Abstract
Several authors have proposed that papionin baboons provide appropriate analogs for early hominin niche differentiation. Savanna-dwelling baboons and australopiths both radiated around the same time after Neogene expansion of C4 grasslands, likely experiencing similar environmental changes and faced with solving similar ecological problems. We explore the insights baboons may provide into dietary ecology of savanna-occupying hominins. We compare dietary information from stable isotope data for feces, hair, and tooth dentine collagen of modern chacma baboons (Papio ursinus) with dietary data for Plio-Pleistocene papionins and hominins from South African savannas. Results confirm that, like the australopiths, baboons consume substantial amounts of C4 food sources. However, the magnitude of inter- and intraindividual variation in baboon diets across different seasons and habitats is less than that from specimens of Australopithecus africanus and Paranthropus robustus analyzed to date. Hominins also consumed greater amounts of C4 resources. Thus, though the data demonstrate that the radiation of both primate groups was closely linked with the spread of C4 grasslands, hominins were even more extreme ecological generalists than baboons were. The absence of a fixed-diet in papionins implies that it was unlikely that the more ecologically flexible hominins evolved specializations for any one food type, an interpretation consistent with recent carbon isotope, dental microwear, and ecomorphological studies. We propose that researchers place less emphasis on resolving the foods that were most important for hominin differentiation; instead, future research should focus on questions related to ecological generalism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding ecological niche differentiation of early hominins relies heavily on comparisons with extant primates, other mammals, and mammalian communities as a whole (Clutton-Brock and Harvey 1979; Peters and O’Brien 1981; Rodman 1999; Walker 1981; Yeakel et al. 2007). Dunbar (1976, 1983), Elton (2006), Jolly (1970, 2001), Lee-Thorp et al. (2003), and Sponheimer et al. (2006a) proposed that baboons (Papio spp. and Theropithecus spp.), as large-bodied savanna-dwelling primates, are a suitable ecological analog for early hominin niche differentiation. Researchers predicted that baboons and hominins occupied a similar range of savanna habitat types (DeVore and Hall 1965; Jolly 2001; Reed 1997), share certain parallels in functional morphology (Jolly 1970, 2001), and have comparable ecological dynamics given their contemporaneous radiations associated with C4 grassland proliferation through the Neogene (Delson 1992; Dunbar 1983; Lee-Thorp et al. 2003; Sponheimer et al. 2006a). However, baboons are extreme generalists, and as a result it has been difficult to characterize ecological traits and draw meaningful predictions for hominin palaeoecology.
Because of their phylogenetic proximity to humans, researchers have given much attention to extant hominoids, especially chimpanzees (Pan troglodytes) in comparisons with hominins. Yet, chimpanzees are a primarily tropical rain forest species (Wrangham et al. 1994), whereas Plio-Pleistocene hominins inhabited more open savanna environments (Reed 1997) and were thus exposed to a different resource base. Indeed, stable carbon isotope analyses, one of few techniques available to test directly hypotheses about early hominin diet, have shown that chimpanzees and australopith diets are disparate. Chimpanzee, even those with access to C4 savanna habitats, feed almost exclusively on C3 food items, e.g., tree leaves and fruit (Schoeninger et al. 1999; Sponheimer et al. 2006a), whereas both Australopithecus africanus and Paranthropus robustus from South African deposits had diets comprising substantial amounts of C4 foods, e.g., grass (Lee-Thorp et al. 1994, 2003; Peters and Vogel 2005; Sponheimer and Lee-Thorp 1999; Sponheimer et al. 2005, 2006b; van der Merwe et al. 2003). By contrast, carbon isotope data from both fossil and extant baboons (Papio spp., Parapapio spp., and Theropithecus spp.) implies that all consumed some C4 foods, in varying proportions that may be similar to that of the early hominin specimens (Codron et al. 2005a, 2006; Lee-Thorp et al. 1989a; Thackeray et al. 1996).
In field studies, Altman (1998), Barton et al. (1993), Byrne et al. (1993), DeVore and Hall (1965), Dunbar and Dunbar (1974), Norton et al. (1987), Whiten et al. (1991), and others revealed that baboon diets, excluding the primarily stenotopic graminivore Theropithecus gelada, vary in extreme ways. Stable isotope ecology is one tool that enables researchers to characterize and to quantify at least some aspects of diet in these and other generalist feeders in easily understandable terms. The basis is that the 13C/12C ratios (depicted as δ13C) of consumer tissues and excreta reflect the carbon isotope composition of food sources from which they are derived (Cerling and Harris 1999; Lee-Thorp and van der Merwe 1987; Tieszen et al. 1979; Vogel 1978). In African and other subtropical savanna environments, the δ13C values of higher plants are bimodally distributed between C3 (dicot trees, shrubs, and forbs) and C4 (grass) photosynthesizing plants. A third photosynthetic pathway, crassulacean acid metabolism (CAM), most common among succulents, has δ13C values that may be intermediate between the 2 end points, or may be indistinguishable from C3 or C4 plants, depending on whether they are obligate CAM-photosynthesizers or capable of switching between C3, C4, and CAM (Keeley and Rundel 2003). In our study areas, i.e., southern African subtropical savanna habitats, most CAM plants have a C4 carbon isotopic signature (Codron et al. 2006). Therefore, the δ13C of herbivores feeding here reflect proportions of C3 to C4/CAM biomass consumed. Stable nitrogen isotopes (15N/14N, or δ15N) are influenced by a more complex combination of environmental parameters, but used in conjunction with δ13C offer additional insights into trophic position, diet, dietary quality, and ecophysiology (Ambrose 1991; Robbins et al. 2005; Schoeninger et al. 1997; Sealy et al. 1987; Sponheimer et al. 2003a). An added benefit of isotopic tools is that one documents diet over different time scales. For example, feces provide information over the short term (several days), hair and teeth yield signals over several months or years, and bone integrates near-lifetime averages (Ambrose and Norr 1993; Passey et al. 2005; Sponheimer et al. 2003b). Serial isotope analysis of incremental tissues such as hair and teeth also provide time-series within individuals (Ayliffe et al. 2004; Sponheimer et al. 2006b).
Comparisons between baboons and australopiths likely will not resolve early hominin diets directly, because in most instances any traits common to both are unlikely to be homologous. Conversely, ecological processes deduced from analogous comparisons merely predict that both groups exhibit similar adaptive propensities in the presence of similar environments or other causative factors (Jolly 2001). Jolly (2001) argued that baboons and hominins share basic anthropoid traits, but are phylogenetically distant enough for homoplastic (analogous) modifications to be easily recognizable. Stable carbon isotope ecology also applies to palaeoenvironmental contexts, so that one can test at least some predictions derived from modern analogs in the fossil record. Thus, baboons are potentially useful simply as indicators of the types of C3 and C4 foods available to a primate foraging in savanna environments, the proportions in which different food items may be consumed, and the types of habitats/microhabitats that lead to increased utilization of C4 resources. We aimed to explore analogies between the ecology of baboons with hominins. We combine new and previously published (Codron et al. 2006; feces only) stable isotope data from feces, hair, and tooth dentine collagen specimens of chacma baboons (Papio ursinus) from several savanna habitats in South Africa to assess the magnitude of variation in their diets and the habitat types that favor C4 consumption. We then use this information to raise possibilities for early hominin ecology, and test the predictions by comparing carbon isotope trends in baboons with the ones reported for early hominins.
Methods
We conducted the study in the Kruger National Park and the Waterberg, South Africa (Codron et al. 2006). Kruger Park is a low-lying (ca. 300 m) semiarid savanna situated in the northeastern corner of South Africa, and the Waterberg is a mountainous region in the northwest on the plateau (>1000 m) of the central interior. The Waterberg is sour bushveld, i.e., an area of low grass productivity and patchy distribution of woody plants and grass (Tainton 1999). Conversely, Kruger Park is a large reserve (2,000,000 ha) that comprises an array of landscapes differing in climate, geology, and vegetation, though one can simplistically divide it into northern (dominated by Colophospermum mopane) and southern (Acacia/Combretum savanna) habitats, and longitudinally into granite-based savanna woodland in the west and basaltic open grasslands in the east.
We collected fresh fecal samples from the northern basalts in Kruger Park at monthly intervals from February 2004 to January 2005. We collected hairs of 9 baboons from Welgevonden Private Game Reserve in the Waterberg in June 2002. We obtained tooth samples of 8 individuals from the Transvaal Museum in Pretoria, including specimens from Blydepoort to the west of Kruger Park and from the D’Nyala Nature Reserve in the Waterberg. In addition, 2 sets of teeth are from Zoetfontein Game Farm in the Waterberg (UCT nos. 9786 and 9787), which the ground staff provided.
We oven-dried feces at 60°C for 24 h and homogenized them via grinding into a fine powder through a 1-mm sieve. We used hair and tooth samples to extract an isotopic time series within individuals. We sectioned each hair strand at ca. 20-mm intervals from root to tip, corresponding to the minimum mass for which we could perform reliable isotopic analyses (ca. 0.5 mg). We removed dentine powder from all 3 molar teeth (where available) at 0.5-mm increments, working from the dentine-enamel junction to the root, using a diamond-tipped microdrill. We isolated collagen in dentine via exposure to a 2.0 M HCl solution (Sealy 1997). We combusted individual feces, hair, and tooth dentine collagen samples in an automated Elemental Analyser (Carlo Erba, Milan), and introduced the resultant CO2 gas to a Finnigan Mat 252 Mass Spectrometer (Finnigan, Bremen) via a continuous flow-through inlet system (Conflo). We report 13C/12C and 15N/14N ratios in conventional delta notation (δ) in per mil (‰), relative to the Vienna PeeDee Belemnite (VPDB) and atmospheric N2 standards, respectively. Standard deviations of repeated measurement of laboratory standards was <0.1‰ for δ13C and 0.3‰ for δ15N. The C/N ratio of all hair and dentine collagen samples was within the expected range (2.7–3.6) for biological proteins (DeNiro 1985).
We conducted statistical comparisons via analysis of variance (ANOVA) and Tukey’s test post hoc for significant differences (p-level 2-tailed = 0.05). We compared feces from the northern basalts of Kruger Park with previously published data for samples from southern granites and southern basalts, Welgevonden, and Zoetfontein, collected in September 2002 and January 2003 (Codron et al. 2006). We converted fecal δ13C values to estimates of %C4 contributions to diet, via a dual-end point mixing model. We assumed diet-feces 13C-discrimination to be −0.9‰, based on controlled-feeding experiments of ungulates (Codron et al. 2005b; Sponheimer et al. 2003b). We used means of local C3 and C4 vegetation as mixing model end points to control for subtle differences in plant δ13C between the different habitats and seasons (April–September = dry season, October–March = wet season: Codron et al. 2006, 2007). We also estimated dietary inputs of different C3 and C4 food items via a dual-isotope multiple-source mixing model (Isosource: Phillips and Gregg 2003). We adjusted feces via diet-feces discriminations reported from controlled-feeding experiments of ungulates, i.e., ·0.9‰ for δ13C and +1.0‰ for δ15N (Sutoh et al. 1987). The food sources in the model (C3 tree and forb foliage, C3 fruit, C3 bark, C3 geophytes, C4 grass, and CAM succulents) are plant groups that are distinguishable on the basis of δ13C, δ15N, or both (p < 0.01). We combined bark and geophytes into a single group, labeled as bark/USOs (underground storage organs), because of similarities in δ13C and δ15N. In Isosource, we adjusted increment and tolerance ranges to 1.0 and 0.1‰, respectively, to control for analytical error and sample variability. We report results of Isosource as 25th–75th percentiles of the feasible intake range.
We compared data between the different materials with previously published results for fossil tooth enamel carbonate of extinct Papio spp. and Parapapio spp. (Codron et al. 2005a) and Australopithecus africanus and Paranthropus robustus from deposits at Makapansgat, Sterkfontein, and Swartkrans (Lee-Thorp et al. 1994; Sponheimer and Lee-Thorp 1999; Sponheimer et al. 2005; van der Merwe et al. 2003). To facilitate the comparisons, we converted δ13C data for all materials to %C4 in diet via the mixing-model, assuming diet-tissue discrimination factors of +3.2‰ for hair, +4.5‰ for collagen, and +13‰ for tooth enamel carbonate (Ambrose and Norr 1993; Cerling and Harris 1999; Lee-Thorp et al. 1989b; Passey et al. 2005; Sponheimer et al. 2003b). For specimens predating 1950, and the fossil data, we applied a correction factor of 1.5‰ to compensate for 20th century 13C-depletion of atmospheric CO2 (van der Merwe et al. 2003). Sponheimer et al. (2006a) showed the conversions to be a successful means for comparing data from different materials with different fractionation effects and reflecting different dietary components.).
Results
Feces: Revisiting Previous Hypotheses
Fecal δ13C data imply that average %C4 in the diets of baboons on the northern basalts is ca. 31% (n = 100; Fig. 1a and b). The data suggest that C4 consumption is greater in the landscape relative to previously published values for baboons on southern basalts (ca. 17%, n = 21; p < 0.001) and southern granites (ca.10%, n = 65; p < 0.0001). Nonetheless, the northern basalts data follow previously reported trends that C4 consumption in Kruger Park is less than in the Waterberg, at least on Welgevonden, where mean %C4 consumed was higher than on northern basalts (ca. 43, n = 135; p < 0.0001). Northern basalts fecal δ13C indicate that baboon diets differ significantly between the dry and wet seasons (p < 0.0001), but %C4 in diet in fact increased from the wet (ca. 24%) to the dry season (ca. 38%), which contrasts with data for the other regions in that seasonal changes were significant only for Welgevonden (p < 0.001), and in that instance C4 intake increased from the dry to the wet season (Fig. 1b).
Regional and seasonal changes in baboon diet from stable isotope data in feces. The plots show differences in (a) fecal δ13C, (b) estimated %C4 in diet, and (c) fecal δ15N between northern basalts (NB), southern basalts (SB), and southern granites (SG) in Kruger Park, and Welgevonden (WG) and Zoetfontein (ZF) game farms in the Waterberg. Dry season data represent samples collected between April and September, and the wet season from October to March. Monthly changes in estimated %C4 in diet (calculated from fecal δ13C) and fecal δ15N for baboons from NB, representing February 2004–January 2005, are in (d). Symbols depict means, and vertical bars ± standard error. Data for SB, SG, WG, and ZF are from Codron et al. (2006).
Fecal δ15N for the northern basalts averaged 6.2 ± 0.1 SE, n = 100 (Fig. 1c). The figure is far higher (p < 0.0001) than previously reported for baboons from southern Kruger Park and the Waterberg (means vary from 3.8‰ ± 0.1 SE on Welgevonden to 4.7‰ ± 0.2 SE on southern granites). The high fecal δ15N of baboons in northern Kruger Park might indicate substantial consumption of 15N-enriched CAM succulents. Seasonally, northern basalt fecal δ15N showed no change from the dry to the wet season (p = 0.93), which contrasts with the general trend for fecal δ15N in the earlier study to increase from the dry to the wet season.
Collections from the northern basalts facilitate a higher resolution study of seasonal variation than was previously available. There are more substantial changes in diet at monthly than at broader dry-versus-wet seasonal scales, with %C4 in diet varying from ca. 60% in March (n = 5) and June (n = 10) to <10% in October (n = 10) and December (n = 10) (Fig. 1d). Similarly, significant changes (p < 0.0001) in mean fecal δ15N are recorded at the monthly scale, monthly means varying from 5.4‰ ± 0.9 SE in July (n = 10) and 5.4‰ ± 0.2 SE in December (n = 10) to 8.7‰ ± 0.2 SE in March). There appears to be no sequential pattern of changes in fecal δ13C (and %C4 intake), or for fecal δ15N, through the seasonal cycle. Instead, the monthly fecal data contain intermittent peaks, e.g., March, June, and November, highlighting the complexity of baboon feeding behavior.
Feces: New Insights from a Dual-isotope Mixing Model (Isosource)
Adjusting fecal δ13C and δ15N for expected diet-feces discrimination effects (Codron et al. 2005b; Sponheimer et al. 2003b; Sutoh et al. 1987) ensured that the means for each of the 5 habitats fell within the range of available food sources distributed in isotope space (Fig. 2a). Results of the multiple source mixing model (Isosource) portray widely divergent diets between baboons from the different regions (Fig. 2b). In Kruger Park landscapes, the predicted dominant food group is C3 foliage (comprising 58–64%, 34–52%, and 47–61% of diet, respectively), whereas in the Waterberg, there appears to be higher consumption of grass (34–45% on Welgevonden and 9–20% on Zoetfontein) and bark/USOs (38–42% on Welgevonden and 15–40% on Zoetfontein). The latter result is consistent with evidence from dental microwear for baboons from the Waterberg region, which imply substantial use of abrasive foods with high grit/dust content, especially USOs (Daegling and Grine 1999). CAM-photosynthesizing succulents make some contribution to baboon diets in the Waterberg (≤14%), as Codron et al. (2006) predicted, but appear to be even more important on northern basalts (29–30%). The model also predicts that C3 fruits are important food items in southern Kruger Park (13–43% on basalts and 11–37% on granites), likely reflecting the more woodland and riparian type habitats here. Fruits appear to make negligible contributions to diet (2–9%) in northern Kruger Park, where open grasslands prevail across the landscape.
Partitioning of multiple food sources in baboon diets from isotope data in feces. The isotope mixing polygon (a) shows the distribution of baboon fecal isotope composition from the 5 habitats in relation to available food sources in isotope space. Fecal data are means for each region adjusted for discrimination effects so that values for plant food types (solid symbols) in the polygons represent 100% intake of the food item. We depict contributions of different food types as 25th –75th percentiles of feasible intake ranges predicted from Isosource (b). NB = northern basalts, SB = southern basalts, and SG = southern granites in Kruger Park; WG = Welgevonden, and ZF = Zoetfontein in the Waterberg.
Hairs and Teeth: Intraindividual Variations
Mean δ13C values of individual baboon hairs range from −19.8‰ (UCT no. 8639) to −18.5‰ (UCT no. 9795; Fig. 3), and the ones for dentine collagen vary from −20.0‰ (UCT nos. 9786, 9787, and TM 911) to −17.9‰ (AZ 806; Fig. 4). The values correspond to diets comprising 21–30% C4 in the case of hair, and 5–30% C4 in dentine collagen. There is a degree of similarity in data for tooth specimens from the Waterberg (5–20% C4), and also across specimens from the Blydepoort and Kruger Park regions (15–30%), complementing results of feces that indicate that though there may be considerable dietary variation between regions, there is less variation within habitats. However, carbon isotope profiles along individual hair strands, teeth, and tooth rows reflect extensive variation even within individuals from the same region. Dietary shifts of 12–32% in terms of C3 versus C4 intake are evinced within individual hairs, and a similar magnitude of variation (10–36%) occurs along teeth and tooth rows of individuals. Again, the observations parallel insights from feces, in that large variations occur from month-to-month through the seasonal cycle.
Serial δ13C profiles along molar teeth and tooth rows of chacma baboons from Blydepoort (BP) and the Waterberg (WB). Changes in %C4 contributions to diet are on the right y-axis. Profiles are in increments of 0.5–1 mm, and the sequences from left-to-right are from dentine-enamel junction to root, i.e., oldest to most recent growth. Accession numbers and date of death are in the graph subtitles.
Comparisons with Fossil Papionins and Hominins
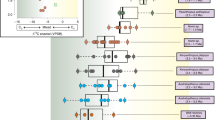
In Fig. 5, we compare variation in baboon C4 consumption, as evinced via δ13C of feces, hair, and dentine collagen, with the variation in individual fossil baboons and hominins from the South African Plio-Pleistocene. In general, the dietary ranges of modern baboons are similar to the ones of both the fossil papionins and early hominins. However, the papionin data, for all tissues, show a narrower range than in hominin taxa, both within individual baboons and across them. For feces, the variation within the Waterberg is only ca. 20–50%, and in southern Kruger Park ca. 10–20%, whereas the hominins, especially Australopithecus africanus, show variations of ≤60% in terms of C3 versus C4 foods consumed. Only when we consider monthly variations, i.e., feces from northern basalts, do baboon dietary variations match the ones across hominin individuals. Similarly, hair and dentine collagen data for modern baboons reflect diets varying from ca. 10 to 40% C4 intake, portraying a smaller magnitude of variation than in hominins. Further, mean %C4 intake in baboons is generally lower than that of early hominins (36% and 31% for Australopithecus africanus and Paranthropus robustus, respectively); only feces from the Waterberg show similarly high C4 consumption (ca. 37%), but even then data from baboon hairs, which reflect diet over a longer time period, imply an average C4 contribution to diet of only ca. 26%.
Dietary variation in early hominins (open squares) and extinct papionins (open circles) based on estimated %C4 intake calculated from δ13Cenamel carbonate, versus that of modern baboons (closed circles) derived from (a) seasonal/monthly and regional mean δ13Cfeces; (b) overall regional mean δ13Cfeces; (c) individual δ13Chair profiles; (d) mean δ13Chair of individuals; (e) individual δ13Cdentine collagen profiles; and (f) mean δ13Cdentine collagen of individuals. Data for Plio-Pleistocene hominin fossils are from Lee-Thorp et al. (1994), Sponheimer and Lee-Thorp (1999), van der Merwe et al. (2003), and Sponheimer et al. (2005), and for fossil papionins from Codron et al. (2005a). WB = Waterberg; SK = southern Kruger Park; NK = northern Kruger Park; KB = Kruger Park and Blydepoort.
Discussion
Our results confirm that baboons, like early hominins, consume significant amounts of C4-based resources. Data from fossil baboons show that C4-feeding has persisted since Plio-Pleistocene times, when baboons lived alongside taxa such as Australopithecus africanus and Paranthropus robustus. Moreover, the range of variation in proportions of C3:C4 foods baboons consume is, in many ways, similar to that of early hominins. The implication is that both groups experienced a similar environmental shift some millions of years ago, and both responded by switching to include C4 food items in their diets.
This is not to argue that baboons and hominins have necessarily identical diets, but instead that there is some benefit in an analogous comparison between the 2; the approach simply dictates that derived traits in 2 taxa are the outcome of a common cause (cf. Jolly 2001), in this instance the likely shift from heavily wooded to more open savanna habitats. In effect, any 2 taxa perceived to share a likeness in apomorphies are analogous, and Laden and Wrangham (2005), Peters and O’Brien (1981), Schaller and Lowther (1969), Ungar et al. (2006), Washburn and DeVore (1961), and Yeakel et al. (2007) used many such cases in attempts to resolve hominin palaeoecology, including chimpanzees and gorillas, cercopithecoid monkeys, platyrrhine monkeys, African carnivores, molerats (Bathyergidae), porcupines (Hystricidae), and others. However, there is a risk of misinterpreting the conclusions that one can derive from the approaches. Jolly (1970) was one of the first to draw analogous comparisons between hominins and a somewhat distantly related baboon taxon, seed-eating geladas (Theropithecus gelada). However, his conclusion, which perhaps was not entirely clear from the outset, was simply that the divergence of geladas from the papionin line entailed an ecological and evolutionary process comparable with that of australopiths from the chimpanzee lineage, i.e., shifting from a diet of larger and/or fibrous foods to one comprising small, hard objects (Jolly 2001). With particular relevance to our study, it is important to note that baboons themselves tell us very little about early hominin diet, but that similarities in isotope data indicate the range of possibilities for a primate in the African savanna, and some of the most illuminating insights can actually be derived from situations in which baboons and hominins differ. In this respect, 2 facets of baboon isotope ecology offer some clues to interpreting the hominin record.
First, a general observation is that baboon diets are typically less isotopically variable than were the ones of australopiths. The 2 groups display an equally broad magnitude of variation only when one considers baboons from multiple habitats, or across subtle temporal scales (monthly). For the most part, baboons within a given region appear to develop a fixed or staple diet, most evinced in the smaller degree of variation between hair and tooth samples than intraindividually (Codron et al. 2006). In field studies of baboons in southern and East African environments, Norton et al. (1987) and Byrne et al. (1993) made similar observations, in that staple food items of populations remain fairly consistent through time with only perimeter items varying in substantial ways The implication is that australopiths were either even more generalist than baboons, or that the variation in early hominin enamel δ13C likely represents individuals from different populations isolated in space or time. Fecal samples collected at monthly intervals portray a similarly high degree of variation as exists across multiple hominin enamel specimens, but the 2 lines of evidence are not strictly comparable because of the short-term nature of feces as dietary indicators. One can make a more appropriate comparison with recently published intraindividual time series of δ13C data along tooth samples of Paranthropus robustus, which revealed short-time fluctuations not unlike the ones we recorded in our monthly fecal data (Sponheimer et al. 2006a). This confirms a similar degree of flexibility in baboon and hominin diets, while also reasserting the idea that the hominin specimens represent different subpopulations within, but not necessarily between, their respective localities (Makapansgat, Sterkfontein, and Swartkrans).
Second, the environmental mechanisms underlying use of C4 foods by baboons are highly complex. For example, increased C4 food intake does not appear to reflect availability; baboons ate relatively high amounts of C4 foods in the Waterberg, where grass productivity is far lower than in most of Kruger Park (Tainton 1999), and within Kruger Park, C4 intake was similarly low on southern granites and southern basalts despite the latter comprising predominantly open grassland savannas (p = 0.38; Codron et al. 2006). Similarly, differences in hominin δ13C between deposits separated by hundreds of thousands of years (Makapansgat M3, ≥3 Ma, Sterkfontein M4, ca. 2.2 Ma, and Swartkrans M1, ca. 1.7 Ma) are minimal (Lee-Thorp et al. 2003); thus increased C4 intake does not appear to be related to progressive increases in C4 availability through time. Therefore, it is far more likely that the switch(es) to C4 foods in hominins was related to complex causes at intraindividual or intrapopulation scales, as is the case in modern baboons.
Researchers have traditionally greatly emphasized the food item(s), either as staples or fallback foods, that had the most substantial impact on early hominin ecological differentiation and functional anatomy. Opinions are divided about whether different types of animal matter, leaves, fruit and nuts, grasses, USOs, or various other resources were fundamental drivers of australopith niche differentiation, adaptation, and evolution. For example, stable carbon isotope data are in many ways incongruent with evidence from dental microwear and ecomorphology, because the latter reveal little evidence for intake of, and adaptations to, the tough, fibrous diets characteristic of most C4-based foods (Lee-Thorp et al. 1994; cf. Scott et al. 2005). For baboons, results of Isosource suggest that the composition of their C3 meal (leaves, fruit, bark) changes substantially across habitats, and that their C4 dietary component includes mainly grass in some cases, but higher proportions of CAM plants in others. Available evidence from field studies is consistent with the results, with authorities divided regarding whether baboons and their functional adaptations are classified as folivores, frugivores, or omnivores. Whiten et al. (1991) reported that leaves comprise 8–53% of baboon diets; fruits, 3–74%, and USOs, 8–53%. Grasses may contribute anywhere between 0 and 40% to bulk intake (DeVore and Hall 1965; Dunbar and Dunbar 1974). In Laikipia, Kenya, baboons incorporate as much foliage into their diets as folivorous colobines do (Barton et al. 1993), and in 1 instance, a baboon troop fed almost exclusively on animal matter (Strum 1975).
The observations are typical of the generalist, somewhat opportunist, feeding behavior predicted for baboons (Altman 1998; Byrne et al. 1993; DeVore and Hall 1965); best elucidated in DeVore and Hall’s (1965) claim that “...it is almost easier to list the items which they do not eat than to describe the items which they do” (p. 43). Regardless, baboons are not as flexible in terms of switching proportions of C3/C4 resource consumption as early hominins were. Both hominin genera also ate more C4 foods overall than those of modern and fossil papionins, excluding Theropithecus gelada (van der Merwe et al. 2003; this study). If no single food source can define baboon diet and functional adaptation, it is even less likely that this occurred in early hominins. The perceived functional adaptations of australopiths to their diets may therefore simply reflect an expansion of their dietary niche(s). Indeed, in most recent tests via isotopic, dental microwear, and other lines of morphological evidence researchers advocate that even Paranthropus robustus was a highly generalist feeder (Scott et al. 2005; Sponheimer et al. 2006a; Wood and Strait 2004), in contrast to most predictions that their enlarged premolars and molar enamel crowns represent a form of dietary specialization that ultimately contributed to their extinction ca. 1 Ma.
We suggest that researchers should devote less attention to resolving the most important food sources and focus more on why hominins shifted to include so much C4 and/or CAM based foods in their diets. Was it a response to perturbations in climate and environmental conditions, did they diversify their diets because competition with sympatric C3-sepcialists forced them to occupy new habitats, or was the dietary shift merely a move toward a more generalist lifestyle that enabled them to adapt and survive further climatic and environmental changes? Future studies of feeding responses to environmental change and fluctuations in biotic interactions in dietary generalists like baboons can help to answer these and other questions, and provide a greatly improved understanding of early hominin palaeoecology.
References
Altman, S. A. (1998). Foraging for survival: Yearling baboons in Africa. Chicago: University of Chicago Press.
Ambrose, S. H. (1991). Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial foodwebs. Journal of Archaeological Science, 18, 293–317.
Ambrose, S. H., & Norr, L. (1993). Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In J. B. Lambert, & G. Grupe (Eds.), Prehistoric human bone: Archaeology at the molecular level (p. 1–37). Berlin: Springer-Verlag.
Ayliffe, L. K., Cerling, T. E., Robinson, T., West, A. G., Sponheimer, M., Passey, B. H., et al. (2004). Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia, 139, 11–21.
Barton, R. A., Whiten, A., Byrne, R. W., & English, M. (1993). Chemical composition of baboon plant foods: Implications for the interpretation of intra- and interspecific differences in diet. Folia Primatologica, 61, 1–20.
Byrne, R. W., Whiten, A., Henzi, S. P., & McCulloch, F. M. (1993). Nutritional constraints on mountain baboons (Papio ursinus): Implications for baboon socioecology. Behavioral Ecology and Sociobiology, 33, 233–246.
Cerling, T. E., & Harris, J. M. (1999). Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia, 120, 347–363.
Clutton-Brock, T. H., & Harvey, P. H. (1979). Comparison and adaptation. Proceedings of the Royal Society of London, 205B, 247–565.
Codron, D., Codron, J., Sponheimer, M., Lee-Thorp, J. A., Robinson, T., Grant, C. C., et al. (2005b). Assessing diet in savanna herbivores using stable carbon isotope ratios of faeces. Koedoe, 48, 115–124.
Codron, D., Lee-Thorp, J. A., Sponheimer, M., & Codron, J. (2007). Stable carbon isotope reconstruction of ungulate diet changes through the seasonal cycle. South African Journal of Wildlife Research, 37, 117–125.
Codron, D., Lee-Thorp, J. A., Sponheimer, M., de Ruiter, D., & Codron, J. (2006). Inter- and intra-habitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C, δ15N and %N. American Journal of Physical Anthropology, 129, 204–214.
Codron, D., Luyt, J., Lee-Thorp, J. A., Sponheimer, M., de Ruiter, D., et al. (2005a). Utilization of savanna-based resources by Plio-Pleistocene baboons. South African Journal of Science, 101, 245–248.
Daegling, D. J., & Grine, F. E. (1999). Terrestrial foraging and dental microwear in Papio ursinus. Primates, 40, 559–572.
Delson, E. (1992). Evolution of old world monkeys. In J. S. Jones, R. D. Martin, D. Pilbeam, & S. Bunney (Eds.), Cambridge encyclopedia of human evolution (pp. 217–222). Cambridge, UK: Cambridge University Press.
DeNiro, M. J. (1985). Postmortem preservation and alterations of in vitro bone collagen isotope ratios in relation to paleodietary reconstruction. Nature, 317, 806–809.
DeVore, I., & Hall, K. R. L. (1965). Baboon ecology. In I. DeVore (Ed.), Primate behavior: Field studies of monkeys and apes (pp. 20–52, 1st ed.). New York: Holt, Rinehart and Winston.
Dunbar, R. I. M. (1976). Australopithecine diet based on a baboon analogy. Journal of Human Evolution, 5, 161–167.
Dunbar, R. I. M. (1983). Theropithecines and hominids: Contrasting solutions to the same ecological problem. Journal of Human Evolution, 12, 647–658.
Dunbar, R. I. M., & Dunbar, E. P. (1974). Ecological relations and niche separation between sympatric terrestrial primates in Ethiopia. Folia Primatologica, 21, 36–60.
Elton, S. (2006). Forty years on and still going strong: The use of hominin-cercopithecid comparisons in palaeoanthropology. Journal of the Royal Anthropological Institute, 12, 19–38.
Jolly, C. J. (1970). The seed-eaters: A new model of hominid differentiation based on a baboon analogy. Man, 5, 1–26.
Jolly, C. J. (2001). A proper study for mankind: Analogies from the papionin monkeys and their implications for human evolution. Yearbook of Physical Anthropology, 44, 177–204.
Keeley, J. E., & Rundel, P. W. (2003). Evolution of CAM and C4 carbon-concentrating mechanisms. International Journal of Plant Science, 164, S55–S77.
Laden, G., & Wrangham, R. (2005). The rise of the hominids as an adaptive shift in fallback foods: Plant underground storage organs (USOs) and australopiths origins. Journal of Human Evolution, 49, 482–498.
Lee-Thorp, J. A., Sealy, J. C., & van der Merwe, N. J. (1989b). Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. Journal of Archaeological Science, 16, 585–599.
Lee-Thorp, J. A., Sponheimer, M., & van der Merwe, N. J. (2003). What do stable isotopes tell us about hominid dietary and ecological niches in the Pliocene? International Journal of Osteoarchaeology, 13, 104–113.
Lee-Thorp, J. A., & van der Merwe, N. J. (1987). Carbon isotope analysis of fossil bone apatite. South African Journal of Science, 83, 712–715.
Lee-Thorp, J. A., van der Merwe, N. J., & Brain, C. K. (1989a). Isotopic evidence for dietary differences between two extinct baboon species from Swartkrans. Journal of Human Evolution, 18, 183–190.
Lee-Thorp, J. A., van der Merwe, N. J., & Brain, C. K. (1994). Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. Journal of Human Evolution, 27, 361–372.
Norton, G. W., Rhine, R. J., Wynn, G. W., & Wynn, R. D. (1987). Baboon diet: A five-year study of stability and variability in the plant feeding and habitat of the yellow baboons (Papio cynocephalus) of Mikumi National Park, Tanzania. Folia Primatologica, 48, 78–120.
Passey, B. H., Robinson, T. F., Ayliffe, L. K., Cerling, T. E., Sponheimer, M., Dearing, M. D., et al. (2005). Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. Journal of Archaeological Science, 32, 1459–1470.
Peters, C. R., & O’Brien, E. M. (1981). The early hominid plant-food niche: Insights from an analysis of plant exploitation by Homo, Pan, and Papio in Eastern and Southern Africa. Current Anthropology, 22, 127–140.
Peters, C. R., & Vogel, J. C. (2005). Africa’s wild C4 plant foods and possible early hominid diets. Journal of Human Evolution, 48, 219–236.
Phillips, D. L., & Gregg, J. W. (2003). Source partitioning using stable isotopes: Coping with too many sources. Oecologia, 136, 261–269.
Reed, K. E. (1997). Early hominid evolution and ecological change through the African Plio-Pleistocene. Journal of Human Evolution, 32, 289–322.
Robbins, C. T., Felicetti, L. A., & Sponheimer, M. (2005). The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia, 144, 534–540.
Rodman, P. S. (1999). Whither primatology? The place of primates in contemporary anthropology. Annual Review of Anthropology, 28, 311–339.
Schaller, G. B., & Lowther, G. R. (1969). The relevance of social carnivore behavior to the study of early hominids. Southwest Journal of Anthropology, 25, 307–341.
Schoeninger, M. J., Iwaniec, U. T., & Glander, K. E. (1997). Stable isotope ratios indicate diet and habitat use in New World monkeys. American Journal of Physical Anthropology, 103, 69–83.
Schoeninger, M. J., Moore, J., & Sept, J. M. (1999). Subsistence strategies of two “savanna” chimpanzee populations: The stable isotope evidence. American Journal of Primatology, 49, 297–314.
Scott, R. S., Ungar, P. S., Bergstrom, T. S., Brown, C. A., Grine, F. E., Teaford, M. F., et al. (2005). Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature, 436, 693–695.
Sealy, J. C. (1997). Stable carbon and nitrogen isotope ratios and coastal diets in the Later Stone Age of South Africa: A comparison and critical analysis of two data sets. Ancient Biomolecules, 1, 131–147.
Sealy, J. C., van der Merwe, N. J., Lee-Thorp, J. A., & Lanham, J. L. (1987). Nitrogen isotopic ecology in southern Africa: Implications for environmental and dietary tracing. Geochimica Cosmochimica Acta, 51, 2707–2717.
Sponheimer, M., & Lee-Thorp, J. A. (1999). Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science, 283, 368–370.
Sponheimer, M., Lee-Thorp, J., de Ruiter, D., Codron, D., Codron, J., Baugh, A., et al. (2005). Hominins, sedges, and termites: New carbon isotope data from the Sterkfontein Valley and Kruger National Park. Journal of Human Evolution, 48, 301–312.
Sponheimer, M., Loudon, J. E., Codron, D., Howells, M. E., Pruetz, J. D., Codron, J., et al. (2006a). Do savanna chimpanzees consume C4 resources? Journal of Human Evolution, 51, 128–133.
Sponheimer, M., Passey, B. H., de Ruiter, D. J., Guatelli-Steinberg, D., Cerling, T. E., & Lee-Thorp, J. A. (2006b). Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science, 314, 980–982.
Sponheimer, M., Robinson, T., Ayliffe, L., Passey, B., Roeder, B., Shipley, L., et al. (2003b). An experimental study of carbon-isotope fractionation between diet, hair, and feces of mammalian herbivores. Canadian Journal of Zoology, 81, 871–876.
Sponheimer, M., Robinson, T., Ayliffe, L., Roeder, B., Hammer, J., Passey, B., et al. (2003a). Nitrogen isotopes in mammalian herbivores: Hair δ15N values from a controlled-feeding study. International Journal of Osteoarchaeology, 13, 80–87.
Strum, S. C. (1975). Primate predation: Interim report on the development of a tradition in a troop of olive baboons. Science, 187, 755–757.
Sutoh, M., Koyama, T., & Yoneyama, T. (1987). Variations of natural 15N abundances in the tissues and digesta of domestic animals. Radioisotopes, 36, 74–77.
Tainton, N. M. (1999). The ecology of the main grazing lands of South Africa: The Savanna biome. In N. M. Tainton (Ed.), Veld management in South Africa (pp. 23–53). Pietermaritzburg, South Africa: University of Natal Press.
Thackeray, J. F., Henzi, S. P., & Brain, C. (1996). Stable carbon and nitrogen isotope analysis of bone collagen in Papio cynocephalus ursinus: Comparison with ungulates and Homo sapiens from southern and East African environments. South African Journal of Science, 92, 209–212.
Tieszen, L., Hein, D., Qvortrup, S., Troughton, J., & Imbamba, S. (1979). Use of δ13C values to determine vegetation selectivity in east African herbivores. Oecologia, 37, 351–359.
Ungar, P. S., Grine, F. E., Teaford, M. F., & El-Zaatari, S. (2006). Dental microwear and diets of African early Homo. Journal of Human Evolution, 50, 78–95.
van der Merwe, N. J., Thackeray, J. F., Lee-Thorp, J. A., & Luyt, J. (2003). The carbon isotope ecology and diet of Australopithecus africanus at Sterkfontein, South Africa. Journal of Human Evolution, 44, 581–597.
Vogel, J. C. (1978). Isotopic assessment of the dietary habits of ungulates. South African Journal of Science, 74, 298–301.
Walker, A. C. (1981). Diet and teeth: Dietary hypothesis and human evolution. Philosophical Transactions of the Royal Society of London, 292B, 57–64.
Washburn, S. L., & DeVore, I. (1961). The social life of baboons. Scientific American, 204, 62–71.
Whiten, A., Bryne, R. W., Barton, R. A., Waterman, P. G., & Henzi, S. P. (1991). Dietary and foraging strategies of baboons. Philosophical Transactions of the Royal Society of London, 334B, 187–197.
Wood, B., & Strait, D. (2004). Patterns of resource use in early Homo and Paranthropus. Journal of Human Evolution, 46, 119–162.
Wrangham, R. W., McGrew, W. C., de Waal, F. B. M., Heltne, P. G., & Marquardt, L. A. (1994). Chimpanzee cultures. Cambridge MA: Harvard University Press.
Yeakel, J. D., Bennett, N. C., Koch, P. L., & Dominy, N. J. (2007). The isotopic ecology of African mole rats informs hypotheses on the evolution of human diet. Proceedings of the Royal Society of London, B274, 1723–1730.
Acknowledgments
We thank James Brink, Lloyd Rossouw, Judith Sealy, and Becky Ackermann for helpful discussions; John Lanham and Ian Newton for assistance with stable isotope analysis; Erwin Leibnitz, Hanno Kilian, and Andre Burger for assistance at Welgevonden; Dieter Mandlmeier and Salthiel Kgomo at Zoetfontein; and Kruger Park Scientific Services. The work is based partly on natural history specimens of the Transvaal Museum (TM), Pretoria, used with permission, and we thank Francis Thackeray, Teresa Kearney, and Stephany Potze of the TM. The Palaeontological Scientific Trust (PAST), the National Research Foundation of South Africa, and the University of Cape Town provided funding for the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Codron, D., Lee-Thorp, J.A., Sponheimer, M. et al. What Insights Can Baboon Feeding Ecology Provide for Early Hominin Niche Differentiation?. Int J Primatol 29, 757–772 (2008). https://doi.org/10.1007/s10764-008-9261-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-008-9261-x