Abstract
Although most colobines feed mainly on leaves and a few feed heavily on seeds, colobine digestive adaptations for folivory are thought to preclude the high use of ripe fleshy fruits. In this long-term study of Semnopithecus vetulus nestor, the endemic western purple-faced langur of Sri Lanka, I investigated the feeding ecology and dietary flexibility for fruit feeding in 2 free-ranging groups (PT1 and R1) living in human-modified environments with abundant cultivated fruit, at Panadura and Piliyandala, for 19 mo and 13 mo respectively, using scan-sampling, vegetation enumeration, and phenological studies. In contrast to folivorous forest-living colobines, including other subspecies of Semnopithecus vetulus, my focal groups used more fruit (>50%) than foliage (PT1: 36%; R1: 34%). Both groups used many plant species (PT1 115; R1 59), but selected their food species, fruits over leaves, and young leaves over mature leaves. Fruit use was independent of young leaf availability. Notably, 78.4% and 83.4% of fruits consumed by PT1 and R1 were fleshy and human-edible, most of which were ripening or ripe (PT1: 72.4%; R1: 94.8%). The main fruit for both groups was Artocarpus heterophyllus (Moraceae; jakfruit), a cultivar with fleshy fruit. These findings differ from previous understanding of colobine diets. I suggest that environmental factors, such as the abundance and nature of available fruits, and the absence of arboreal-primate fruit competitors, could influence the use of ripe fleshy fruits by colobines strongly, highlighting the need to review the dietary and digestive flexibility of this group in changed and changing natural environments to formulate effective conservation action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most colobines are predominantly folivorous (Clutton-Brock 1975; Davies et al. 1999; Hoang et al. 2009; Li et al. 2003; Marsh 1981; Matsuda et al. 2009; Oates 1988; Solanki et al. 2008; Struhsaker 1975; Wong et al. 2006; Zhou et al. 2006), which is made possible by their specialized gastrointestinal adaptations for feeding on leaves that distinguish them from other primates (Bauchop 1978; Chivers 1994; Oates and Davies 1994). These adaptations include a multichambered stomach and foregut fermentative digestion by bacteria (Bauchop 1978; Chivers 1994), facilitated by an alkaline environment (pH 5.0–7.0) in the foregut, which is structurally separated from the more acidic fundic region (Bauchop 1978; Kay and Davies 1994). Bacterial action is vital for colobines to digest structural carbohydrates in leaves (Bauchop 1978; Kay and Davies 1994), detoxify plant food (Kay and Davies 1994; McKey et al. 1981), biosynthesize protein and vitamins, and conserve water in the host (Bauchop 1978; Kay and Davies 1994; Oxnard 1969). Because leaves are fibrous and hard to digest (Waterman and Kool 1994), the capacious colobine stomach increases fermentative volume and prolongs the passage of food through the gut to enable efficient digestion (Bauchop 1978; Chivers 1994) and detoxification (Chivers 1994; Kay and Davies 1994; Mckey 1978). Such adaptations support an efficient strategy for digestion of foliage (Milton 1981), but are essentially different from the requirements of a frugivore feeding on easily digested but protein-poor fruit flesh that needs to be rapidly passed through the gut to maximize nutrient gain (Kay and Davies 1994: Milton 1981).
Despite the needs of a folivorous digestive strategy, colobines have more dietary flexibility than initially thought. Colobines with greater stomach and large intestine capacity tend to be more folivorous, whereas species with relatively capacious small intestines ingest more fruit parts (Chivers 1994). Although some colobines consume fruit parts in amounts similar to or greater than foliage (Bennett 1983; Bennett and Sebastian 1988; Brugiere et al. 2002; Davies 1991; Gurmaya 1986; Hladik 1977; McKey et al. 1981), and a few folivores show seasonally elevated fruit use (Dasilva 1994; Fashing 2001; Hoang et al. 2009; Kool 1993; Stanford 1991; Yeager 1989), colobine frugivory is in fact mainly semenivory or seed eating (Bennett 1983; Bennett and Sebastian 1988; Brugiere et al. 2002; Chivers 1994; Davies 1991; Kay and Davies 1994; Maisels et al. 1994; McKey et al. 1981), and there are no reports of free-ranging colobines feeding year-round mainly on fleshy whole fruits, or feeding heavily on ripening or ripe fleshy fruits (Clutton-Brock 1975; Dasilva 1994; Fashing 2001; Matsuda et al. 2009; Oates 1988; Ungar 1995; Yeager 1989).
Two explanations evoked for the dearth of feeding on ripening or ripe fleshy fruits are as follows: 1) The colobine digestion lacks the flexibility to process large quantities of fruit flesh, due to restrictions imposed by foregut fermentation and microbial digestion (Davies 1991; Kay and Davies 1994). Ripe fruit flesh, which is high in nonstructural carbohydrates and organic acids, tends to be swallowed quickly, precluding buffering action by saliva, and is also digested rapidly, increasing the possibility of rapid and heavy accumulation of volatile fatty acids (VFAs) that may lower forestomach pH and cause hyperacidity of forestomach fluids (Kay and Davies 1994), and even death due to acidosis (Kay and Davies 1994) or bloat (Bennett 1983). 2) Fleshy fruits adapted for animal dispersal may contain bactericidal substances to protect fruits from microbes and to preserve palatability for fruit dispersal agents (Janzen 1978), which may impair forestomach fermentation (Kay and Davies 1994). Accordingly, it is thought that colobines living in areas of poor leaf quality have adapted to the paucity of edible leaves by feeding heavily on seeds—considered suited for the colobine digestive system—instead of on fleshy whole fruits (Bennett 1983; Davies 1991; Kay and Davies 1994; McKey 1978).
Prior studies on forest living Semnopithecus vetulus, a highly arboreal colobine endemic to Sri Lanka (Dela 1998; Hill 1934), place it among the most folivorous colobines, based on studies of Semnopithecus vetulus monticola and S. vetulus philbricki (Hladik 1977; Rudran 1970) in the dry forests at Polonnaruwa and the wet montane forests at Horton Plains (Sri Lanka) respectively. This is supported by gastrointestinal features with pronounced adaptations for folivory (Amerasinghe et al. 1971; Chivers 1994). In sharp contrast, my studies of the diet and feeding behavior of 2 free-ranging groups of Semnopithecus vetulus nestor living in village gardens and rubber monocultures in the western wet lowlands of Sri Lanka show high year-round use of fruits (Dela 2007). Both sites had abundant human edible fleshy (HEF) fruits (Dela 2007) that were likely to be nontoxic, easily digested, and high in nonstructural carbohydrates, and there were no sympatric arboreal primate competitors for fruit. This first study on Semnopithecus vetulus nestor provided an excellent opportunity to investigate the langurs’ dietary flexibility in relation to food availability, diet, food selection, and fruit use and to test whether the diet and feeding behavior of the langurs were determined by local environmental conditions or the colobine adaptations for folivory. In accordance with previously held views that “Animals with voluminous sacculated guts cannot suddenly adopt dietary strategies predicted on fast rates of passage, . . .” (Milton 1981, p. 503) or that colobine digestive adaptations preclude feeding heavily on fleshy, ripe, and ripening fruits (Chivers 1994, Kay and Davies 1994), the langurs should have ignored fleshy fruits and fed selectively on the young leaves and seeds that were also available. However, because both groups showed a high year-round use of seasonal food, composed mainly of fruits (Dela 2007), I hypothesized that the langurs had adopted a frugivorous dietary strategy to exploit the whole fruits available in their environments, despite colobine digestive adaptations, and predicted that:
-
1)
The langurs were feeding more heavily and selectively on whole fruits than on foliage or seeds, despite the availability of edible leaves and seeds, and
-
2)
The langurs were feeding selectively on ripening or ripe whole fleshy fruits over other fruits.
Methods
Focal Taxon
Five subspecies of Semnopithecus vetulus are recognized (Brandon-Jones et al. 2004; Deraniyagala 1955). Of these, Semnopithecus vetulus nestor is the smallest (Hill 1934), with a maximum recorded mass of ca. 5–6 kg (Dela unpubl. data). A recent survey confirmed its range in the western lowlands of Sri Lanka to be an area with a high human population density and very low forest cover (Dela unpubl. data). The latter is attributed to degradation and total clearing of forests in this region (Gunatilleke and Gunatilleke 1991) during the past 500 yrs (NARESA 1991). Logging in the island’s natural forests is now banned, but the forests available to Semnopithecus vetulus nestor are very fragmented, degraded, small, and isolated, with varying degrees of human interference (Dela pers. obs. 2007–2010). The langurs’ main habitats are modified environments: village gardens and rubber plantations that are also now undergoing rapid change (Dela pers. obs. 2002–2010). Consequently, Semnopithecus vetulus nestor was listed as one of the 25 most endangered primates in the world (Mittermeier et al. 2005).
Subjects
I studied the feeding behavior of 2 habituated, free-ranging reproductive groups of Semnopithecus vetulus nestor from August 1985 to February 1987 at Panadura and Piliyandala, as part of an 8-yr behavioral study. During the feeding study, the Panadura group (PT1) ranged from 13 to 16 individuals, and the Piliyandala group (R1) ranged from 12 to 14. Infants (<12 mo) ranged from 2 to 4 in PT1, and 0 to 2 in R1 (Dela 2007). The home ranges of PT1 and R1 were 9.6 ha and 3.9 ha, respectively (Dela 2007).
Sites
My 2 study sites were located within Sri Lanka’s lowland wet zone, which receives rainfall throughout the year, and heavy monsoon rains typically from May to September and December to February (Wijesinghe et al. 1993). Both sites had similar climatic features, an elevation <100 m, and a flat terrain. Mean rainfall at the closest weather recording stations maintained by the Department of Meteorology was 2290 mm and 2269 mm for Panadura and Piliyandala, respectively, and the mean daily temperature was 27.6°C at both sites during the study (Dela 2007). The site at Panadura, a small coastal town located ca. 20 km south of Colombo, had a mosaic of village gardens (with 12 houses/ha) and a small (0.25 ha) plot of rubber (Hevea brasilliensis). The canopy was ca. 20 m in height and fairly continuous, and the stem density was 402/ha (Dela 1998). The more rural Piliyandala site had a well maintained rubber plantation of ca. 32 ha, fringed by village gardens with a density of 3 houses/ha (Dela 2007). The stem density here was 411/ha, and the canopy was fairly closed at ca 20–25 m height (Dela 1998). The village gardens at the Panadura study site were older than those of Piliyandala (Dela 2007), which probably accounted for a significantly greater tree species richness per unit area than at Piliyandala (Dela 2007). A few species dominated the vegetation at both sites, and only 11 (85.3% of stems and 86.4% of biomass) at Panadura and 13 (91.7% of stems and 90.4% of biomass) species at Piliyandala had relative densities ≥1 (Dela 2007). The Piliyandala site had pronounced fluctuation in the supply of seasonal food (fruits, flowers, flush) owing to the prevalence of rubber monoculture in the area, and a more fluctuating fruit supply than at Panadura (Dela 2007). There were no forests within or near both sites, but village gardens at each site had a few forest tree species.
Ten of 94 households in the area occupied by PT1 provisioned the group occasionally, but this had no religious or cultural significance. The recipient was often the adult male of the group. Neither study site had other nonhuman primates. Use of fruits from common village garden fruit species, such as Musa paradisiaca (banana), Mangifera indica (mango), and Artocarpus incisus (breadfruit), by the langurs caused food conflicts between PT1 and householders at Panadura (Dela in press). The use of fruits of Artocarpus heterophyllus (jak) caused most food conflicts for R1 at Piliyandala (Dela in press), where there were few mature trees of Musa paradisiaca, Mangifera indica, and Artocarpus incisus (Dela 1998, 2007). Humans harvested many HEF fruits as they ripened. For example, fruits of Musa paradisiaca, Mangifera indica, and Artocarpus incisus were harvested by humans as they ripened, and were rarely left on the trees until they became fully ripe (Dela 1998).
Vegetation
I conducted preliminary studies from February 1985 to July 1985 at Panadura, and to January 1986 at Piliyandala, to 1) select and habituate study groups, 2) provisionally ascertain their home ranges, 3) identify individuals, and 4) conduct a detailed vegetation survey of areas they entered. I staked out contiguous quadrats of 0.25 ha in the areas entered by the 2 groups with the help of field assistants, and sampled all woody trees and plants of Musa paradisiaca with stems ≥30 cm girth at breast height (GBH). I numbered the trees (except Musa paradisiaca) with paint or metal tags and measured their GBH. I identified common village garden species in the field, and made herbarium specimens of doubtful species for identification at the National Herbarium in Peradeniya by a botanist colleague, Neela de Zoysa Simon. Likewise, I later sampled new quadrats the groups entered during the behavioral study. During data analysis I excluded all peripheral quadrats with trees clearly outside the home ranges of the 2 focal groups and therefore not available to them. I used the remaining 28 × 0.25 ha quadrats at Panadura (7 ha) and 16 × 0.25 ha quadrats at Piliyandala (4 ha) to assess spatial food availability (Dela 2007). I follow Senaratna (2001) for nomenclature and taxonomy of plants.
Phenology
I conducted monthly productivity sampling of 39 species (N = 234 individuals) at Panadura, and 33 species (N = 168 individuals) at Piliyandala to determine food availability, excluding Musa paradisiaca (Musaceae), for which I could not collect monthly data from the same individual. I sampled potential food species that had 1) high relative density and/or high frequency in the vegetation quadrats, or 2) individuals producing seasonal plant parts covering >50% of the canopy/tree at a given time. I selected individuals for sampling at random from all enumerated trees, regardless of canopy size, tree height, or maturity, but excluded trees that were constantly pruned, unless the species was habitually maintained as a shrub. I conducted productivity studies at each site on 2 consecutive days immediately before the monthly 5-d behavior sample periods.
I modified methods used by W. P. J Dittus (pers. comm. 1985) to record the monthly availability of vegetative (leaves) and reproductive (fruits and flowers) plant parts. I scored the presence of leaves, fruits, and flowers in different age classes (buds, young, mature, old) on a scale of 0–10; total absence scored 0. I awarded a maximum score of 10 for all leaves on a single individual, and a maximum collective score of 10 for both fruits and flowers on a tree as they occupied the same position in the canopy. I classified flowers in inflorescences, very small green fruits, and leaves of uncertain age as age-undetermined. During data analysis I pooled leaf buds and young leaves as young leaves, all young fruits before maturing as immature fruits, and all age classes of flowers as 1 floral category. I use the term “mature fruits” here as used by local people to refer to ripening fruits that were clearly different from young/immature fruits in size, color and texture. I also used taste to identify HEF fruits that were ready for harvesting. I present monthly availability of plant parts for each study group as a composite of the tree species that provided their respective monthly top 3 specific items during the feeding study, with the exception of 2 species (basal area = 0.30%) at Panadura and 1 at Piliyandala (basal area 2%).
Diet
I collected feeding data from August 1985 to February 1987 (N = 19 mo) on PT1 at Panadura, and from February 1986 to February 1987 (N = 13 mo) on R1 at Piliyandala, during typical monthly sample periods of 5 consecutive days at each site. I collected feeding data via 5-min scans (Altmann 1974) beginning on the hour at 06:00 h, and commencing at 15-min intervals until light failed at dusk, usually between 18:00 and 19:00 h. I took a break between 12:20 and 13:40 h. I targeted all visible individuals one after the other, as visibility permitted, using a pair of Optolyth 8 × 56 binoculars. I targeted an individual only once during a single scan, and recorded the first activity of each target individual to last ≥5 s to decrease observer bias toward conspicuous activities. When the activity was feeding—defined as handling food for consumption, chewing, or ingesting—I recorded the food item, and if it was of plant origin, I recorded the species and age category of the plant part (item). I excluded feeding records for infants (<12 mo) during data analysis, because they were highly dependent on food use by the mother.
I tasted fruits edible to humans and noted whether they were sweet or sour, and whether they were starchy when raw or cooked. I used percentage feeding records on a particular food species or plant part to represent its proportionate feeding time and dietary contribution. Except when specifically stated, my references to diet mean plant diet and exclude nonplant matter and provisioned food; fruit use excludes seeds picked up from the ground; and whole fruit use includes seeds ingested with fruit flesh. All seeds mean seeds picked up from the ground and seeds from fruits ingested apart from other fruit parts. When mature (ripening) fleshy fruits were not distinctly different in color from immature fruits I classified then as immature, as in the case of the somewhat tart-tasting green fruits that were ready for human harvesting and considered mature by local people, but were very different in taste and color from ripe/ripening fruits. I avoided purposefully interacting with the focal groups during sampling, and did not interfere when householders provisioned PT1 or chased langurs off food trees. I opportunistically noted the consistency of feces when these were markedly different from usual in texture, color, and consistency.
Analysis of Food Availability and Diet
I computed the Importance Value Index (IVI) of a tree species as its relative density + relative basal area + relative frequency in sample quadrats (Dela 1998).
I used species richness (N S ) to compare the number of plant species in 1) the vegetation samples at the 2 sites and 2) the diets of the 2 groups, where S is the total number of species.
I computed species diversity in the vegetation samples and monthly diets using the Shannon–Wiener Index of Diversity using the equation \( H\prime = - \sum\nolimits_1^S {{{\text{p}}_i}1{\text{n}}\;{{\text{p}}_i}} \) wherein p i is the proportion of the total number of individuals consisting of the ith species and S is the total number of species.
I used the basal area (BA) of a tree species as an indicator of biomass following Kool (1993), assuming a circular cross-section of the trunk, and computed: 1) the BA of a tree species and 2) its relative basal area as: \( \left. 1 \right)\,{\text{B}}{{\text{A}}_i} = \pi \times \left( {D_i^2/4} \right) \times {N_i}\,{\text{and}}\,\left. 2 \right)\,{\text{B}}{{\text{A}}_i}/{\text{B}}{{\text{A}}_t} \times 100,\,{\text{respectively}}, \) where D i is the mean diameter of a tree species at breast height calculated from the measured GBH values, N i is the total number of individuals of the ith species in the sample, and BA t is the basal area of all trees in the sample (Dela 1998).
I computed selection ratios (SR) for species contributing ≥1% to the diet of each focal group and species contributing ≥1% to the enumerated vegetation sample at each study site to determine whether the langurs selected their food species or fed on what was most abundant. I used the formula used by Kool (1993) and Bennett (1983):
assuming that a plant species with a SR >1 is preferentially selected, and eaten more than would be expected if they were feeding at random (Kool 1993), or in relation to its availability, whereas a species with SR < 1 is under-used, and therefore not selected.
I used Mann-Whitney U-tests (Lehner 1979) to compare the monthly use of different parameters of fruits, foliage, and seeds in the 2 focal groups, and Spearman’s rank correlation (Lehner 1979) to test the relationship between different parameters of monthly diet and food availability. I also used Spearman’s rank correlation to test for selective feeding by comparing 1) the proportionate feeding records on selected species used by the 2 focal groups with the corresponding BA of such species and 2) the monthly proportionate use of ripe/ripening fruits and immature fruits of top fruit species with their corresponding monthly productivity scores. I also examine selective feeding on: 1) fruits, mature leaves, and young leaves and 2) ripe and ripening fruits over immature fruits of top fruit species, by graphically comparing the monthly proportionate feeding records for the 2 focal groups with their corresponding monthly productivity scores.
Results
Food Availability
Both study sites were dominated by a few cultivated species (Table I). Cocos nucifera, Areca catechu, Artocarpus heterophyllus, and Musa paradisiaca ranked among the 5 species with highest IVI at both sites, but Hevea brasiliensis (Euphorbiaceae) ranked top for IVI at Piliyandala because of the rubber plantation. Among the 116 species recorded from both sites, 71 (61%) were restricted to 1 site, but >75% of individuals at each site were from species common to both. Despite this, tree species richness and biomass of species common to both sites differed considerably between the 2 study sites (Table I). Overall, Panadura had a richer flora, with 101 species from 39 plant families, vs. 60 species from 29 plant families at Piliyandala. Arecaceae (= Palmae), Moraceae, Anacardiaceae, and Euphorbiaceae were among the top 5 plant families at both study sites in terms of tree biomass (BA), amounting to 81.7% at Panadura and 83.6% of BA at Piliyandala. Arecaceae ranked top for BA at Panadura, followed by Moraceae. The latter was notably less common at Piliyandala, where Arecaceae ranked at the top, followed by Euphorbiaceae. Fabaceae ranked 6th for BA at Panadura and 4th at Piliyandala. Panadura had 31 tree species (80.1% of BA) that yielded HEF fruits vs. 23 tree species (51% of BA) at Piliyandala, which had more rubber monoculture. Several common village-garden fruit species yielding fleshy fruits at Panadura, such as Artocarpus heterophyllus (Moraceae), Artocarpus incisus (Moraceae), and Mangifera indica (Anacardiaceae), had notably lower IVI at Piliyandala because of fewer individuals and lower biomass (Table I). Piliyandala also had only 1 fruiting tree of Artocarpus incisus and Mangifera indica; and though Musa paradisiaca (Musaceae) ranked 5th for IVI at this site, most individuals were young and nonfruiting. Both sites had a few wet zone forest tree species such as Trema orientalis, Dillenia retusa, and Artocarpus nobilis with HEF fruits.
Diet Composition
Plant food comprised almost all of the diet of PT1 and R1 (Table II). Fruit parts, including all seeds, made up 52.8% of all food ingested by PT1 and 59.4% ingested by R1. Although 10 households provisioned PT1 with very small amounts of mature fruit kernel of Cocos nucifera, ripe yellow fruits of Musa paradisiaca, ripening fruit flakes of Artocarpus heterophyllus, bread, and flour-based cooked food—including very oily local sweets—provisioned food was negligible in the total diet (1%). Use of nonplant matter (PT1 = 0.53%; R1 = 0.30%), comprising bread, earth-related substances (PT1 = 0.1%; R1 = 0.2%), and rotten wood devoid of insects or larvae, was also negligible. Bread use was rare, although PT1 appeared to relish fresh bread, and once the adult male pilfered bread from a house. Animal food was not important for either group and never sought. Although on a few occasions I saw langurs feed on red ants (Oecophylla sp.) and mosquitoes, this was very rare, and occurred only when the langurs were bitten/stung by these insects.
Nonprovisioned Plant Food
PT1 had a richer plant diet composed of 215 specific items from >115 plant species, vs. 106 specific items from >59 species for R1 (Tables III and IV). Fruits and young leaves were the main dietary items. Mature leaves and flowers contributed <10% to the diet of each group (Tables III and IV), although flower use rose during the seasonal availability of flowers of Ceiba pentandra (for PT1), and flowers of Hevea brasiliensis (for R1). R1 consumed a very small amount (0.06%) of exudates (gum) of Anacardium occidentale. Both groups consumed very small amounts (<1%) each of bark, yams left exposed on the ground by householders, and stems of small branches. Vines, nonwoody plants, and trees <30 cm GBH contributed 7.6% and 3.7% to the plant diet of PT1 and R1, respectively, indicating the importance of large food trees for this arboreal species.
PT1 obtained fruits from more species (42) than R1 (28). Even so, fruits comprised a similar high proportion in the nonprovisioned plant diet of PT1 (52.2%) and R1 (53.9%). Seeds from dehisced fruits of Hevea brasiliensis and ripe fruits of Artocarpus heterophyllus from the ground contributed only a further 1.5% and 5.6% to their respective diets. Use of foliage (leaves of all ages and leaf petioles) was less important than fruits (PT1 = 35.6% and R1 = 33.6%). Use of whole fruits by PT1 and R1 amounted to 50.7% and 53.8% of the diet, respectively (PT1: mean ± SD, 50.7 ± 6.1%; range, 39.7–63.1, N = 19; R1: mean ± SD, 53.8 ± 20.7%, range, 9.6–74.2, N = 13) and exceeded the use of foliage (PT1: M-W, U = 14, p < 0.001, N 1 = 19, N 2 = 19; R1: M-W, U = 33, p = 0.008, N 1 = 13, N 2 = 13). Although young leaves were second to fruits in importance for both focal groups, neither group showed a significant relationship between monthly use of fruits and young leaf availability (PT1: R s = 0.001, p = 0.997, N = 19; R1: R s = 0.434, p = 0.138, N = 13), or monthly young leaf use and fruit availability (PT1 R s = −0.126, p = 0.606; R1: R s = −0.269, p = 0.374).
Food Selection
Plant Parts
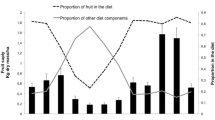
Both groups consumed significantly more fruits than foliage monthly (PT1: M-W, U = 12, p < 0.001, N 1 = 19, N 2 = 19; R1: M-W, U = 32, p = 0.007, N 1 = 13, N 2 = 13), although leaves were more available than fruits at all times (Fig. 1) and both groups used more species for leaves than fruits (PT1: M-W, U = 0, p < 0.001, N 1 = 19, N 2 = 19; R1: M-W, U = 13.5, p < 0.001, N 1 = 13, N 2 = 13). Fruits were used selectively year round by both groups, except briefly by R1 from August to October 1986 (Fig. 1), when midyear fruit production declined owing to the sharp seasonal drop in fruits of Artocarpus heterophyllus. Conversely, R1 underused young leaves during February and March 1986 (Fig. 1), when there was mass leaf flush in the rubber monoculture, and in August 1986 when young leaf availability was very low. Both focal groups, however, selected young leaves over mature leaves, by using significantly more young leaves than mature leaves monthly (PT1: M-W, U = 13, p < 0.001, N 1 = 19, N 2 = 19; R1: M-W, U = 6, p < 0.001, N 1 = 13, N 2 = 13), and used more species for young leaves than mature leaves (PT1: M-W, U = 79, p = 0.003, N 1 = 19, N 2 = 19; R1: M-W, U = 30.5, p = 0.005, N 1 = 13, N 2 = 13), although mature leaves were more spatially abundant and temporally consistent (Fig. 1). Both groups also fed selectively on only the leaf petioles of fibrous or tough mature leaves, particularly from Anacardiaceae, Bombacaceae, Euphorbiaceae, and Combretaceae.
Food Species
PT1 and R1 used food species irrespective of their biomass availability (Table V), as there was no significant correlation between relative BA of tree species and their proportionate contribution to the diet (PT1: R s = 0.196, p = 0.435, N = 18; R1: R s = 0.184, p = 0.529, N = 14). For example, neither group selected Cocos nucifera with a very high biomass at both sites, or Musa paradisiaca, which was used exclusively for fruits but had a high BA because of a large number of young nonfruiting individuals. Hevea brasiliensis, with highest biomass at Piliyandala, and the second most exploited species by R1, was also not selected. Conversely, species contributing <1% to the total biomass, such as Terminalia catappa, Macaranga peltata, Ceiba pentandra, and Carica papaya (PT1 at Panadura) and Trema orientalis and Garcinia zeylanica (R1 at Piliyandala) were highly selected. Artocarpus heterophyllus, the top food species for both groups and their top source of fruits, was selected by both focal groups, but this was more pronounced at Piliyandala, where it had lower biomass. The langurs were also selective of the leaf species they consumed, as only 6.8% (N = 7/103) of leaf species used by PT1 and 15.9% (N = 7/44) used by R1 contributed >1% each to their respective diets.
Plant Food Families
PT1 used 44 and R1 used 32 plant families for food, but only a few families provided most of the food they used. Moraceae accounted for 35.6% and 46.4% of the diets of PT1 and R1, respectively; and also provided 50.4% and 68.7% of all fruits consumed by PT1 and R1, respectively. PT1 used Fabaceae mostly for fruits (6.6% of the diet) and less for leaves (2.9% of the diet), as HEF fruits of Tamarindus indica comprised 5.8% of the plant diet, and fruits comprised >60% of the Fabaceae food intake. There were no species producing HEF fruits of Fabaceae at Piliyandala, where this plant family provided 3% of the plant diet and was used mainly for leaves. Moraceae, Anacardeaceae, Combretaceae, and Euphorbiaceae provided 68.1% of young leaves for PT1 vs. 7.2% from Fabaceae, while Moraceae and Euphorbiaceae provided 58.9% of young leaves for R1. Artocarpus heterophyllus (Moraceae), Artocarpus incisus (Moraceae), and Mangifera indica (Anacardiaceae) were the main sources of young leaves for PT1, and provided 51.3% of young leaves in the diet. Likewise, Artocarpus heterophyllus (Moraceae) and Hevea brasiliensis (Euphorbiaceae) provided 53.7% of young leaves consumed by R1. Arecaceae (palms) dominated the vegetation at both sites, but both groups avoided eating the very fibrous palm leaves.
Type of Fruit in Diet
Fruits used by both groups included very large/medium fleshy whole fruits, very small whole fruits, and dry fruits used only for seeds. They did not avoid bright or dark-colored fruits or seeds. For instance, they fed on the purple-colored arils of Sterculia balanghas (Sterculiaceae) of dehisced bright red fruits and on many HEF fruits that became red as it ripened, or had yellow or orangey flesh when ripe. Most of the fruits eaten by the 2 groups were HEF whole fruits that were ripening or ripe (Fig. 2). Both groups also consumed several fleshy and ripe non-HEF whole fruits, e.g., Morinda citrifolia (Rubiaeae) and Horsfieldia irya (Myristicaceae).
HEF Fruit vs. OWF HEF whole fruits comprised 78.4% of all fruits eaten for PT1 and 83.4% for R1 (Fig. 2), amounting to 40.9% and 44.9% of the plant diets of the 2 groups, respectively. Both focal groups used more HEF (whole) fruits than all other whole fruits (OWF) and all seeds (PT1: M-W, U = 1, p < 0.001, N 1 = 19, N 2 = 19; R1: M-W, U = 29, p = 0.004, N 1 = 13, N 2 = 13). PT1 and R1 used HEF fruits heavily and consistently, except a few months for R1 (Fig. 3) when HEF fruit availability declined. PT1 and R1 consumed 23 and 13 HEF fruit species respectively (Table VI), which amounted to almost all HEF fruit species with fruiting individuals in their environments. Although R1 used fewer species for HEF fruit than PT1 (Table VI), there was no significant difference in the proportionate monthly use of HEF fruit by the 2 groups (M-W, U = 102, p = 0.409, N 1 = 19, N 2 = 13).
Both groups consumed OWF (Fig. 3) that became seasonally available, mainly young fruits of Ceiba pentandra (Bombacaceae), very small green fruits of Macaranga peltata (Euphorbiaceae) and Trema orientalis (Ulmaceae), and small green non-HEF fruits of Ficus sp. (Moraceae) and Vitex pinnata (Verbanaceae). Notably, both groups fed only on soft young OWF fruits that became hard or fibrous when ripe. For example, they fed on the soft endosperm of young green fruits of Areca catechu (Arecaceae) (>97%), but avoided the hard mature/ripe fruits. They used fleshy fruits of a hard consistency for their green exocarp, e.g., the young green fruits of Hevea brasiliensis (Euphorbeaceae) and Calophyllum inophyllum (Clusiaceae).
Seeds
Both focal groups consumed seeds along with the flesh of many HEF fruit and OWF, and chewed some seeds noticeably. Both groups consumed seeds only from nonfleshy, e.g., Leucaena leucocephala, Pericopsis mooniana, Adenanthera pavonina (Fabaceae), or dry fibrous fruit, and foraged for rubber and jakfruit seeds on the ground. However, both groups used whole fruits (mainly HEF fruits) much more than seeds (Fig. 3), and this difference was significant (PT1: M-W, U = 0, p < 0.001, N 1 = 19, N 2 = 19; R1: M-W, U = 5, p < 0.001, N 1 = 13, N 2 = 13). For example, both groups fed on seeds of Artocarpus heterophyllus (starchy, hard) with mature fruit flakes, but discarded seeds of ripe fruits when they fed on the succulent fruits, although the langurs fed on ripe seeds from the ground during fruit scarce periods. The langurs also ignored the dry fruit (nut/seed) of Anacardium occidentale (Anacardeaceae) and fed only on the ripe reddish HEF fruit pedicels. Likewise, the langurs discarded hard, medium to large (≥1.5 cm) seeds when feeding on some HEF fruits, e.g., Nephelium lappaceum (Sapindaceae), ripening/ripe fruits of Spondias dulcis, and Mangifera indica (Anacardiaceae). PT1 often spat out the small hard seeds of Tamarindus indica when feeding on the fully ripe pulpy HEF fruit, though they consumed the softer seeds of immature and ripening fruit with fruit flesh. Conversely, PT1 consumed only seeds of fibrous old/dehisced fruits of Ceiba pentandra, but fed on the soft fruit flesh and the soft seeds of immature fruit.
Whereas there was no relationship between use of all seeds (only seeds from fruit and seeds from the ground) and monthly HEF fruit use for PT1 (R s = 0.032, p = 0.898, N = 19), there was a significant negative correlation between use of all seeds and HEF fruits for R1 (R s = −0.904, p < 0.001, N = 13) owing to heavy use of seeds of Hevea brasiliensis (rubber) during the mid-year fruit shortage at Piliyandala when there was low use of HEF fruit (Fig. 3). The seasonal seeds of Hevea brasiliensis (rubber) formed the top source of seeds for both groups, for which they foraged on the ground and fed on the soft kernel. Seeds of Hevea brasiliensis, together with leaf petioles of H. brasiliensis, became top food items for R1 during fruit scarcity of Artocarpus heterophyllus from June to November 1986 at Piliyandala.
Ripe and Ripening HEF Fruits
Most HEF fruits consumed were mature (ripening) or fully ripe (Table VI), amounting to 72.4% of HEF fruit consumed by PT1 and 94.8% by R1. Neither group avoided ripe HEF fruit, with the exception of the starchy and sticky yellow fruit flesh of Chrysophyllum roxburgii (Sapotaceae), which was also hardly used by local people. About 30% of HEF fruits consumed by PT1 and 16% by R1 also ranged from mild to very acidic. The latter fruits included the pulpy legume of Tamarindus indica (tamarind) and ripe watery berry of Avarrhoa bilimbi (cucumber tree) that are used for food souring and preserving by local people. I have also subsequently seen other langurs at the Panadura site feed on the rind of lemon fruits (Citrus limon) and the ripe, sour, and succulent fruits of Averhoa carambola (Oxalidaceae). Neither study group fed on mature/ripe fruits of Cocos nucifera—a fibrous drupe with a leathery epicarp, a thick fibrous mesocarp, and a stony endocarp—off the trees, but PT1 fed avidly on the sweet and slightly-oily endosperm of opened mature/ripe fruits found on the ground or when provisioned by local people. Both groups fed occasionally on young (immature) fruits of Cocos nucifera off the trees and licked the sweet-tasting liquid that seeped out.
Proportionate use vs. availability of the 2 top fruit species for PT1 and R1, respectively (Fig. 4), indicates that the langurs fed more selectively on mature (ripening) or ripe fruits rather than on immature fruits of these species. Artocarpus heterophyllus (Moraceae) made up 44.3% of all fruits consumed by PT1 and 67.1% by R1, of which 98% (PT1) and 99% (R1) were mature and ripe fruit. Both groups avoided immature fruits (white flesh) with soft seeds that were more abundant during most months (Figs. 4a and b), and fed selectively on mature and ripe fruits, except briefly when they were seasonally absent at Piliyandala. The langurs fed avidly on the sweet ripe fleshy fruit flakes, when their feces became paler and more watery than usual. The gummy white latex of Artocarpus heterophyllus fruits of all ages did not deter langurs from feeding on mature and ripe fruits. The mature fleshy yellow fruit flakes (perianths) and starchy and fairly hard large (ca. 3 cm) seeds of Artocarpus heterophyllus were eaten boiled or cooked by local people; and the fully ripe sweet succulent fruit flakes were consumed by fruit bats and eaten raw by humans.
PT1 consumed the strictly seasonal (Fig. 4c) sour (ripening) and sour and sweetish (very ripe) fleshy legumes of Tamarindus indica and the very sour and tart-tasting immature fruits equally (MW = 161.5, p = 0.52, N 1 = 19, N 2 = 19); there is also a positive correlation between proportionate monthly use and availability of both mature/ripe (R s = 0.598, p = 0.007, N = 19) and immature (R s = 0.578, p = 0.01, N = 19) fruits of Tamarindus indica. However, monthly use of all fruits of Tamarindus indica, including unclassed fruits, significantly and positively correlates only with proportionate availability of mature/ripe fruits (R s = 0.726, p < 0.001, N = 19), and not with immature fruits (R s = 0.383, p = 0.106, N = 19), suggesting that the langurs fed more selectively on mature/ripe fruits than on immature fruits. When the langurs fed very heavily on ripe fruits of Tamarindus indica, their feces often became lighter in color and more watery than usual.
The mature and fully ripe fruits of Dillenia retusa—also consumed by parakeets and occasionally by village children—was used by both focal groups, but more heavily by R1, which had access to less HEF cultivated fruit species than PT1. The sweetish, sour, and juicy and rather fibrous mature/ripe fruits comprised 95% of total fruit intake of Dillenia retusa by R1, although immature fruit was more available (Fig. 4d). There is a strong positive correlation between monthly use and availability for mature/ripe fruits of Dillenia retusa (R s = 0.791, p = 0.001, N = 13), but not for immature fruits (R s = 0.209, p = 0.494, N = 13).
The availability of many mature or ripe HEF fruits for the langurs was severely limited by human harvesting, as they were harvested when ripening, i.e., deemed mature, to protect crops from langurs. This particularly affected availability of mature/ripe fruits of Mangifera indica (mango), Artocarpus incisus (breadfruit), Musa paradisiaca (banana), and Nephelium lappaceum (rambutan) for the langurs, which probably accounted for their low use (Table VI), as the langurs fed on them avidly when they were available. Conversely, there was a surplus of mature/ripe fruits of Artocarpus heterophyllus after human harvesting during the fruiting season at both study sites. The local people also harvested ripe fruits of Tamarindus indica mostly from the ground, and never harvested fruits of Dillenia retusa for food because it was eaten only very rarely by village children.
Discussion
Frugivory and Selective Use of Fruits
This study provides clear evidence that Semnopithecus vetulus nestor living in environments modified by humans and with abundant sources of cultivated fruits had adopted a frugivorous dietary strategy unlike that of any other colobine studied to date. Fruits comprised the main dietary item of both groups and were eaten consistently more than foliage, despite greater availability of leaves than fruits in the environment, and the use of more species for leaves than fruits year-round. The langurs also used significantly more whole fruits than foliage and more whole fruits (mainly HEF fruits) than seeds. Notably, the proportion of whole fruits in the diet for the 2 focal groups is higher than recorded for any colobine frugivore (Bennett 1983; Bennett and Sebastian 1988; Brugiere et al. 2002; Davies 1991; Maisels et al. 1994; McKey et al. 1981). Both focal groups also had similar proportionate use of 1) HEF whole fruits, despite difference in biomass and tree species richness of species producing HEF fruits between sites; and 2) fruits and leaves, despite differences in: available food species, tree biomass, temporal variation of plant food for the 2 groups (Dela 2007), and species richness of their respective diets (Dela 1998).
These results raise the question of whether the use of whole fruits by the langurs is due to the lack of edible leaves and seeds in their environment. Although both study sites had a high biomass of Arecaceae with fibrous leaves, and Moraceae, Anacardiaceae, and Euphorbiaceae that are characterized by resins, latex, or other plant secondary compounds (Curtin and Chivers 1978) that may limit leaf use, Moraceae, Anacardiaceae, and Euphorbiaceae were top leaf sources for both focal groups. Moreover, both groups used leaves from these families more than legume (Fabaceae) leaves, which are regarded as high-quality colobine food (Bennett and Davies 1994), and Fabaceae contributed more HEF fruits than leaves for PT1. This differs from the strategies adopted by colobines living in inhospitable areas with poor quality leaves that adapt either by 1) feeding very selectively on young leaves and edible mature leaf parts as their main dietary item (Curtin and Chivers 1978), or 2) feeding heavily on seeds (Bennett 1983; Davies 1991; McKey 1978). Although both focal groups fed selectively on young leaves over mature leaves as do many colobines (Chapman et al. 2002; Fashing 2001; Hoang et al. 2009; Li et al. 2003; Oates 1988; Ruhiyat 1983; Struhsaker 1975; Zhou et al. 2006), fruit (mostly fleshy whole fruits) was their main dietary item. Their fruit use was not determined by young leaf availability, indicating that use of fruits was not due to a shortage of young leaves. Further, R1, with less leaf sources than PT1, underused young leaves during peak abundance, and both groups had access to more species for leaves than fruits. Thus it appears that these langurs do not employ the first strategy. The langurs also did not use the second strategy, as R1 increased seed use when fruit availability dropped in an environment where HEF fruit availability was seasonal, and both groups consumed more HEF whole fruits than seeds, i.e., seeds only from fruits and from the ground, despite both sites having nutritious edible seeds. For example, a single fruit of Artocarpus heterophyllus may contain up to 100 medium-sized edible seeds that are rich in protein (Bobbio et al. 1978) and nonstructural carbohydrates (Ananthasubramaniam et al. 1978), and richer in crude protein than flakes (Ananthasubramaniam et al. 1978). The langurs also discarded the seeds when feeding on ripe sweet fruit flesh of Artocarpus heterophyllus, but used seeds from the ground when ripening/ripe fruits were in short supply. Because ripe fruit flesh and seeds are available together, the langurs appear to prefer ripe fruit flesh of Artocarpus heterophyllus to seeds. Conversely, I have seen langurs in more fruit-deprived village environments feed on both ripe fruit flesh and seeds of Artocarpus heterophyllus (Dela pers. obs.). As such, the findings of this study suggest a preference for whole fruits over foliage or seeds, and support my prediction that the langurs were feeding more heavily and selectively on whole fruits (mainly HEF fruits) than on foliage or seeds to adopt a frugivorous feeding strategy, despite the availability of edible leaves and seeds.
Is Frugivory a Requirement for Semnopithecus vetulus nestor?
This exceptionally high use of whole fleshy fruits by the small-bodied Semnopithecus vetulus nestor is in sharp contrast to pronounced folivory demonstrated by forest-living S. vetulus philbricki and S. vetulus monticola (Hladik 1977; Rudran 1970) that have a mass >9 kg (Hill 1934). Leaves comprised ca. 60% of the diet of Semnopithecus vetulus philbricki, and mature leaves amounted to two thirds of its leaf intake, reaching 80% of the diet on some days (Hladik 1977). Although it is proposed that high metabolic requirements of small-bodied colobines may preclude their adopting low-nutrient, high-fiber diets with low energy turnover, and that the use of food with a low protein/fiber ratio increases with body size (Bennett 1983; Bennett and Sebastian 1988; Kay and Davies 1994), no clear relationship exists (Yeager 1989), as the small-bodied Procolobus verus and Presbytis aygula are folivorous (Oates 1988; Ruhiyat 1983), whereas the large-bodied Semnopithecus entellus and Nasalis larvatus feed heavily on fruit parts (Bennett and Sebastian 1988; Hladik 1977; Yeager 1989). I have also observed Semnopithecus vetulus vetulus, which is larger than S. vetulus nestor, and large-bodied intermediate forms of S. vetulus nestor and S. vetulus monticola feed avidly on mature/ripe fruits of Artocarpus heterophyllus during a 2007–2010 range survey of S. vetulus nestor (Dela pers. obs.). As such, there is no evidence that the high use of fleshy fruits by Semnopithecus vetulus nestor (<6 kg) is a direct requirement of its small body size.
Selective Use of Ripe and Ripening Fleshy Whole Fruits
The high use of ripe and ripening fleshy fruits by both focal groups is in sharp contrast to previous reports of colobine fruit eating which consists of semenivory (Bennett and Davies 1994; Kay and Davies 1994); the use of unripe, nonfleshy, fibrous, and desiccated or dry and bitter whole fruits (Bennett and Sebastian 1988; Clutton-Brock 1975; Dasilva 1994; Fashing 2001; Hladik 1977; Matsuda et al. 2009; Oates 1988; Ungar 1995; Yeager 1989); and avoidance of acidic fruits (Ungar 1995). My focal groups fed mainly on ripe and ripening HEF whole fruits, and more on HEF fruits than on OWF and seeds, with mature/ripe HEF fruits ranging up to a maximum of 50% and 71% of the monthly diet for PT1 and R1, respectively. Neither group avoided HEF sweet and sour ripening and ripe fruits that could be regarded as unsuited for the colobine digestion (Kay and Davies 1994), but underused fruits with a tough exocarp and fruits that were dry, hard or fibrous. The fruit of Artocarpus heterophyllus, which provided PT1 and R1 with much of their fruit intake (Dela 2007), is a large syncarp (Moncur 1985), ranging up to 20 kg (Bobbio et al. 1978). These fruits have a large number of edible seeds per fruit, and fleshy fruit flakes that are starchy when mature (ripening) and sweet and succulent when fully ripe. The fruit extract, though not acidic, contains volatile essential oils and esters that give the fruit a prominent odor (Sulit and Ganaban 1968). The ripe succulent sweet fruit flesh also contains ca. 7.5% sugar on a dry weight basis, and both flakes and seeds are rich in soluble carbohydrates (Ananthasubramaniam et al. 1978). Despite such characteristics that should preclude colobines feeding on it, almost all fruits of Artocarpus heterophyllus consumed by both focal groups were ripening or ripe, and were selected by the langurs over the immature fruits. Likewise, langurs selectively used ripe or ripening sweetish and acidic fruits of Tamarindus indica and Dillenia retusa over sour immature fruits that were more available, suggesting that the ripe sweet and sour fruits were preferred. These findings support my prediction that the langurs were feeding selectively on ripening or ripe whole fleshy fruits over other fruits.
Dietary Flexibility
My findings do not support previous arguments that 1) colobine digestive adaptations restrict the use of fruit flesh (Bennett 1983; Kay and Davies 1994; McKey et al. 1981) or that 2) digestive adaptations for feeding on leaves are unsuited for easily digested ripe fruit flesh that needs a fast rate of passage through the gut (Kay and Davies 1994; Milton 1981). The suggestion that the gut morphology of colobines feeding on fleshy fruits could differ from that of other colobines (Stanford 1991) is also not tenable because the gastrointestinal features of Semnopithecus vetulus place it among the most folivorous colobines (Chivers 1994). A major difference in the digestive system of populations of Semnopithecus vetulus nestor exploiting cultivated fruits from that of forest-living populations and subspecies is unlikely because the extensive clearing of tropical wet evergreen lowland rain forests within the range of S. vetulus nestor for human settlements and agriculture date back only a few hundred years (NARESA 1991). Both focal groups also demonstrated ability to feed on very toxic foods, such as seeds of Hevea brasiliensis (Mallika 1991), which require functional microbial detoxification. These findings demonstrate that Semnopithecus vetulus nestor has sufficient dietary flexibility to feed heavily and consistently on ripe and ripening fleshy fruits despite the colobine digestive adaptations for leaf and seed eating. In addition, 1) most groups of forest-living Semnopithecus vetulus nestor throughout its range come into adjacent villages to feed on mature/ripe fruits of Artocarpus heterophyllus and other HEF fruits, and 2) the langurs feed heavily on mature/ripe fruits of Artocarpus heterophyllus within forest patches subjected to enrichment planting by the Forest Department (Dela pers. obs.). I have also observed the use of boiled rice, bee honey, ripe bananas, alcoholic beverages, and tea by captive Semnopithecus vetulus nestor with no immediate side effects (Dela unpubl. data), and PT1 group fed on bread and oily sweetmeats when provisioned. Likewise, other colobine species in captivity feed on honey, sultanas, and chocolates (Caton 1999). I have also observed captive Semnopithecus vetulus nestor (N = 2) emit a foul-smelling gas through the mouth after feeding heavily on ripening and ripe fruits of Artocarpus heterophyllus (Dela pers. obs.); wild colobines may be less prone to acidosis than captives (Kay and Davies 1994), and colobines can to some extent limit forestomach VFA buildup by increasing absorption rates and can buffer acidity of stomach pH (Kay and Davies 1994). This suggests that colobine digestive adaptations are more flexible than previously evinced. If so, why do forest colobines avoid ripe and ripening fleshy whole fruits?
Possible Limitations to the Use of Fruit Flesh
There are several possible explanations for avoidance of fruit flesh by colobines. First, fruit use by a primate can be limited by spatial and temporal variations in fruit availability (Milton 1980; Rudran 1978; Stanford 1991) and sympatry with primate fruit competitors (Gautier-Hion 1983 in Stanford, 1991; Rudran 1978). Colobines fed on fleshy whole fruit at Polonnaruwa (Rudran 1970), Kakamega (Fashing 2001), Tiwai (Dasilva 1994), and Makandé Forest, Gabon (Brugiere et al. 2002), but this decreased when fruits ripened and were increasingly consumed by sympatric cercopithecines (Brugiere et al. 2002; Dasilva 1994; Fashing 2001; Rudran 1978). Likewise, both focal groups used ripe or ripening HEF fruits mainly from species that were not subject to heavy human fruit harvesting or crop (fruit) protection measures, indicating the influence of food competition as a determinant in limiting the use of ripening and ripe fleshy whole fruits.
Second, because primate frugivores have to consume large quantities of easily digested ripe fruit flesh rapidly (Kay and Davies 1994; Milton 1981), filling the capacious colobine stomach (Chivers 1994) with ripe fleshy fruits may be difficult in the presence of other primate fruit competitors, particularly for large-bodied colobines with capacious stomachs. This may necessitate supplementing the colobine fruit diet by feeding heavily on leaves. Because ripe fruit flesh can reduce the efficiency of foregut microflora (Kay and Davies 1994), ad hoc mixing of ripe fruits with large quantities of low-quality leaves may not be viable for a primate folivore dependent on bacterial detoxification (Glander 1975). However, PT1 and R1 had abundant HEF fruits and no arboreal primate fruit competitors, enabling them to feed heavily year-round on ripe and ripening fleshy fruits, supplemented during fruit scarcity with other seasonal plant parts such as edible young leaves, seeds, and flowers (Dela 2007). In this context, the relatively small stomach of Semnopithecus vetulus nestor, owing to its small body size, may also have helped a switch to frugivory.
Third, a colobine frugivore feeding on fleshy ripening/ripe fruits would need to feed very selectively on foliage compatible with the fast rates of passage in the gut required for fruit flesh (Bennett pers. comm. 1998). This may not be possible for most forest colobines, especially in tropical rain forests. However, it is in keeping with the greater use of young leaves than mature leaves, and the use of leaf petioles over leathery mature leaf laminae, by both focal groups. Compared with mature leaves, young leaves are more nutritious and easily digested, lower in fiber, higher in protein (McKey et al. 1981; Milton 1979; Mowry et al. 1996; Oates et al. 1980), and have a lower processing cost (Waterman and Kool 1994). Leaf petioles have less protein than the lamina, but contain vascular bundles with nutritious sap and higher water content, and are easier to chew and digest than mature leaves (Oates et al. 1980). Both focal groups, despite their differences in leaf sources, hardly used mature leaves. Likewise, the folivorous Trachypithecus auratus, which selectively consumes sweet fruits, shows very low use of mature leaves (Kool 1993). The use of most leaf species in very small amounts by the 2 focal groups also evokes a generalist herbivore strategy to prevent a buildup of individual toxins to lethal levels by diversifying leaf sources (Freeland and Janzen 1974), which is relevant when ingesting leaves with fruits. This may also explain the relatively high species richness of the diet of Semnopithecus vetulus nestor with a large number of leaf sources vs. the species-poor diets of forest-living folivorous S. vetulus philbricki (Hladik 1977). However, both focal groups consumed seeds of Hevea brasiliensis that are high in toxic cyanogenetic glucosides (Mallika 1991). Likewise, Colobus satanas consumed seeds possibly high in secondary substances, but avoided leaves rich in such substances (McKey 1978; McKey et al. 1981). This strategy may reflect the tradeoff required of a folivore consuming food high in secondary compounds, nutrients, and digestibility (Estrada 1984) because the cost of maintaining a detoxification system declines for nutrient- and protein-rich food (Freeland and Janzen 1974). Cyanogenetic glucosides in rubber seeds may also decrease with age (Mallika 1991), and both focal groups fed only on seeds from dehisced fruits.
Finally, forest colobines may not have access to fruits that meet colobine nutritional requirements. Fruit quality can determine a fruit diet (Kay and Davies 1994), as also suggested by colobine fruit use in Moraceae-dominated forests (Fashing 2001). Although the nature of fruit flesh varies widely (Kay and Davies 1994), the low protein content of most fleshy fruits may prevent colobines from adopting high-fruit diets (Kay and Davies 1994). Other primate frugivores consume invertebrates (Dew 2005; Robinson 1986; Rudran 1978) to enhance their protein intake, but colobines rely on leaves, supplemented by microbial protein (Bauchop 1978; Kay and Davies 1994), necessitating the use of protein-rich plant food (Wasserman and Chapman 2003). Neither focal group sought animal protein, but had access to seeds and fruit flakes of Artocarpus heterophyllus that contain 12.6% and 4.5% of crude protein, respectively (Ananthasubramaniam et al. 1978). Likewise, seeds of Hevea brasiliensis contain ca 18.4% protein (Mallika 1991). The high intake of young leaves, and seeds of Hevea brasiliensis, by the 2 groups, and the similar leaves/fruits ratio in the diets of both focal groups despite vegetation differences between sites (Dela 2007) suggest meeting a protein threshold. Thus the quality of fruit from cultivated species and other seasonal food at the 2 study sites may also have enabled PT1 and R1 to adopt high-fruit diets.
My findings thus suggest strongly that the low use of fleshy ripening or ripe fruits by colobines is more complex than thought previously, owing to the interplay of many external environmental factors and intrinsic features. There could be many other unexplored factors that influence fruit use by colobines, such as site-specific differences in colobine foregut microflora, external site-specific factors such as human tolerance where langurs coexist with humans that limits access to HEF fruit (Dela 1998), fruit taste (Stevenson 2004), and even soil chemistry (Fashing et al. 2007). Both focal groups consumed very small quantities of earth-based material and rotten wood that could be related to fruit eating, as geophagy and related food may help adsorption of digestive inhibitors and toxins, serve an antacid function for buffering forestomach pH (Kay and Davies 1994; Waterman and Kool 1994), or supply minerals (Li et al. 2003). However, the data from this study are insufficient to explore these possibilities.
Conclusion
I demonstrate exceptionally high use of fruits (mainly whole fleshy fruits) in Semnopithecus vetulus nestor, and an unusual propensity to feed heavily and selectively on ripe and ripening fleshy whole fruits for a colobine, particularly from a species thought to be predominantly folivorous. The findings of this study show that the 2 focal groups fed selectively and consistently more on fruits than on foliage or seeds, despite the availability of edible leaves and seeds, and suggest that the langurs fed preferentially on whole fruits over foliage and seeds. Frugivory may have been possible owing to 1) continual high availability of ripening and ripe HEF fruit likely to be energy-rich, nontoxic, easily digested, and nutritious; 2) absence of other arboreal primate fruit competitors; 3) availability of other high-quality seasonal food year-round to consume with fruit flesh; and 4) a relatively small stomach size owing to the small body size of this colobine. Whatever the impetus for frugivory, this study demonstrates that Semnopithecus vetulus nestor has sufficient dietary flexibility to feed continuously and heavily on ripe and ripening fleshy whole fruit despite digestive adaptations for leaf and seed eating, and suggests that 1) colobine digestive systems are more flexible than previously believed and 2) the low use of fleshy ripening or ripe whole fruits by colobines is more complex than presently understood. An important question relevant for captive breeding and conservation of endangered colobines remains: whether individual colobines or populations adapted to a frugivorous diet can in the short term shift back to a highly folivorous diet in natural forests. My study thus highlights the need to review feeding behavior and digestive flexibility among colobines, particularly in changing and changed environments, for conservation of species that are increasingly threatened by loss and degradation of their natural habitats.
References
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49, 227–264.
Amerasinghe, F. P., Van Cuylenberg, B. W. B., & Hladik, C. M. (1971). Comparative histology of the alimentary tract of Ceylon primates in correlation with the diet. Ceylon Journal of Sciences (Biological Sciences), 9, 75–87.
Ananthasubramaniam, C. R., Menachery, M., & Chandrasekharan Nair, A. M. (1978). Nutritive value of Jack (Artocarpus heterophyllus, Lam) fruit waste for cattle. The Indian Journal of Nutrition and Dietetics, 15, 12–16.
Bauchop, T. (1978). The significance of micro-organisms in the stomach of non-human primates. World Review of Nutrition and Dietetics, 332, 198–212.
Bennett, E. L. (1983). The banded langur: Ecology of a colobine in a west Malaysian rain-forest. Ph.D. thesis, University of Cambridge.
Bennett, E. L., & Davies, A. G. (1994). The ecology of Asian colobines. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour, and evolution (pp. 129–171). Cambridge: Cambridge University Press.
Bennett, E. L., & Sebastian, A. C. (1988). Social organization and ecology of proboscis monkeys (Nasalis larvatus) in mixed coastal forest in Sarawak. International Journal of Primatology, 9, 233–255.
Bobbio, F. O., El-dash, A. A., Bobbio, P. A., & Rodrigues, L. R. (1978). Isolation and characterization of the physicochemical properties of the starch of jackfruit seeds (Artocarpus heterophyllus). Cereal Chemistry, 55, 505–511.
Brandon-Jones, D., Eudey, A. A., Geissmann, T., Groves, C. P., Melnick, D. J., Morales, J. C., et al. (2004). Asian primate classification. International Journal of Primatology, 25, 97–164.
Brugiere, D., Gautier, J. P., Moungazi, A., & Gautier-Hion, A. (2002). Primate diet and biomass in relation to vegetation composition and fruiting phenology in a rain forest in Gabon. International Journal of Primatology, 23(5), 999–1024.
Caton, J. M. (1999). Digestive strategy of the Asian colobine genus Trachypithecus. Primates, 40, 311–325.
Chapman, C. A., Chapman, L. J., Bjorndal, K. A., & Onderdonk, D. A. (2002). Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. International Journal of Primatology, 23(2), 283–310.
Chivers, D. J. (1994). Functional anatomy of the gastrointestinal tract. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 205–257). Cambridge: Cambridge University Press.
Clutton-Brock, T. H. (1975). Feeding behaviour of red colobus and black and white colobus in East Africa. Folia Primatologica, 23, 165–207.
Curtin, S. H., & Chivers, D. J. (1978). Leaf-eating primates of Peninsular Malaysia: the siamang and the dusky leaf-monkey. In G. G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 441–464). Washington, DC: Smithsonian Institution Press.
Dasilva, G. L. (1994). Diet of Colobus polykomos on Tiwai Island: selection of food in relation to its seasonal abundance and nutritional quality. International Journal of Primatology, 15, 655–668.
Davies, A. G. (1991). Seed-eating by red leaf monkeys (Presbytis rubicunda) in a dipterocarp forest of northern Borneo. International Journal of Primatology, 12, 119–144.
Davies, A. G., Oates, J. F., & Dasilva, G. L. (1999). Patterns of frugivory in three West African colobine monkeys. International Journal of Primatology, 20, 327–357.
Dela, J. D. S. (1998). The ecology and social biology of a selected population of the western purple-faced leaf monkey (Trachypithecus vetulus nestor = Presbytis senex nestor). Ph.D. thesis, University of Peradeniya.
Dela, J. D. S. (2007). Seasonal food use strategies of Semnopithecus vetulus nestor, at Panadura and Piliyandala, Sri Lanka. International Journal of Primatology, 28(3), 607–626.
Dela, J. D. S. (in press). Impact of monkey-human relationships on Semnopithecus vetulus nestor in human modified environments. Journal of the National Science Foundation of Sri Lanka.
Deraniyagala, P. E. P. (1955). A new race of leaf monkey from Ceylon. Spolia Zeylanica, XXVII(11), 293–294.
Dew, J. L. (2005). Foraging, food choice, and food processing by sympatric ripe-fruit specialists: Lagothrix lagotricha poeppigii and Ateles belzebuth belzebuth. International Journal of Primatology, 26(5), 1107–1135.
Estrada, A. (1984). Resource use by howler monkeys (Alouatta palliata) in the rain forest of Los Tuxlas, Veracruz, Mexico. International Journal of Primatology, 5, 105–131.
Fashing, P. J. (2001). Feeding ecology of guerezas in the Kakamega forest, Kenya: the importance of Moraceae fruit in their diet. International Journal of Primatology, 22(4), 579–609.
Fashing, P. J., Dierenfeld, E. S., Christopher, B., & Mowry, C. B. (2007). Influence of plant and soil chemistry on food selection, ranging patterns, and biomass of Colobus guereza in Kakamega Forest, Kenya. International Journal of Primatology, 28(3), 673–703.
Freeland, W. J., & Janzen, D. H. (1974). Strategies in herbivory by mammals: the role of plant secondary compounds. American Naturalist, 108, 269–289.
Glander, K. E. (1975). Habitat description and resource utilization: A preliminary report on mantled howling monkey ecology. In R. H. Tuttle (Ed.), Socioecology and psychology of primates (pp. 37–57). The Hague: Mouton and Co.
Gunatilleke, I. A. U. N., & Gunatilleke, C. V. S. (1991). Threatened woody endemics of the wet lowlands of Sri Lanka and their conservation. Biological Conservation, 55, 17–35.
Gurmaya, K. J. (1986). Ecology and behavior of Presbytis thomasi in Northern Sumatra. Primates, 27, 151–172.
Hill, W. C. O. (1934). A monograph on the purple-faced leaf monkeys (Pithecus vetulus). Ceylon Journal of Science (B), xix (PT1), 23–88.
Hladik, C. M. (1977). A comparative study of the feeding strategies of two sympatric species of leaf monkeys: Presbytis senex and Presbytis entellus. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 323–353). London: Academic Press.
Hoang, M. D., Baxter, G. S., & Page, M. J. (2009). Diet of Pygathrix nigripes in southern Vietnam. International Journal of Primatology, 30, 15–28.
Janzen, D. H. (1978). Complications in interpreting the chemical defences of trees against tropical arboreal plant-eating vertebrates. In G. G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 73–85). Washington, DC: Smithsonian Institution Press.
Kay, R. N. B., & Davies, A. G. (1994). Digestive physiology. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 229–249). Cambridge: Cambridge University Press.
Kool, K. M. (1993). The diet and feeding behavior of the silver leaf monkey (Trachypithecus auratus sondaicus) Indonesia. International Journal of Primatology, 14, 667–670.
Lehner, P. M. (1979). Handbook of ethological methods. Garland Series in Ethology. New York: Garland STPM Press.
Li, Z., Wei, Y., & Rogers, E. (2003). Food choice of white-headed langurs in Fusui, China. International Journal of Primatology, 24, 1189–1205.
Maisels, F., Gautier-Hion, A., & Gautier, J. P. (1994). Diets of two sympatric colobines in Zaire: more evidence on seed-eating in forests on poor soils. International Journal of Primatology, 15, 681–701.
Mallika, G. V. (1991). Applied chemical studies on rubber seed to support its industrial utilization. M.Phil thesis, University of Sri Jayawardenapura, Nugegoda.
Marsh, C. W. (1981). Diet choice among red colobus (Colobus badius rufomitratus) on the Tana River, Kenya. Folia Primatologica, 35, 147–178.
Matsuda, I., Tuuga, A., & Higashi, S. (2009). The feeding ecology and activity budget of proboscis monkeys. American Journal of Primatology, 71, 1–15.
McKey, D. B. (1978). Soils, vegetation, and seed-eating by black colobus monkeys. In G. G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 423–438). Washington, DC: Smithsonian Institution Press.
McKey, D. B., Gartlan, J. S., Waterman, P. G., & Choo, G. M. (1981). Food selection by black colobus monkeys (Colobus satanas) in relation to food chemistry. Biological Journal of the Linnaean Society, 16, 115–146.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. American Naturalist, 114, 362–378.
Milton, K. (1980). The foraging strategy of howler monkeys: A study in primate economics. New York: Columbia University Press.
Milton, K. (1981). Food choice and digestive strategies by two sympatric primate species. American Naturalist, 117, 496–505.
Mittermeier, R. A., Ratsimbazafy, J., Rylands, A. B., Williamson, L., Oates, J. F., Mbora, D., et al. (2005). Primates in peril: the World’s 25 Most Endangered Primates 2006–2008. Primate Conservation, 22, 1–40.
Moncur, M. W. (1985). Floral ontogeny of the jackfruit Artocarpus heterophyllus. Lam (Moraceae). Australian Journal of Botany, 33, 585–593.
Mowry, C. B., Decker, B. S., & Shure, D. J. (1996). The role of phytochemistry in dietary choices of Tana River red colobus monkeys (Procolobus badius rufomitratus). International Journal of Primatology, 17, 63–84.
NARESA (1991). Natural resources of Sri Lanka: Conditions and trends. In M. F. Baldwin (Ed.), Keels Business Systems, Education Centre. Colombo.
Oates, J. F. (1988). The diet of the olive colobus monkey, Procolobus verus, in Sierra Leone. International Journal of Primatology, 9, 457–478.
Oates, J. F., & Davies, A. G. (1994). What are the colobines? In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 1–9). Cambridge: Cambridge University Press.
Oates, J. F., Waterman, P. G., & Choo, G. M. (1980). Food selection by the south Indian leaf-monkey, Presbytis johnii, in relation to leaf chemistry. Oecologica (Berlin), 45, 45–56.
Oxnard, C. E. (1969). A note on the ruminant-like digestion of langurs. Laboratory Primate Newsletter, 8, 24–25.
Robinson, J. G. (1986). Seasonal variation in use of time and space by the wedge-capped capuchin monkey Cebus olivaceus: implications for foraging theory. Smithsonian Contribution to Zoology, 431.
Rudran, R. (1970). Aspects of ecology of two sub-species of purple-faced langurs (Presbytis senex). M.Sc. thesis, University of Ceylon.
Rudran, R. (1978). Socioecology of the blue-monkey (Ceropithecus mitis stuhlmanni) of the Kibale Forest, Uganda. Smithsonian Contribution to Zoology, 249.
Ruhiyat, Y. (1983). Socio-ecological study of Presbytis aygula in West Java. Primates, 24(3), 344–359.
Senaratna, L. K. (2001). A checklist of the flowering plants of Sri Lanka. Sri Lanka: National Science Foundation.
Solanki, G. S. K., Kumar, A., & Shama, B. K. (2008). Feeding ecology of Trachypithecus pileatus in India. The diet of the capped langur (Presbytis pileata) in a moist deciduous forest in Bangladesh. International Journal of Primatology, 12, 199–216.
Stanford, C. B. (1991). The diet of the capped langur (Presbytis pileata) in a moist deciduous forest in Bangladesh. International Journal of Primatology, 12(3), 199–121.
Stevenson, P. R. (2004). Fruit choice by woolly monkeys in Tinigua National Park, Colombia. International Journal of Primatology, 25(2), 367–381.
Struhsaker, T. T. (1975). The red colobus monkey. Chicago: University of Chicago Press.
Sulit, J. I., & Ganaban, E. V. (1968). Studies of the utilization of non-edible portion of ripe nangka (Artocarpus integrifolia) fruit for jelly-making. Araneta Journal of Agriculture, 15, 213–228.
Ungar, P. S. (1995). Fruit preferences of four sympatric primate species at Ketambe, Northern Sumatra, Indonesia. International Journal of Primatology, 16(2), 221–224.
Wasserman, M. D., & Chapman, C. A. (2003). Determinants of colobine monkey abundance: the importance of food energy, protein and fibre content. Journal of Animal Ecology, 72, 650–659.
Waterman, P. G., & Kool, K. M. (1994). Colobine food selection and plant chemistry. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 125–284). Cambridge: Cambridge University Press.
Wijesinghe, L.C. A. de S., Gunatilleke, I. U. A. N., Jayawardene, S. D. G., Kotagama, S. W., & Gunatilleke, C. V. S. (1993). Biological conservation in Sri Lanka. A status report. Colombo: IUCN Sri Lanka.
Wong, S. N. P., Saj, T. L., & Sicotte, P. (2006). Comparison of habitat quality and diet of Colobus vellerosus in forest fragments in Ghana. Primates, 47, 365–373.
Yeager, C. P. (1989). Feeding ecology of the proboscis monkey (Nasalis larvatus). International Journal of Primatology, 10, 453–497.
Zhou, Q., Wei, F., Li, M., Huang, C., & Luo, B. (2006). Diet and food choice of Trachypithecus francoisi in the Nonggang Nature Reserve, China. International Journal of Primatology, 27(5), 1441–1460.
Acknowledgments
Financial support for the feeding study was provided by the Natural Resources Energy and Science Authority of Sri Lanka (presently the National Science Foundation) through Grant MAB/85/1. I am deeply grateful to the late Mr. L. de Alwis and Prof S. W. Kotagama for the help given for me to secure funds and their support. I offer special thanks to my chief Ph.D. supervisor, Dr. W. P. J. Dittus, for advice and critical comments, and to Mr. Leslie Wijesinghe, the late Prof. W. R. Breckenridge, and the late Dr. R. P. Jayawardena for their encouragement when it was most needed. I also thank the Public Trustee and the manager and staff of the Regidale Estate for permission to conduct this work at Piliyandala, and all householders who allowed me to conduct this research. I thank my dedicated field assistants: Ms. M. L. Soma Perera, Jane Nona, Aslin, and Badra. Special thanks are due to Ms. Neela de Zoysa-Simon for identification of plant specimens; Ms. Gayathree Jayasinghe for statistical advice and data entry; Ms. Piyandani Dissanayake, Ms. Anne Moldrich, and the late Ms. Vasumathi Somasundaram for help with data entry; and Ms. Elaine Wijesinha for patient help with checking data tables. I am grateful for comments in relation to future publications by my Ph.D. examiner, Dr. Elizabeth Bennett, and for comments on this paper from Dr. Peter Fashing and an anonymous reviewer. I thank Dr. Joanna Setchell for very constructive editorial suggestions. I also thank Primate Conservation Incorporated, The Ministry of Environment (Sri Lanka), and MathHydropower (Pvt) Limited for supporting the range survey of Semnopithecus vetulus nestor, and the Forest Department of Sri Lanka for help with forest surveys, which provided valuable insight on feeding habits throughout the range of the western purple-faced langur.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jinie D. S. Dela is a visiting academic of the Open University of Sri Lanka.
A comment to this article is available at http://dx.doi.org/10.1007/s10764-015-9838-0.
Rights and permissions
About this article
Cite this article
Dela, J.D.S. Western Purple-faced Langurs (Semnopithecus vetulus nestor) Feed on Ripe and Ripening Fruits in Human-modified Environments in Sri Lanka. Int J Primatol 33, 40–72 (2012). https://doi.org/10.1007/s10764-011-9538-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-011-9538-3