and provisioning relatively less important to females.
These two general scenarios are often construed as mutually exclusive owing to the reliance of the former on male philopatry (for cooperative hunting) and the reliance of the latter on female philopatry (for the operation of kin selection). If one considers limited dispersal by both sexes to be the ancestral condition for hominins, however (cf. Strier 2001), then one can posit a scenario that includes elements of both (cf. Key 2000; Key and Aiello 1999). This scenario is contingent on the emergence of a multilevel structure in Homo erectus, allowing both male and female bonding as elements of a complex social system. Most probably, female-bonded, cooperative kin groups were followed by cooperative bonds between males and females. Bonds among males would remain, but at a higher level of social structure.
Social Evolution in Homo erectus
Selection for Female Subgrouping
As noted previously, ecological changes following global cooling and drying ca. 2–1.5 Ma would have placed stronger selective pressures on Homo erectus with regard to energy acquisition, at least during parts of the year, leading to group fissioning for hours, days, or weeks at a time. In addition, females were faced with greater costs of reproduction under these conditions. This dual selective pressure on females would have favored small, cooperative breeding units that may have included multiple generations of related females (O’Connell et al. 1999; Fig. 1). Female cooperation in these units, possibly for food procurement but most importantly for breeding, would have been favored via both kin selection and reciprocal altruism (Hrdy 2009).
These small female groups may have been more likely to occur at resource-rich locations, e.g., “favored places,” cf. Plummer (2004), and may have moved among these locations while tracking seasonally changing resources. As a means of coping with these drier, more heterogeneous habitats, foraging strategies probably involved increased consumption of animal protein; increased exploitation of underground storage organs (USOs), perhaps as seasonally important fallback foods; and possibly the use of fire to cook USOs, thereby increasing their digestibility and nutritive breadth (Aiello and Wells 2002; Plummer 2004; Wrangham et al. 1999).
The Role of Males
The close social cohesion of Homo erectus female kin groups combined with their variable spatial distribution, at least seasonally, may have placed selective pressures on males to keep track of a limited number of these groups to facilitate sexual access. Once a male had invested in a group to the point of siring offspring, it would be advantageous to remain associated with that group so as to protect his progeny. Eventually selection would have favored males who succeeded in becoming the resident or alpha male of these small groups to maintain exclusive sexual access via male competition —and possibly female coercion— and protect offspring from potentially infanticidal conspecifics. Although often spatially discrete, especially while foraging, these groups must have also maintained social ties and fused into larger groups at times, especially if risk of predation, sexual coercion, or infanticide was high and/or if resident/alpha males left groups temporarily to engage in cooperative hunting. This combination of small female kin groups, with males variably attached, and larger associations for protection would have been the initial stages of a multilevel social system.
Although single males may have been able to defend small groups of females when females were widely dispersed and encounter rates with other males low, it is unlikely that males would have been able to keep this up year-round without some elements of male cooperation and inhibition. The classic example of such inhibition is the “respect for possession” of females by hamadryas baboon males (Kummer et al. 1974). Like hamadryas, male Homo erectus may have retained a network of male relatives, similar to the clan of hamadryas baboons, within which males exchanged females (cf. Pines et al. 2011; Schreier and Swedell 2009). Male–male bonds within clans would have been beneficial for defense of females, defense of resources, and carcass acquisition through either hunting or active scavenging. Males may even have allowed younger male relatives into the social sphere of the one-male group to provide additional protection and childcare assistance for the females (akin to follower males in hamadryas OMUs). If the younger male was a close relative and contributed to protection of females and dependents, then any offspring he managed to sire would be compensated for by increased offspring survival and inclusive fitness benefits to the resident male. The presence of a younger, related male ally would also have allowed the resident male to be away for extended periods of time, e.g., while acquiring food via hunting, without leaving his female(s) and offspring vulnerable to sexual coercion or infanticide. This could have involved an age-graded dominance hierarchy in which the alpha male sired most, but not all, offspring born into the OMU, akin to the “two-male team” found in some hamadryas baboons (Colmenares 1992).
These changes in male strategies would have involved a transition from a promiscuous, roaming strategy (as in chimpanzees) to an exclusion or semiexclusion strategy (as in gorillas or hamadryas baboons). Instead of competing over access to females only when receptive, males would have competed over “rights” to females and attempted to maintain those rights, just as hamadryas males defend their “possession” of females (Kummer 1968). Patterns of variation in other taxa suggest that the smaller these female groups, the more likely that one male can successfully monopolize them (Emlen and Oring 1977; Nunn 1999). This scenario highlights the importance of male contest competition during human evolution, which is arguably more likely than other mechanisms of sexual selection to have shaped modern human behavior (Puts 2010). Interestingly, recent evidence from specimens of Paranthropous robustus from the same general time period as Homo erectus suggests that males had an extended period of growth, suggestive of intense male competition after attainment of reproductive maturity (Lockwood et al. 2007).
Male competition is often thought to have been less important for Homo erectus owing to the decreased sexual dimorphism in this taxon compared to earlier hominins (though this notion has recently been questioned; see Antón 2003), and this has further been interpreted as suggestive of a transition from polygamy to monogamy (Plummer 2004) owing to the general association between monogamy and monomorphism in nonhuman primates (cf. Plavcan 1999). However, sexual dimorphism may have decreased in Homo erectus for reasons unrelated to sexual selection. Selection for increased body size in females, which would effectively reduce sexual dimorphism, may have derived from the increased predator encounter rates faced by individuals in a more open habitat with wider ranging in smaller foraging parties, as these selective pressures would have acted more strongly on the smaller sex (Aiello 1996; McHenry 1994). Moreover, an increase in brain size may have favored larger body mass as the proportion of a female’s energy budget allocated to her offspring during gestation and lactation decreases with larger body size (Antón 2003; Charnov and Berrigan 1993). The decreased relative body size of male Homo erectus may thus be illusory, as the increase in female size may be a result of ecological pressures that bear no relationship to male–male competition, and male H. erectus were in fact larger than males from earlier hominin species. In addition, Pleistocene hominins were likely more reliant than earlier hominins on the use of tools as weapons, reducing the importance of large body size in and of itself as the major determinant of success in male competition (Darwin 1871). Bercovitch (2001), for example, pointed out that the primary physiological difference (not directly related to reproduction) between modern human males and females is upper body strength, which likely facilitated the use of weapons for male–male competition. Similarly, Puts (2010) argued that the relatively low degree of sexual dimorphism in modern humans underestimates sex differences in the traits most important in contest competition: strength, speed, and secondary sexual characteristics such as beards and deep voices. Regardless, at no point during hominin evolution did body size sexual dimorphism ever decrease to anything approaching that of monogynous nonhuman primates (e.g., Hylobates, Aotus, Callicebus), suggesting that some degree of male–male competition and polygyny has always characterized the hominin lineage (McHenry 1994; Plavcan 1999, 2000).
Male–Female Pair-Bonding
With male defense of small groups of females, selection would have favored males that formed bonds with each female via grooming, infant caretaking, and possibly provisioning (though male provisioning is not a necessary component of this scenario; cf. Bird 1999) in exchange for some degree of female sexual fidelity and paternity certainty, an association of traits that characterizes many monogamous mammals (Kleiman 1977). The scenario we present here differs from previous models of pair bonds as economic arrangements (e.g., Lovejoy 1981), however, in two main ways: 1) males provided mainly protection rather than food for females, and 2) males formed pair bonds with multiple females. Wide variation in size and composition of these groupings is likely, probably at least in part determined by patterns of kinship among females; it is notable within this context that hamadryas OMUs vary in size from 1 to 9 and 30 % are monogamous pairs rather than polygynous units (Swedell et al. 2011). This would likely have differed for Homo erectus, though, as the role of intrasexual relationships would have been far greater for female H. erectus than for female hamadryas, which make very few efforts to remain with kin. Male–female bonds in social units of Homo erectus would function to produce, provision, and protect offspring, with female (kin) groups focusing on cooperative child rearing, cooperative foraging, and defense of offspring and resources. Females may also have been polyandrous to some degree; by copulating occasionally with secondary males (as in hamadryas: Swedell and Saunders 2006) they could guard against potential infanticide and effectively combine a predominant anti-infanticide strategy of paternity concentration with a secondary strategy of paternity confusion (cf. Swedell and Saunders 2006; van Schaik et al. 1999). Male investment in offspring care and protection would have then varied according to female sexual fidelity, paternity certainty, and the availability of the female’s kin to aid in child-rearing and protection (Hrdy 2009), as well as the number of females in the group and the number of females and offspring among whom resources were being distributed.
Resistance to Male Sexual Coercion
In hamadryas baboons, female kin are broken up by coercive transfer of females among OMUs (cf. Clutton-Brock and Parker 1995; Smuts and Smuts 1993; Swedell and Schreier 2009). Hamadryas females do not form coalitions to resist male coercion, perhaps because, in an environment with nonmonopolizable, widely distributed resources and no need for assistance with reproduction, there are no overriding competitive or cooperative benefits to females of forming alliances with their kin (Barton 2000). In Homo erectus, however, bonds among females would have been strongly selected for, owing to the high costs of reproduction and the benefits of cooperative infant rearing (Aiello and Key 2002; Hrdy 2009; Key 2000). Female kin groups in Homo erectus would thus have been less susceptible to this type of male coercion than in hamadryas, as both sexes would have benefitted from the maintenance of groups in which female kin provided crucial assistance with child-rearing. Female Homo erectus may thus have played a larger role in OMU formation and mate selection than do female hamadryas baboons. Even if female kin groups of Homo erectus were sometimes broken up by males, 1) cooperative infant rearing among a small group of females, some unrelated, could have been selected for via reciprocal altruism given the likely costs to females of attempting to reproduce without assistance; and 2) any breakup of female kin groups by males may have occurred infrequently enough so as to preserve female kin bonding as a general pattern. Importantly, Hrdy (2009) argues that male investment in care of offspring would have, as in modern humans, varied according to the availability of female kin as allomothers, suggesting that males would have benefitted from the maintenance of female kin groups so as to reduce obligate investment in their offspring.
The Evolution of Cooperative Relationships
A scenario that begins with cooperation among females and leads to cooperation by males is supported by agent-based modeling by Cathy Key and Leslie Aiello (Key 2000; Key and Aiello 1999, 2000), who found that, when energetic costs of reproduction for females are high, cooperation is more likely to evolve among females than between the sexes. Key and Aiello also found that the likelihood of both types of cooperation increases with the cost of reproduction for females. This strongly suggests that the increased costs of reproduction faced by female Homo erectus would have led first to cooperation among females, probably among kin, and then later to cooperative relationships between the sexes. Key and Aiello’s (2000) model also demonstrates that nonreciprocated male cooperation with females will evolve when 1) female energetic costs are far higher than male costs, and 2) males who do not cooperate are punished. This fits well with a scenario of high reproductive costs for female Homo erectus, low costs for males, and a female strategy by which they remain faithful to males who cooperate and unfaithful to those who do not. According to Key and Aiello’s model, actual paternity certainty is not required for the initial development of cooperation by males, but it does heighten male cooperation later in the evolutionary process.
The Outcome: Multilevel Social Organization of Homo erectus
Ultimately, this evolutionary scenario would give rise to a multilevel social system with multiple types of social and reproductive bonds at different levels of social structure, each with varying reproductive and ecological functions (Fig. 1). Such a scenario is consistent with a sexual division of labor, which may have also emerged at this point in hominin evolution (Bird 1999; Marlowe 2010; Zihlman 1978; Zihlman and Tanner 1978). The smallest social grouping, the one-male (or two-male) unit, would have been a (mostly) polygynous one-male, multifemale group with alloparenting by both sexes, a cooperative reproductive arrangement among and between the sexes. Beyond the reproductive unit, a larger social network of male kin, analogous to the hamadryas clan, could have enabled exchange and defense of females and cooperative hunting (cf. Rodseth 2012). Even larger social groupings would likely have been selected for as well, for ecological reasons, though possibly less cohesive than a hamadryas band and more similar to a chimpanzee community. These larger social units, comprising individuals that tolerate one another and coalesce when necessary, would have been beneficial for protection from predation at night or during other times of increased predator pressure, competition with large carnivores over prey (given the large guild of African predators during the late Pliocene–early Pleistocene; Plummer 2004), defense of limited or accumulated food resources from conspecifics, and protection for females and offspring during male hunting expeditions (Aiello and Dunbar 1993; van Schaik 1983). However, given that reciprocity and cooperation operate best on a small scale and decrease with increasing numbers of individuals (Aiello and Dunbar 1993; Dunbar 1992b, 1998), small networks at the OMU and clan levels would have been crucial for the maintenance of cooperation and reciprocity via strong social bonds, with larger networks for more ecological functions. Marlowe et al. (2008) demonstrated that third-party punishment, a mechanism to reinforce cooperation by punishing defectors, occurs more in larger, more complex societies. This suggests that the evolution of a multilevel social structure would have the added effect of reinforcing cooperative relationships.
In this scenario, it is likely that both males and females sometimes dispersed among social units, but only to a limited degree. While OMUs were probably more likely to consist of female relatives than nonrelatives, some females may have been transferred among units by males via sexual coercion. Males, on the other hand, were probably more likely to remain in their natal clan because of the benefits of cooperation with kin, though may have occasionally dispersed in search of available females. Limited bisexual dispersal does not preclude gene flow and outbreeding, as dispersal of even a small number of individuals of either or both sexes per generation is enough to maintain genetic diversity at the population level (Wright 1931). The fact that male kin networks and female kin networks operate at different levels of social structure would reduce the likelihood of inbreeding, assuming that the tenure of a resident male in an OMU is typically shorter than a female’s pre-reproductive life. This is not necessarily the case for hamadryas (in which females are transferred among OMUs before they have the opportunity to reproduce with their presumed fathers), but it would have been more likely for Homo erectus given longer life history parameters in hominoids compared to baboons, especially if growth rates were slightly slower in H. erectus compared to earlier hominins (Dean et al. 2001).
It is important to note here that we are not proposing a one-to-one correspondence between the multilevel societies of hamadryas baboons and those of Plio-Pleistocene hominins. Key elements of Plio-Pleistocene society that differentiate it from a simple baboon model include the increased role of females, extensive alloparenting, cooperative foraging, a sexual division of labor, and kin ties within and among OMUs. Chapais (2008) discusses the importance of bilateral kinship and reciprocal exogamy in modern human social organization, i.e., the maintenance of kin bonds postdispersal across multiple generations through both the maternal and paternal lines. This is a feature of hominin societies that does not characterize baboons, but that would have been enabled by a multilevel social system that operated in a similar way to that of hamadryas baboons. Most importantly, multilevel societies in hominins would have been built upon a foundation of higher cognitive power that would have increased their benefits to both sexes with regard to reproduction and food acquisition. The evolution of a multilevel social organization in a cognitively sophisticated hominin would thus have provided the raw material for bilateral kin bonds to evolve in the context of regular exchange of females among reproductive units, and for a sexual division of labor to enhance foraging success while strengthening social ties.
Cognitive Implications
Larger group sizes impose a potential cognitive cost in that they increase the number of dyadic relationships that each individual will benefit from monitoring (Dunbar 1992b, 1998). Larger groups may also constrain collective decision-making abilities, which depend on some form of shared information among all individuals in a group (Fischer and Zinner 2011; Sueur et al. 2011). Johnson (1983) suggested that collective decision-making abilities in humans are reduced in groups of six or more, suggesting that a multilevel social structure would be advantageous in limiting the very closest set of relationships to a manageable size and reducing this cognitive burden. However, a multilevel society also simultaneously increases the total number of individuals and relationships that each individual must monitor. This additional social monitoring, if it occurs, is arguably a further constraint on cognition (Aiello and Dunbar 1993; Byrne and Whiten 1988; Dunbar 1992b, 1998) and may underlie the uniformity in scaling ratio among the sizes of successive levels of organization in modern hunter-gatherer (Hamilton et al. 2007) and mammalian multilevel societies (Hill et al. 2008; Schreier and Swedell 2012a). The commonality in scaling ratio across species, with each level about three to four times larger than the one beneath it, suggests that societies may self-organize to an optimal scaling ratio to maintain social cohesion and communication within cognitive limitations (Kudo and Dunbar 2001).
For Homo erectus in particular, wider social networks beyond the female kin group and even beyond the male clan may have selected for larger brain size and complexity to allow recognition and tracking of large numbers of individuals over time (Fig. 1). Further brain expansion for these purposes would have been energetically feasible because of the higher-quality diet of Homo erectus compared to that of earlier hominins (Aiello and Wheeler 1995; Leonard et al. 2003; Milton 1999). Whether this diet was high in animal tissue, or simply included enough meat to allow a focus on calorie-rich plant foods such as underground storage organs (cf. Milton 1999; Plummer 2004), or whether cooking in particular increased diet quality (cf. Wrangham et al. 1999), is as yet unclear. What is clear is that this new diet was sufficient to support the brain and body size enlargement that characterized later hominins. This unique combination of an already more sophisticated cognitive apparatus, i.e., that characterizing early Homo, a higher-quality diet, and a multilevel social structure would have allowed even greater social and ecological possibilities.
Hrdy (2009) expands on the cognitive implications of cooperative breeding by arguing that the necessity for clear communication of needs between infants and alloparents would have provided a selective pressure for the evolution of empathy. From this perspective, a multilevel social organization and cooperative breeding have together shaped hominin cognition and laid the groundwork for further expansion of brain size, the evolution of language, and the origin of modern human emotions (Aiello and Dunbar 1993; Hrdy 2009).
Future Research
Key tests of the applicability of the hamadryas model will involve further exploration of the role of kin selection in shaping hamadryas behavior and the benefits to both males and females of all three types of social bonds outlined here. For example, we expect to find that females are more often transferred among male kin than among nonkin and that males help their kin defend females, e.g., via leader–follower relationships. Evidence of male kin cooperation in this way, combined with evidence for the adaptive value of sociality among hamadryas females (cf. Silk 2007a, b), would show that it is indeed possible for a social system to include fitness-enhancing bonds among males, among females, and between the sexes prior to the further cognitive expansion found in modern humans, thereby laying the groundwork for the reciprocal exogamy configuration described by Chapais (2008).
The applicability of this model can be evaluated further with additional paleontological and archeological data. Clarification of the degree of body size dimorphism of Homo erectus may allow inferences about the intensity of male–male competition and the probability of a polygynous mating system. Additional fossil finds might clarify whether there is unequivocal skeletal evidence for endurance walking and/or running in Homo erectus (Bramble and Lieberman 2004), consistent with the greater ranging behavior postulated here for this taxon.
The paleoenvironmental context of the landscapes used by Homo erectus can be reconstructed with a variety of faunal and geochemical techniques, including the reconstruction of vegetation structure through the stable isotopic analysis of pedogenic carbonates (Plummer et al. 2009). These data could then be combined with data on artifact distribution and density to infer the range of habitats used and the intensity of use. We suspect that new archaeological data will continue to show the trend of previous work: that Homo erectus ranged widely through a variety of habitats, frequently in relatively open, grassy settings (Sikes et al. 1999).
Likewise, zooarchaeological analysis, lithic raw material sourcing, and methods for inferring stone tool function (such as the investigation of artifact edges for plant phytoliths and use-wear analysis of artifacts) would allow for reconstruction of hominin ranging, faunal acquisition strategies, types of prey accumulated and their nutritional yield, and the types of plant foods being processed (Plummer 2004). Evidence for increased ranging by Homo erectus through a broad spectrum of habitats relative to earlier hominins, as well as a reliance on difficult to acquire or process animal and plant foods in packages large enough to be shared, would support the view that juvenile Homo erectus would have needed and could have received assistance via provisioning.
Conclusions
The key differences between the model of evolution of Homo erectus outlined in this article and most previous models can be summarized as follows: 1) a multilevel social structure in which different levels of society serve different social, reproductive, and ecological functions; 2) the possibility of both female kin bonding (and the grandmother hypothesis) and male kin bonding (and kin selected cooperation among males), with each kinship network operating at a different level of society; and 3) not single but multiple pair bonds between the sexes in polygynous one- or two-male groups.
Male–male bonding and female–female bonding have traditionally been juxtaposed as largely mutually exclusive, as have pair-bonding and same-sex bonding (Fisher 1983; Foley 1989, 1996; Lovejoy 1981). Hamadryas baboons, however, display all of these features to varying degrees, as do modern humans (Chapais 2008; Rodseth et al. 1991). For the reasons we outline here, it is likely that the origins of this complexity in the human lineage can be found in a Plio-Pleistocene hominin such as Homo erectus.
References
Abegglen, J.-J. (1984). On socialization in hamadryas baboons. London: Associated University Presses.
Achenbach, G. G., & Snowdon, C. T. (2002). Costs of caregiving: Weight loss in captive adult male cotton-top tamarins (Saguinus oedipus) following the birth of infants. International Journal of Primatology, 23, 105–122.
Aiello, L. C. (1996). Hominine preadaptations for language and cognition. In: P. Mellars & K. Gibson (Eds.), Modelling the early human mind (pp. 89–99). Cambridge: McDonald Institute Monographs.
Aiello, L. C., & Dunbar, R. I. M. (1993). Neocortex size, group size, and the evolution of language. Current Anthropology, 34, 184–193.
Aiello, L. C., & Key, C. (2002). Energetic consequences of being a Homo erectus female. American Journal of Human Biology, 14, 551–565.
Aiello, L. C., & Wells, J. C. K. (2002). Energetics and the evolution of the genus Homo. Annual Review of Anthropology, 31, 323–338.
Aiello, L. C., & Wheeler, P. (1995). The expensive tissue hypothesis. Current Anthropology, 36, 199–221.
Alberts, S. C., & Altmann, J. (1995). Balancing costs and opportunities: Dispersal in male baboons. American Naturalist, 145, 279–306.
Alberts, S. C., & Altmann, J. (2006). The evolutionary past and the research future: Environmental variation and life history flexibility in a primate lineage. In L. Swedell & S. R. Leigh (Eds.), Reproduction and fitness in baboons (pp. 277–303). New York: Springer.
Aldrich-Blake, F. P. G., Bunn, T. K., Dunbar, R. I. M., & Headley, P. M. (1971). Observations on baboons, Papio anubis, in an arid region in Ethiopia. Folia Primatologica, 15, 1–35.
Alexander, R. D., & Noonan, K. M. (1979). Concealment of ovulation, parental care, and Human social evolution. In N. A. Chagnon & W. Irons (Eds.), Evolutionary biology and human social behavior (pp. 436–453). North Scituate, MA: Duxbury Press.
Anderson, C. M. (1983). Levels of social organization and male–female bonding in the genus Papio. American Journal of Physical Anthropology, 60, 15–22.
Antón, S. C. (2003). Natural history of Homo erectus. Yearbook of Physical Anthropology, 46, 126–170.
Antón, S. C. (2008). Framing the question: Diet and evolution in early Homo. In C. Vinyard, M. J. Ravosa, & C. Wall (Eds.), Primate craniofacial function and biology (pp. 443–482). Amsterdam: Springer.
Antón, S. C., & Swisher, C. C. (2004). Early dispersals of Homo from Africa. Annual Review of Anthropology, 33, 271–296.
Antón, S. C., Leonard, W. R., & Robertson, M. L. (2002). An ecomorphological model of the initial hominid dispersal from Africa. Journal of Human Evolution, 43, 773–785.
Archie, E. A., Moss, C. J., & Alberts, S. C. (2006). The ties that bind: Genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proceedings of the Royal Society of London B: Biological Sciences, 273, 513–522.
Baab, K. L. (2008). The taxonomic implications of cranial shape variation in Homo erectus. Journal of Human Evolution, 54, 827–847.
Barton, R. (2000). Socioecology of baboons: The interaction of male and female strategies. In P. M. Kappeler (Ed.), Primate males: Causes and consequences of variation in group composition (pp. 97–107). Cambridge, UK: Cambridge University Press.
Bedaso, Z., Wynn, J. G., Alemseged, Z., & Geraads, D. (2010). Paleoenvironmental reconstruction of the Asbole fauna (Busidima Formation, Afar, Ethiopia) using stable isotopes. Geobios, 43, 165–177.
Begun, D. J., & Walker, A. (1993). The endocast. In A. Walker & R. Leakey (Eds.), The Nariokotome Homo erectus skeleton (pp. 326–358). Cambridge, MA: Harvard University Press.
Bercovitch, F. B. (1990). Female choice, male reproductive success, and the origin of one male groups in baboons. In M. Thiago de Mello, A. Whiten, & R. W. Byrne (Eds.), Baboons: Behaviour and ecology, use and care. Selected Proceedings of the XIIth Congress of the International Primatological Society (pp. 61–76). Brasilia, Brasil.
Bercovitch, F. B. (2001). Reproductive ecology of Old World monkeys. In P. T. Ellison (Ed.), Reproductive ecology and human evolution (pp. 369–396). New York: Aldine de Gruyter.
Bergman, T. J. (2010). Experimental evidence for limited vocal recognition in a wild primate: Implications for the social complexity hypothesis. Proceedings of the Royal Society of London B: Biological Sciences, 277, 3045–3053.
Bird, R. (1999). Cooperation and conflict: The behavioral ecology of the sexual division of labor. Evolutionary Anthropology, 8, 65–75.
Bobe, R., & Behrensmeyer, A. K. (2004). The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeography, Palaeoclimatology, Palaeoecology, 207, 399–420.
Bramble, D. M., & Lieberman, D. E. (2004). Endurance running and the evolution of Homo. Nature, 432, 345–352.
Bromage, T. G., & Dean, M. C. (1985). Re-evaluation of the age at death of immature fossil hominids. Nature, 317, 525–527.
Byrne, R. W., & Whiten, A. (Eds.). (1988). Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford: Oxford University Press.
Cerling, T., Wynn, J., Andanje, S., Bird, M., Korir, D., Levin, N., Mace, W., Macharia, A., Quade, J., & Remien, C. (2011). Woody cover and hominin environments in the past 6 million years. Nature, 476, 51–56.
Chapais, B. (2008). Primeval kinship: How pair-bonding gave birth to human society. Cambridge, MA: Harvard University Press.
Charnov, E. L., & Berrigan, D. (1993). Why do female primates have such long lifespans and so few babies? or Life in the slow lane. Evolutionary Anthropology, 1, 191–194.
Clutton-Brock, T. H., & Parker, G. A. (1995). Sexual coercion in animal societies. Animal Behaviour, 49, 1345–1365.
Codron, D., Lee-Thorp, J. A., Sponheimer, M., de Ruiter, D., & Codron, J. (2008). What insights can baboon feeding ecology provide for early hominin niche differentiation? International Journal of Primatology, 29, 757–772.
Colmenares, F. (1991a). Greeting behaviour between male baboons: Oestrus females, rivalry and negotiation. Animal Behaviour, 41, 49–60.
Colmenares, F. (1991b). Greeting, aggression, and coalitions between male baboons: Demographic correlates. Primates, 32, 453–463.
Colmenares, F. (1992). Clans and harems in a colony of hamadryas and hybrid baboons: Male kinship, familiarity and the formation of brother-teams. Behaviour, 121, 61–94.
Colmenares, F. (2004). Kinship structure and its impact on behavior in multi-level societies. In B. Chapais & C. M. Berman (Eds.), Kinship and behavior in primates (pp. 242–270). New York: Oxford University Press.
Connor, R. C., Mann, J., Tyack, P. L., & Whitehead, H. (1998). Social evolution in toothed whales. Trends in Ecology & Evolution, 13, 228–232.
Copeland, S. R., Sponheimer, M., de Ruiter, D. J., Lee-Thorp, J. A., Codron, D., le Roux, P. J., Grimes, V., & Richards, M. P. (2011). Strontium isotope evidence for landscape use by early hominins. Nature, 474, 76–79.
Coqueugniot, H., Hublin, J.-J., Veillon, F., Houët, F., & Jacob, T. (2004). Early brain growth in Homo erectus and implications for cognitive ability. Nature, 431, 299–302.
Darwin, C. R. (1871). The descent of man and selection in relation to sex. London: John Murray.
Deacon, T. W. (1997). The symbolic species. New York: W. W. Norton.
Dean, M. C. (2006). Tooth microstructure tracks the pace of human life-history evolution. Proceedings of the Royal Society London B, 273, 2799–2808.
Dean, M. C., & Smith, B. H. (2009). Growth and development of the Nariokotome youth, KNM-WT 15000. In F. E. Grine, J. G. Fleagle, & R. E. Leakey (Eds.), The first human: Origin and early evolution of the genus Homo (pp. 101–120). New York: Springer.
Dean, C., Leakey, M. G., Reid, D., Schrenk, F., Schwartz, G. T., Stringer, C., & Walker, A. (2001). Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature, 414, 628–631.
deMenocal, P. (2004). African climate change and faunal evolution during the Pliocene-Pleistocene. Earth and Planetary Science Letters, 220, 3–24.
DeVore, I., & Washburn, S. L. (1963). Baboon ecology and human evolution. In F. C. Howell & F. Bourlière (Eds.), African ecology and human evolution (pp. 335–367). Chicago: Aldine.
Dominguez-Rodrigo, M., & Pickering, T. R. (2003). Early hominid hunting and scavenging: A zooarcheological review. Evolutionary Anthropology, 12, 275–282.
Dominguez-Rodrigo, M., Serrallonga, J., Juan-Tresserras, J., Alcala, L., & Luque, L. (2001). Woodworking activities by early humans: A plant residue analysis on Acheulian stone tools from Peninj (Tanzania). Journal of Human Evolution, 40, 289–299.
Dunbar, R. I. M. (1983). Relationships and social structure in gelada and hamadryas baboons. In R. A. Hinde (Ed.), Primate social relationships: An integrated approach (pp. 299–307). Sunderland, MA: Sinauer Associates.
Dunbar, R. I. M. (1988). Primate social systems. Ithaca, NY: Cornell University Press.
Dunbar, R. I. M. (1992a). A model of the gelada socio-ecological system. Primates, 33, 69–83.
Dunbar, R. I. M. (1992b). Neocortex size as a constraint on group size in primates. Journal of Human Evolution, 22, 469–493.
Dunbar, R. I. M. (1998). The social brain hypothesis. Evolutionary Anthropology, 6, 178–190.
Dunbar, R., & Dunbar, P. (1975). Social dynamics of gelada baboons. Basel: S. Karger.
Egeland, C. P., & Dominguez-Rodrigo, M. (2008). Taphonomic perspectives on hominid site use and foraging strategies during Bed II times at Olduvai Gorge, Tanzania. Journal of Human Evolution, 55, 1031–1052.
Ellison, P. T. (2008). Energetics, reproductive ecology, and human evolution. PaleoAnthropology, 2008, 172–200.
Elton, S. (2006). Forty years on and still going strong: The use of hominin-cercopithecid comparisons in palaeoanthropology. Journal of the Royal Anthropological Institute (N.S.), 12, 19–38.
Emlen, S. T., & Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science, 197, 215–223.
Fischer, J., & Zinner, D. (2011). Communication and cognition in primate group movement. International Journal of Primatology, 32, 1279–1295.
Fisher, H. (1983). The sex contract: The evolution of human behavior. New York: Quill.
Foley, R. A. (1989). The evolution of hominid social behaviour. In V. Standen & R. A. Foley (Eds.), Comparative socioecology: The behavioural ecology of humans and other mammals (pp. 473–494). Oxford: Blackwell.
Foley, R. A. (1996). An evolutionary and chronological framework for human social behaviour. In W. G. Runciman, J. Maynard Smith, & R. I. M. Dunbar (Eds.), Evolution of social behaviour patterns in primates and man. Oxford: Oxford University Press.
Foley, R., & Gamble, C. (2009). The ecology of social transitions in human evolution. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 364, 3267–3279.
Foley, R. A., & Lee, P. C. (1989). Finite social space, evolutionary pathways, and reconstructing hominid behavior. Science, New Series, 243, 901–906.
Foley, R. A., & Lee, P. C. (1991). Ecology and energetics of encephalization in hominid evolution. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 334, 223–232.
Franciscus, R. G., & Trinkaus, E. (1988). Nasal morphology and the emergence of Homo erectus. American Journal of Physical Anthropology, 75, 517–527.
Galat-Luong, A., Galat, G., & Hagell, S. (2006). The social and ecological flexibility of Guinea baboons: Implications for Guinea baboon social organization and male strategies. In L. Swedell & S. R. Leigh (Eds.), Reproduction and fitness in baboons: Behavioral, ecological, and life history perspectives (pp. 105–121). New York: Springer.
Graves, R. R., Lupo, A. C., McCarthy, R. C., Wescott, D. J., & Cunningham, D. L. (2010). Just how strapping was KNM-WT 15000? Journal of Human Evolution, 59, 542–554.
Grueter, C. C., Chapais, B., & Zinner, D. (2012). Evolution of multilevel social systems in nonhuman primates and humans. International Journal of Primatology, 33.
Grüter, C. C., & Zinner, D. (2004). Nested societies: Convergent adaptations of baboons and snub-nosed monkeys? Primate Report, 70, 1–98.
Gurven, M., & Walker, R. (2006). Energetic demand of multiple dependents and the evolution of slow human growth. Proceedings of the Royal Society of London B: Biological Sciences, 273, 835–841.
Hamilton, M. J., Milne, B. T., Walker, R. S., Burger, O., & Brown, J. H. (2007). The complex structure of hunter-gatherer social networks. Proceedings of the Royal Society of London B: Biological Sciences, 274, 2195–2202.
Hammond, R. L., Lawson Handley, L. J., Winney, B. J., Bruford, M. W., & Perrin, N. (2006). Genetic evidence for female-biased dispersal and gene flow in a polygynous primate. Proceedings of the Royal Society of London B: Biological Sciences, 273, 479–484.
Hapke, A., Zinner, D., & Zischler, H. (2001). Mitochondrial DNA variation in Eritrean hamadryas baboons. Behavioral Ecology and Sociobiology, 50, 483–492.
Harvati, K., Frost, S. R., & McNulty, K. P. (2004). Neanderthal taxonomy reconsidered: Implications of 3D primate models of intra- and interspecific differences. Proceedings of the National Academy of Sciences of the USA, 101, 1147–1152.
Hawkes, K., O’Connell, J. F., & Blurton Jones, N. G. (1997). Hadza women's time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Current Anthropology, 38, 551–577.
Hawkes, K., O’Connell, J. F., Blurton Jones, N. G., Alvarez, H., & Charnov, E. L. (2000). The grandmother hypothesis and human evolution. In L. Cronk, N. Chagnon, & W. Irons (Eds.), Adaptation and human behavior (pp. 237–258). New York: Aldine De Gruyter.
Hill, R. A., Bentley, A., & Dunbar, R. I. M. (2008). Network scaling reveals consistent fractal pattern in hierarchical mammalian societies. Biology Letters, 4, 748–751.
Hrdy, S. B. (2009). Mothers and others: The evolutionary origins of mutual understanding. Cambridge, MA: Harvard University Press.
Isaac, G. (1978). The food-sharing behaviour of proto-human hominids. Scientific American, 238, 90–108.
Isbell, L. A., & Van Vuren, D. (1996). Differential costs of locational and social dispersal and their consequences for female group-living primates. Behaviour, 133, 1–36.
Iwamoto, T. (1979). Feeding ecology. In M. Kawai (Ed.), Ecological and sociological studies of gelada baboons, vol. 16 (pp. 280–335). Basel: Karger.
Jack, K. M., & Isbell, L. A. (2009). Dispersal in primates: Advancing an individualized approach (Preface to Special Issue). Behaviour, 146, 429–436.
Johnson, G. A. (1983). Decision-making organization and pastoral nomad camp size. Human Ecology, 11, 175–199.
Jolly, C. J. (1963). A suggested case of evolution by sexual selection in primates. Man, 222, 177–178.
Jolly, C. J. (1970). The seed-eaters: A new model of hominid differentiation based on a baboon analogy. Man, 5, 1–26.
Jolly, C. J. (1993). Species, subspecies, and baboon systematics. In W. H. Kimbel & L. B. Martin (Eds.), Species, species concepts, and primate evolution (pp. 67–107). New York: Plenum Press.
Jolly, C. J. (2001). A proper study for mankind: Analogies from the Papionin monkeys and their implications for human evolution. Yearbook of Physical Anthropology, 44, 177–204.
Jolly, C. J. (2009). Fifty years of looking at human evolution: Backward, forward, and sideways. Current Anthropology, 50, 187–199.
Kaplan, H., Hill, K., Lancaster, J., & Hurtado, A. M. (2000). A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology, 9, 156–185.
Kawai, M., Dunbar, R., Ohsawa, H., & Mori, U. (1983). Social organization of gelada baboons: Social units and definitions. Primates, 24, 13–24.
Key, C. A. (2000). The evolution of human life history. World Archaeology, 31, 329–350.
Key, C. A., & Aiello, L. C. (1999). The evolution of social organization. In R. Dunbar, K. Chris, & C. Power (Eds.), The evolution of culture (pp. 15–33). Piscataway, NJ: Rutgers University Press.
Key, C., & Aiello, L. C. (2000). A prisoner's dilemma model of the evolution of paternal care. Folia Primatologica, 71, 77–92.
Key, C. A., & Ross, C. (1999). Sex differences in energy expenditure in non-human primates. Proceedings of the Royal Society of London B: Biological Sciences, 266, 2479–2485.
Kingston, J. D. (2007). Early human evolutionary paleoecology. Yearbook of Physical Anthropology, 50, 20–58.
Kleiman, D. G. (1977). Monogamy in mammals. The Quarterly Review of Biology, 52, 39–69.
Krützen, M., Sherwin, W. B., Connor, R. C., Barré, L. M., Van de Casteele, T., Mann, J., & Brooks, R. (2003). Contrasting relatedness patterns in bottlenose dolphins (Tursiops sp.) with different alliance strategies. Proceedings of the Royal Society of London B: Biological Sciences, 270, 497–502.
Kudo, H., & Dunbar, R. I. M. (2001). Neocortex size and social network size in primates. Animal Behaviour, 62, 711–722.
Kummer, H. (1968). Social organization of hamadryas baboons: A field study. Chicago: University of Chicago Press.
Kummer, H. (1971). Primate societies. Chicago: Aldine.
Kummer, H. (1990). The social system of hamadryas baboons and its presumable evolution. In M. Thiago de Mello, A. Whiten, & R. W. Byrne (Eds.), Baboons: Behaviour and ecology, use and care. Selected Proceedings of the XIIth Congress of the International Primatological Society (pp. 43–60). Brasilia, Brasil.
Kummer, H., Götz, W., & Angst, W. (1974). Triadic differentiation: An inhibitory process protecting pair bonds in baboons. Behaviour, 49, 62–87.
Laden, G., & Wrangham, R. (2005). The rise of the hominids as an adaptive shift in fallback foods: Plant underground storage organs (USOs) and australopith origins. Journal of Human Evolution, 49, 482–498.
Layton, R. H., O’Hara, S., & Bilsborough, A. (2012). Antiquity and social functions of multi-level social organisation among human hunter-gatherers. International Journal of Primatology, 33
le Roux, A., Beehner, J. C., & Bergman, T. J. (2011). Female philopatry and dominance patterns in wild geladas. American Journal of Primatology, 73, 422–430.
Leakey, M. D. (1971). Olduvai Gorge: Excavations in Beds I and II, 1960–1963. Cambridge, UK: Cambridge University Press.
Leonard, W. R., & Robertson, M. L. (1997). Comparative primate energetics and hominid evolution. American Journal of Physical Anthropology, 102, 265–281.
Leonard, W. R., Robertson, M. L., Snodgrass, J. J., & Kuzawa, C. W. (2003). Metabolic correlates of hominid brain evolution. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 136, 5–15.
Lockwood, C. A., Menter, C. G., Moggi-Cecchi, J., & Keyser, A. W. (2007). Extended male growth in a fossil hominin species. Science, 318, 1443–1446.
Lovejoy, C. O. (1981). The origin of man. Science, 211, 341–350.
Lovejoy, C. O. (2009). Reexamining human origins in light of Ardipithecus ramidus. Science, 326, 74.
Marlowe, F. W. (2010). The Hadza: Hunter-gatherers of Tanzania. Berkeley: University of California Press.
Marlowe, F. W., Berbesque, J. C., Barr, A., Barrett, C., Bolyanatz, A., Cardenas, J. C., Ensminger, J., Gurven, M., Gwako, E., Henrich, J., Henrich, N., Lesorogol, C., McElreath, R., & Tracer, D. (2008). More ‘altruistic’ punishment in larger societies. Proceedings of the Royal Society of London B: Biological Sciences, 275, 587–590.
Maryanski, A. (1996). African ape social networks: A blueprint for reconstructing early hominid social structure. In J. Steele & S. Shennan (Eds.), The archaeology of human ancestry (pp. 67–90). London: Routledge.
Matsuda, I., Zhang, P., Swedell, L., Mori, U., Tuuga, A., Bernard, H., & Sueur, C. (2012). Comparisons of inter-individual relationships among non-human primates living in multi-level social systems. International Journal of Primatology, 33.
McHenry, H. M. (1994). Behavioral ecological implications of early hominid body size. Journal of Human Evolution, 27, 77–87.
McHenry, H. M. (1996). Sexual dimorphism in fossil hominids and its socioecological implications. In J. Steele & S. Shennan (Eds.), The archaeology of human ancestry (pp. 91–109). London: Routledge.
McHenry, H. M., & Coffing, K. (2000). Australopithecus to Homo: Transformations in body and mind. Annual Review of Anthropology, 29, 125–146.
Milton, K. (1999). A hypothesis to explain the role of meat-eating in human evolution. Evolutionary Anthropology, 8, 11–21.
Mitani, J. C., Watts, D. P., & Muller, M. N. (2002). Recent developments in the study of wild chimpanzee behavior. Evolutionary Anthropology, 11, 9–25.
Monahan, C. M. (1996). New zooarchaeological data from Bed II, Olduvai Gorge, Tanzania: Implications for hominid behavior in the Early Pleistocene. Journal of Human Evolution, 31, 93–128.
Nunn, C. L. (1999). The number of males in primate social groups: A comparative test of the socioecological model. Behavioral Ecology and Sociobiology, 46, 1–13.
O’Connell, J. F., Hawkes, K., & Blurton Jones, N. G. (1999). Grandmothering and the evolution of Homo erectus. Journal of Human Evolution, 36, 461–485.
O’Connell, J. F., Hawkes, K., Lupo, K. D., & Blurton Jones, N. G. (2002). Male strategies and Plio-Pleistocene archaeology. Journal of Human Evolution, 43, 831–872.
Palombit, R. A. (2003). Male infanticide in wild savanna baboons: Adaptive significance and intraspecific variation. In C. B. Jones (Ed.), Sexual selection and reproductive competition in primates: New perspectives and directions (pp. 3–47). Norman, OK: The American Society of Primatologists.
Pante, M. C. (2010). The larger mammal fossil assemblages from Beds III and IV, Olduvai Gorge, Tanzania: Implications for the feeding behavior of Homo erectus. Ph.D. thesis, Rutgers University.
Parker, S. T. (1990). Why big brains are so rare: Energy costs of intelligence and brain size in anthropoid primates. In S. T. Parker & K. R. Gibson (Eds.), “Language” and intelligence in monkeys and apes (pp. 129–154). Cambridge, UK: Cambridge University Press.
Parker, S. T. (2004). The cognitive complexity of social organization and socialization in wild baboons and chimpanzees: Guided participation, socializing interactions, and event representation. In A. E. Russon & D. R. Begun (Eds.), The evolution of thought (pp. 45–60). Cambridge, UK: Cambridge University Press.
Patzelt, A., Zinner, D., Fickenscher, G., Diedhiou, S., Camara, B., Stahl, D., & Fischer, J. (2011). Group composition of Guinea baboons (Papio papio) at a water place suggests a fluid social organization. International Journal of Primatology, 32, 652–668.
Pereira, M. E. (1993). Juvenility in animals. In M. E. Pereira & L. A. Fairbanks (Eds.), Juvenile primates: Life history, development, and behavior (pp. 17–27). New York: Oxford University Press.
Phillips-Conroy, J. E., Jolly, C. J., Nystrom, P., & Hemmalin, H. A. (1992). Migration of male hamadryas baboons into Anubis groups in the Awash National Park, Ethiopia. International Journal of Primatology, 13, 455–476.
Pines, M., & Swedell, L. (2011). Not without a fair fight: Failed abductions of females in wild hamadryas baboons. Primates, 52, 249–252.
Pines, M., Saunders, J., & Swedell, L. (2011). Alternative routes to the leader male role in a multi-level society: Follower versus solitary male strategies and outcomes in hamadryas baboons. American Journal of Primatology, 73, 679–691.
Plavcan, J. M. (1999). Mating systems, intrasexual competition and sexual dimorphism in primates. In P. C. Lee (Ed.), Comparative primate socioecology (pp. 241–260). Cambridge, UK: Cambridge University Press.
Plavcan, J. M. (2000). Inferring social behavior from sexual dimorphism in the fossil record. Journal of Human Evolution, 39, 327–344.
Plummer, T. (2004). Flaked stones and old bones: Biological and cultural evolution at the dawn of technology. Yearbook of Physical Anthropology, 47, 118–164.
Plummer, T. W., Ditchfield, P. W., Bishop, L. C., Kingston, J. D., Ferraro, J. V., Braun, D. R., Hertel, F., & Potts, R. (2009). Oldest evidence of tool-making hominins in a grassland-dominated ecosystem. PLoS One, 4, e7199.
Pobiner, B. L., Rogers, M. J., Monahan, C. M., & Harris, J. W. K. (2008). New evidence for hominin carcas processing strategies at 1.5 Ma, Koobi Fora, Kenya. Journal of Human Evolution, 55, 103–130.
Pontzer, H. (in press). Ecological energetics in early Homo. Current Anthropology
Pontzer, H., & Kamilar, J. M. (2009). Greater ranging associated with greater reproductive investment in mammals. Proceedings of the National Academy of Sciences of the USA, 106, 102–106.
Potts, R. (1991). Why the Oldowan? Plio-Pleistocene toolmaking and the transport of resources. Journal of Anthropological Research, 47, 153–176.
Potts, R. (1994). Variables versus models of early Pleistocene hominid land use. Journal of Human Evolution, 27, 7–24.
Potts, R. (1998). Environmental hypotheses of hominin evolution. Yearbook of Physical Anthropology, 41, 93–136.
Puts, D. A. (2010). Beauty and the beast: Mechanisms of sexual selection in humans. Evolution and Human Behavior, 31, 157–175.
Reed, K. E. (1997). Early hominid evolution and ecological change through the African Plio-Pleistocene. Journal of Human Evolution, 32, 289–322.
Ren, R., Garber, P. A., & Li, M. (2012). Fission–fusion behavior in Yunnan snub-nosed monkeys (Rhinopithecus bieti) in Yunnan, China. International Journal of Primatology, 33.
Rodseth, L. (2012) From bachelor threat to fraternal security: Male associations and modular organization in human societies. International Journal of Primatology. doi:10.1007/s10764-012-9593-4.
Rodseth, L., Wrangham, R. W., Harrigan, A. M., & Smuts, B. B. (1991). The human community as a primate society. Current Anthropology, 32, 221–254.
Rogers, M. J., Harris, J. W. K., & Feibel, C. S. (1994). Changing patterns of land use by Plio-Pleistocene hominids in the Lake Turkana Basin. Journal of Human Evolution, 27, 139–158.
Rose, M. D. (1976). Bipedal behavior of olive baboons (Papio anubis) and its relevance to an understanding of the evolution of human bipedalism. American Journal of Physical Anthropology, 44, 247–262.
Rubenstein, D. I. (2010). Ecology, social behavior, and conservation in zebras. Advances in the Study of Behavior, 42, 231–258.
Ruff, C. B. (2002). Variation in human body size and shape. Annual Review of Anthropology, 31, 211–232.
Ruff, C. B. (2008). Femoral/humeral strength in early African Homo erectus. Journal of Human Evolution, 54, 383–390.
Ruff, C. B. (2009). Relative limb strength and locomotion in Homo habilis. American Journal of Physical Anthropology, 138, 90–100.
Schick, K. D., & Toth, N. (1993). Making silent stones speak. New York: Simon and Schuster.
Schreier, A. (2010). Feeding ecology, food availability and ranging patterns of wild hamadryas baboons at Filoha. Folia Primatologica, 81, 129–145.
Schreier, A., & Swedell, L. (2009). The fourth level of social structure in a multi-level society: Ecological and social functions of clans in hamadryas baboons. American Journal of Primatology, 71, 948–955.
Schreier, A., & Swedell, L. (2012a). The socioecology of network scaling ratios in the multilevel society of hamadryas baboons. International Journal of Primatology. doi:10.1007/s10764-011-9572-1.
Schreier, A., & Swedell, L. (2012b). Ecology and sociality in a multilevel society: Ecological determinants of social cohesion in hamadryas baboons. American Journal of Physical Anthropology. doi:10.1002/ajpa.22076.
Shultz, S., Opie, C., & Atkinson, Q. D. (2012). Stepwise evolution of stable sociality in primates. Nature, 479, 219–222.
Sigg, H., Stolba, A., Abegglen, J.-J., & Dasser, V. (1982). Life history of hamadryas baboons: Physical development, infant mortality, reproductive parameters and family relationships. Primates, 23, 473–487.
Sikes, N. E., Potts, R., & Behrensmeyer, A. K. (1999). Early Pleistocene habitat in Member 1 Olorgesailie based on paleosol stable isotopes. Journal of Human Evolution, 37, 721–746.
Silk, J. B. (2007a). The adaptive value of sociality in mammalian groups. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 362, 539–559.
Silk, J. B. (2007b). Social components of fitness in primate groups. Science, 317, 1347–1351.
Silk, J. B., Beehner, J. C., Bergman, T. J., Crockford, C., Engh, A. L., Moscovice, L. R., Wittig, R. M., Seyfarth, R. M., & Cheney, D. L. (2009). The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society of London B: Biological Sciences, 276, 3099–3104.
Simpson, S. W., Quade, J., Levin, N. E., Butler, R., Dupont-Nivet, G., Everett, M., & Semaw, S. (2008). A female Homo erectus pelvis from Gona, Ethiopia. Science, 322, 1089–1092.
Smith, B. H., & Tompkins, R. L. (1995). Toward a life history of the hominidae. Annual Review of Anthropology, 24, 257–279.
Smolker, R., Richards, A. F., Connor, R. C., & Pepper, J. W. (1992). Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour, 123, 38–69.
Smuts, B. B. (1985). Sex and Friendship in Baboons. New York, Aldine Publishing Company.
Smuts, B. B., & Smuts, R. W. (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Advances in the Study of Behavior, 22, 1–63.
Snyder-Mackler, N., Beehner, J. C., & Bergman, T. J. (2012). Defining higher levels in a gelada multilevel society. International Journal of Primatology, 33.
Strier, K. B. (1992). Atelinae adaptations: Behavioral strategies and ecological constraints. American Journal of Physical Anthropology, 88, 515–524.
Strier, K. B. (2001). Beyond the apes: Reasons to consider the entire primate order. In F. B. M. de Waal (Ed.), Tree of origin: What primate behavior can tell us about human social evolution (pp. 69–93). Cambridge, MA: Harvard University Press.
Strum, S. C. (1987). Almost human. New York: W. W. Norton.
Strum, S. C., & Mitchell, W. (1987). Baboon models and muddles. In W. G. Kinzey (Ed.), The evolution of human behavior: Primate models (pp. 87–104). Albany: State University of New York Press.
Sueur, C., King, A. J., Conradt, L., Kerth, G., Lusseau, D., Mettke-Hofmann, C., Schaffner, C. M., Williams, L., Zinner, D., & Aureli, F. (2011). Collective decision-making and fission-fusion dynamics: A conceptual framework. Oikos, 120, 1608–1617.
Swedell, L. (2002). Affiliation among females in wild hamadryas baboons (Papio hamadryas hamadryas). International Journal of Primatology, 23, 1205–1226.
Swedell, L. (2006). Strategies of sex and survival in hamadryas baboons: Through a female lens. Upper Saddle River, NJ: Pearson Prentice Hall.
Swedell, L. (2011). African Papionins: Diversity of social organization and ecological flexibility. In C. J. Campbell, A. Fuentes, K. C. MacKinnon, S. K. Bearder, & R. M. Stumpf (Eds.), Primates in perspective (2nd ed., pp. 241–277). New York: Oxford University Press.
Swedell, L., & Saunders, J. (2006). Infant mortality, paternity certainty, and female reproductive strategies in hamadryas baboons. In L. Swedell & S. R. Leigh (Eds.), Reproduction and fitness in baboons (pp. 19–51). New York: Springer.
Swedell, L., & Schreier, A. (2009). Male aggression towards females in hamadryas baboons: Conditioning, coercion, and control. In M. N. Muller & R. W. Wrangham (Eds.), Sexual coercion in primates and humans (pp. 244–268). Cambridge, MA: Harvard University Press.
Swedell, L., & Tesfaye, T. (2003). Infant mortality after takeovers in wild Ethiopian hamadryas baboons. American Journal of Primatology, 60, 113–118.
Swedell, L., Hailemeskel, G., & Schreier, A. (2008). Composition and seasonality of diet in wild hamadryas baboons: Preliminary findings from Filoha. Folia Primatologica, 79, 476–490.
Swedell, L., Saunders, J., Schreier, A., Davis, B., Tesfaye, T., & Pines, M. (2011). Female “dispersal” in hamadryas baboons: Transfer among social units in a multilevel society. American Journal of Physical Anthropology, 145, 360–370.
Uchino, B. N. (2006). Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29, 377–387.
Ungar, P. S., Krueger, K. L., Blumenschine, R. J., Njau, J., & Scott, R. S. (2011). Dental microwear texture analysis of hominins recovered by the Olduvai Landscape Paleoanthropology Project, 1995–2007. Journal of Human Evolution. doi:10.1016/j.jhevol.2011.04.006.
van der Merwe, N. J., Masao, F. T., & Bamford, M. K. (2008). Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. South African Journal of Science, 104, 153–155.
van Schaik, C. P. (1983). Why are diurnal primates living in groups? Behaviour, 87, 120–144.
van Schaik, C. P., van Noordwijk, M. A., & Nunn, C. L. (1999). Sex and social evolution in primates. In P. C. Lee (Ed.), Comparative primate socioecology (pp. 204–231). Cambridge, UK: Cambridge University Press.
Vrba, E. S. (1985). Ecological and adaptive changes associated with early hominid evolution. In E. Delson (Ed.), Ancestors: The hard evidence (pp. 63–71). New York: Alan R. Liss.
Vrba, E. S. (1988). Late Pliocene climatic events and hominid evolution. In F. E. Grine (Ed.), Evolutionary history of the “robust” australopithecines (pp. 405–426). New York: Aldine.
Walker, A., & Ruff, C. (1993). The reconstruction of the pelvis. In A. Walker & R. Leakey (Eds.), The Nariokotome Homo erectus skeleton (pp. 221–233). Cambridge, MA: Harvard University Press.
Washburn, S. L., & DeVore, I. (1961a). Social behavior of baboons and early man. In S. L. Washburn (Ed.), Social life of early man (pp. 91–105). Chicago: Aldine.
Washburn, S. L., & DeVore, I. (1961b). The social life of baboons. Scientific American, 204, 62–71.
Wheeler, P. E. (1984). The evolution of bipedality and loss of functional body hair in hominids. Journal of Human Evolution, 13, 91–98.
Wittemyer, G., Douglas-Hamilton, I., & Getz, W. M. (2005). The socioecology of elephants: Analysis of the processes creating multitiered social structures. Animal Behaviour, 69, 1357–1371.
Wrangham, R. W. (1980). An ecological model of female-bonded primate groups. Behaviour, 75, 262–300.
Wrangham, R. W. (1987). The significance of African apes for reconstructing human social Evolution. In W. G. Kinzey (Ed.), The evolution of human behavior: Primate models (pp. 51–71). Albany: State University of New York Press.
Wrangham, R., & Conklin-Brittain, N. (2003). Cooking as a biological trait. Comparative Biochemistry and Physiology A, 136, 35–46.
Wrangham, R., Holland Jones, J., Laden, G., Pilbeam, D., & Conklin-Brittain, N. L. (1999). The raw and the stolen: Cooking and the ecology of human origins. Current Anthropology, 40, 567–594.
Wrangham, R., Cheney, D., Seyfarth, R., & Sarmiento, E. (2009). Shallow-water habitats as sources of fallback foods for hominins. American Journal of Physical Anthropology, 140, 630–642.
Wright, S. (1931). Evolution in Mendelian populations. Genetics, 16, 97–259.
Zhang, P., Watanabe, K., Li, B., & Tan, C. L. (2006). Social organization of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, Central China. Primates, 47, 374–382.
Zhang, P., Li, B., Qi, X., MacIntosh, A. J. J., & Watanabe, K. (2012). A proximity-based social network of the Sichuan snub-nosed monkey (Rhinopithecus roxellana). International Journal of Primatology, 33.
Zihlman, A. L. (1978). Women in evolution, part II: Subsistence and social organization among early hominids. Signs, 4, 4–20.
Zihlman, A. L., & Tanner, N. M. (1978). Gathering and the hominid adaptation. In L. Tiger & H. T. Fowler (Eds.), Female hierarchies (p. 163). Chicago: Beresford Book Service.
Zinner, D., Groeneveld, L. F., Keller, C., & Roos, C. (2009). Mitochondrial phylogeography of baboons (Papio spp.) – Indication for introgressive hybridization? BMC Evolutionary Biology, 9, 83.
Zuckerman, S. (1932). The social life of monkeys and apes. London: Routledge & Kegan Paul.
Zuckerman, S., & Parkes, A. S. (1939). Observations on secondary sexual characters in monkeys. Journal of Endocrinology, 1, 430–439.
Acknowledgments
We thank Leslie Aiello, Susan Antón, Cliff Jolly, Eric Delson, Becky Ackermann, Julian Saunders, and Sue Taylor Parker for helpful discussions during the development of these ideas, and we are especially grateful to Cliff Jolly, Fred Bercovitch, and Shirley Strum for numerous constructive and insightful comments on a previous version of this manuscript. We are also indebted to two anonymous reviewers, the editors of this special issue, and Joanna Setchell for additional helpful comments that improved this manuscript. We thank the Anthropology Section of the New York Academy of Sciences and the organizers of the Darwin’s Legacy: Human Evolution in Africa conference sponsored by the New York Consortium in Evolutionary Primatology (NYCEP) for inviting L.Swedell to deliver earlier versions of this article as public lectures. Finally, we thank the Leakey Foundation, the National Geographic Society, and the Wenner-Gren Foundation for their financial support of the Filoha Hamadryas Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swedell, L., Plummer, T. A Papionin Multilevel Society as a Model for Hominin Social Evolution. Int J Primatol 33, 1165–1193 (2012). https://doi.org/10.1007/s10764-012-9600-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-012-9600-9