Abstract
We present a brief overview of the literature on biological applications and experimental data on the effects of THz radiation. The region of the electromagnetic spectrum from 0.1 to 10 THz is a frontier area for research in physics, chemistry, biology, materials science and medicine. This area has recently begun to be filled by a variety of sources of high quality radiation with a wide range of new technologies related to it. New sources have led to new science in many areas, as scientists begin to become aware of the opportunities for research progress in their fields using THz radiation. Therefore the opportunities for THz science in chemistry and biology are wide ranging. Some of them will extend the range of already established work, many others have not yet been realized but show great promise, and the rest fall somewhere in between.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
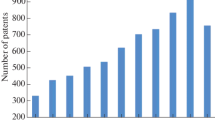
The spectral region from 0.1 to 10 THz represents an opportunity for research in physics, chemistry, biology, and medicine [1–4]. If we look at about eighty conferences and workshops dealing with THz science held in the last five years, we can observe a significant increase of biological applications in 2007 despite the total number of conferences being lower (Fig. 1). In 2004-2005 most of the presented papers regarded the applications of THz in defence and security area, while THz applications, more dedicated to basic research in biology and medicine, have gradually increased in 2006-2007, until last September when THz applications in biology and medicine became the first topic at the 2008-IRMMW-THz Conference. This increase of interest in biological applications is because sources of high quality radiation are now available both in continuous wave (CW) and pulsed form, down to single-cycles or less, with peak powers up to 10 MW [5–7], and because of the unique sensitivity of this spectral region to the global nuclear motions, which are important in biomolecular processes. More specifically, it is important to point out that THz radiation is generally non-invasive. THz waves have low photon energies (1 THz = 4.1 meV), one million times weaker than x-rays, and do not cause harmful photoionization in biological tissues. THz radiation interrogates vibrational modes that extend across large portions of the biomolecular framework with length scales that extend over tens of angstroms. These extend motions are important for the functioning of proteins/RNA/DNA that undergo transformations in secondary and tertiary structures. THz frequencies provide access to timescales in the sub-picoseconds domain which are difficult to attain using other methods. Ultimately, much larger systems than proteins and DNA strands will be accessible, particularly with the coupling of THz science with near-field probes. One envisions label-free measurement of protein-protein interactions as cellular activity is occurring in live cells. The opportunities for THz science in chemistry and biology are, therefore, wide ranging (Fig. 2).
Number of conferences and workshops on topics related to THz science. The graph is based on counts of conferences and workshops announced on the web from 2004 to March 2009 (total number = 81). On the y-axis is reported the number of conferences. The percentage value of biological applications is also reported (red bar).
2 The opportunities for THz science in biology
Biology, for our purpose, can be considered to exist in the realm of large, complex macromolecules up to viruses, cells and tissue. Biology can be further characterized as consisting of evolved molecules which have specific functions, often by means of biological networks which involve the highly specific interactions of molecular complexes in a sequential manner. The basic molecular building blocks of biomolecules is now well known: What is far less understood is the specificity and control that biomolecules have as “nanomachines” [8]. The dynamics of these nanomachines are important over a huge range of time scales: from the sub-picoseconds time domains to the ultraslow conformational relaxation. Clearly the fast, picosecond timescale, conformational dynamics of proteins is strongly coupled to THz frequencies.
2.1 Applications in biocatalysis and photobiology
THz science offers unprecedented opportunity to analyse fast protein vibrations. The experimental identification of fast (sub-picosecond) small-scale promoting vibrations and coupled motions will provide a step forward in the understanding of the physical basis of enzyme catalysis. In fact it appears that something is missing in the physical understanding of enzyme catalysis. Current physical models (e.g., transition state theory) only account for ~106 fold increase in reaction rate, and do not account for 1021 fold increase in reaction rate over reference reaction in absence of an enzyme. In this context, new emerging theory indicates a fast tunnelling models for enzyme catalysis: proton and electron transfer [9]. In this dynamical and quantum mechanical view of enzyme catalysis, protein motion (millisecond to sub picosecond) modulates barrier properties (i.e. ‘squeezing’) to facilitate tunnelling. Further detailed insight, particularly at the atomic level, comes from theory and simulation—such studies are crucial to corroborate and extend findings that emerge from experimental studies. Recently, an atomic-level description of the reaction chemistry of tryptamine oxidation by aromatic amine dehydrogenase dominated by proton tunnelling has been presented [10]. Combining experiments and molecular simulations the authors show that the proton transfer occurs predominantly to oxygen O2 of Asp128 β (aspartic acid) in a reaction dominated by tunnelling over ~ 0.6 angstroms. In this enzyme system, tunnelling is promoted by a short-range motion modulating proton-acceptor distance and no long-range coupled motion is required (Fig. 3, top). Further, a ternary electron transfer complex was studied showing extensive motion at the protein interface [11, 12]. This complex comprises the iron-sulfur flavoprotein trimethylamine dehydrogenase and electron transferring flavoprotein (ETF) from Methylophilus methylotrophus. A dual interaction of ETF with trimethylamine dehydrogenase provides for dynamical motion at the protein interface: one site acts as an anchor, thereby allowing the other site to sample a large range of interactions, some compatible with rapid electron transfer (Fig. 3, bottom).
THz science and the understanding of the physical basis of the enzyme catalysis. Top : Schematic overview of the ADDH reaction with the tryptamine. The ADDH structure is represented in cartoon form with the α subunit are depicted in blue, the β subunit in yellow, and TTQ cofactor in magenta sphere. The oxidative half-reaction consists of two consecutive long-range electron transfer events to the single-electron carrier azurin, depicted in green, with the copper atom as an orange sphere. (From Fig. 1 of L. Masgrau, et al., Atomic description of an enzyme reaction dominated by proton tunnelling- Science 312,237-241 © 2006 by The American Association for the Advancement of Science). Bottom: Representation of the model for the hydrogen transfer reaction. The motions of the environment modulate the symmetry of the double well, thus allowing the system to reach a configuration with (nearly) degenerate quantum states (q=q *), from which the hydrogen is able to tunnel. The difference between panels a and b is a gating motion that reduces the distance between the two wells along the HC-axis (r H) away from its equilibrium value (r 0). This motion increases the probability of tunnelling at the (nearly) degenerate configuration q *. (From Fig. 1 of M. J. Sutcliffe, et al.,-Hydrogen tunnelling in enzyme catalysed H-transfer reactions: flavoprotein and quinoprotein systems- Phil Trans R Soc Lond B Biol Sci. 361: 1375–1386- © 2006 by The Royal Society London).
THz science offers opportunities also for studying the photoisomerization of the retinal chromophore. The visual cycle is the biological conversion of a photon into an electrical signal in the retina. This process occurs via G-protein coupled receptors called opsins which contains the chromophore, 11-cis retinal. This is the light absorption portion and is covalently linked to the opsin. The retinal chromophores occur in different isomeric forms depending on the physical function of the protein. When struck by a photon, 11-cis retinal undergoes photoisomerization to all-trans retinal which changes the conformation of the opsin leading to signal transduction cascades. The cis-trans isomerization is the fastest known biological photochemical reaction (ca. 200 fs). Vibrational coherence of the isomerization may be responsible for the speed of this reaction. Walther et al. [13] have used THz–Time Domain Spectroscopy (TDS) to measure the far infrared spectra of three isomers of retinal: all-trans, 13-cis and 9-cis. Retinal has shown to exhibit absorptivity in the 0.3-3 THz region showing distinct differences between the low frequency vibrational spectra of three isomers. Jones et al. [14] provided molecular modelling fitting of these experimental data, indicating new opportunities for future combination of structural, spectral and biochemical studies.
THz-TDS is sensitive in discriminating the different conformational states of protein with similar structure. In the study of Qu et al.[15] although the secondary and tertiary structures of native CP43 and CP47, that are photosynthesis membrane proteins, are very similar, it was possible to discriminate the different conformational state through the THz absorption spectra. This difference was maintained also after treatment with guanidine hydrochloride indicating that the proteins denatured are in different unfolding states. In addition, Balu et al. [16] found that the nature of the vibrational motions activated by terahertz radiation is surprisingly similar between two structurally similar proteins, i.e. rhodopsin and bacteriorhodopsin, confirming the importance of THz spectroscopy studies.
In the emerging new branch of time resolved THz emission experiments, the transient radiation is generated within the sample itself, allowing monitoring of the underlying ultrafast light-induced polarization processes. This method has been successfully applied to biological samples allowing direct information about the mechanism of primary charge translocation in retinal proteins as well photosynthetic systems. Groma et al. [17] were able to observe light-induced coherent terahertz radiation from bateriorhodopsin with femtosecond time resolution. The detected THz signal was analyzed by numerical simulation by different models for the elementary polarization processes, revealing correlated sequence of primary electron transfer inside the retinal chromophore, and proton transfer, i.e. redistribution of a H bond near the retinal (Fig. 4).
Near-field terahertz emission detected from the native (A) and the acid blue form (B) of bacteriorhodopsin and compared with the results of simulations based on model I (gray line), model II (blue lines), and model III (red lines) for the elementary polarization. (From Fig. 3 of G.I. Groma, et al., -THz radiation from bacteriorhodopsin reveals correlated primary electron and proton transfer process- PNAS 105:6888-6893 © 2008 by National Academy of Sciences, U.S.A.).
2.2 Applications in proteomics and pharmaceutical screenings
It is currently possible to distinguish several amino acids based on their THz spectra, particularly in the crystalline form. THz absorption spectra (THz-FTIR) of the pure Tryptophan D and L enantiomers and of a single fluorine-substituted analogue, which generates a completely different spectrum compared to the native amino acids, have been reported [see review 4, 18]. In this way it is possible to extract ”contaminant” concentrations a task that is difficult using other methods. Polypeptides have been also investigated using THz-infrared spectroscopy. The spectra for several di- and tri-peptides have shown the variability in spectral content and uniqueness for each structure [see review 4].
The vibrational modes corresponding to protein tertiary structural motion lay in the far infrared and terahertz frequency range. These collective large scale motions depend on the global protein structure and thus they are necessarily perturbed by ligand binding events. It is worth noting that, the determination of protein-protein and protein-ligand binding is a key component of pharmaceutical development. Andrea Markelz’s group [19] has studied conformational change using THz-TDS through identifying protein-ligand binding in solution. In this case the authors used lysozyme, an enzyme that naturally protect from bacteria. The experimental observation of these collective modes require many identically prepared molecules in which the large-scale motions are coherent. This can be achieved in an experiment which starts with molecules that are cold and therefore in their ground state. These would then be excited with a powerful terahertz pulse that is not resonant with any modes except the soft mode of interest. Probing the molecules at different times after the excitation should lead to the observation of the consequent changes in their dynamics while the ensemble rattles in concert. The authors have demonstrated that the overall change in the dielectric response of lysozyme was due to the binding with n-acetyl glucosamine, a competitive inhibitor of lysozyme. This enzyme consists of two domains connected by a hinging region, which form the cleft where the inhibitor binds. Thus the binding strongly inhibits this hinging motion and, consequently all the collective modes associated with it, causing the decrease in terahertz response. The same authors were also able to use THz-TDS to identify the oxidation state of cytochrome c. They reported a remarkable difference between the oxidized and reduced forms of cytochrome c indicating that the THz dielectric response is sensitive for this transition. In addition, the clear UV/VIS absorbance changes between the oxidized and reduced forms of this system allowed having a second parallel method to verify its oxidation state [20]. These works are successful examples of how terahertz spectroscopy can be used to quickly assay protein binding for proteomics and pharmaceutical research.
2.3 Applications as label-free sensors
Several recent papers have demonstrated the ability of THz radiation to detect single and double stranded DNA sequences. The transmission change in the terahertz region (i.e. 0.1-10 THz) is greatly increased if the DNA is hybridised - that is, two complementary strands bind to each other and the hydrogen bonds are placed in between the two chains (annealing) – in comparison if the DNA is denatured (when the annealing is reversed by heating and the two chains are not linked together by hydrogen bonds). The DNA hybridization is recognized by the change of the THz spectrum caused by the hydrogen-bond vibration mode of -NH2 structure between complementary DNA. The internal motions, dependent on the weak hydrogen bonds of the double-helix base pairs, are extremely sensitive to DNA composition and topology and have an impact on the transfer of genetic information. Refinement of this work will lead to label-free sensors based on complementary hybridization [21].
In addition, by using THz-TDS, hybridization studies can detect single point mutations associated with the disease hereditary hemochromatosis that is characterized by excessive absorption of dietary iron [22]. The spectra reveal clear differences in resonance peaks for the three hybridized oligonucleotides with a trend toward lower transmission and lower resonant frequencies with greater sequence differences in hybridized pairs. The sensitivity of these measurements was such that femtomoles of DNA were enough to detect the mutation.
2.4 THz applications as an in situ imaging technique
A novel biological application of terahertz absorption, ionic contrast terahertz near-field microscopy, has recently been proposed as an in situ imaging technique, with the potential of direct observation of neuron swelling induced by temperature change or neurotoxin poisoning. This imaging modality depends on the extent to which ionic solutes modulate the terahertz absorption of water solutions and is suggested to provide a real time image of functionally relevant variations of ion concentrations in biological samples. Using this approach, Masson et al. [23] have reportedly observed physiologically relevant changes in the intra- and extra-cellular concentrations of potassium and sodium chloride in and around living neurons. They reported that the terahertz absorption of a solution is highly sensitive to its salt content, and that concentration variation as small as 10 mM can be readily measured using incident radiation between 0.1 and 2 THz. However, other authors have not supported this proposed imaging application [24].
2.5 Critical aspects in THz biological application
A critical aspect of biological molecules is their close coupling with water [25]. Proteins must be in an aqueous environment to function, that is, undergo their conformational switching motions. Bulk water continues to be a difficult substance to understand, particularly in the THz frequency region. Several recent studies have addressed the use of THz spectroscopy to probe biomolecules in aqueous environments. Martina Havenith’s group [26] found that the THz region is a sensitive probe to evaluate changes induced by the solute in the network of hydrogen bonds of water in the vicinity of a biomolecule, i.e. lactose. The lactose molecules and the water in the hydration shell oscillate coherently relative to one another, and the coherent oscillation of water in the hydration shell absorbs more strongly in the THz region than comparatively does in the bulk. Recently the same authors reported studies [27] in which THz spectroscopy provides a window on protein-water rearrangements during the folding process. In fact proteins change the properties of water to perform particular tasks in different parts of our cells. The new approach, using KInetic THz Absorption (KITA) at ultra fast laser pulses, provided a look at the motion of water molecules in real time during the folding process.
3 THz radiation effects on biological systems
Beside a particular interest in biology, the fact that proteins respond to THz radiation may have implications for human exposure. Very little is known of the effects of THz radiation on biological systems and about potential damages that could be induced after the absorption of this radiation by these systems. Testing t-ray quantities that would be needed for bodily imaging, researchers have found no indication of permanent, x-ray-like tissue damage, as expected by the low photon energies involved (1 THz = 4.1 meV).
However, at the cellular level, a manifold number of effects of THz radiation have been reported [reviewed in 28–30]. Cell membrane may be of significance in governing the interaction with this radiation. Membranes of biological systems usually have a thickness of about 10-6 cm, across which an electrical potential difference of 100 mV is maintained. An enormous electric field of 105 V/cm thus acts on the membrane which, therefore, is strongly polarized electrically. In this context, exposure experiments in the frequency range from 30 to 300 GHz which can alter membrane structural and functional properties have been reported [31–36]. However, the interpretation of these biological effects is a matter of controversy, especially regarding whether low intensity high frequency radiation can produce biological effects via non-thermal mechanism [37–39].
Considerable impulse to the investigation of interaction of THz radiation with biological systems has been provided by the studies performed in the framework of the EU-THz-BRIDGE project [40]. In this context, we studied the effects of THz radiation on the permeability of a membrane model system (liposome), which, in contrast to living cells, is well defined and only has a limited number of parameters, allowing an accurate evaluation of membrane effects induced by electromagnetic radiation [41]. The effects induced by 0.13 THz radiation pulse-modulated at low frequencies of 5, 7 or 10 Hz, and at time-averaged incident intensity (IAV) up to 17 mW/cm2 was studied in real-time during the irradiation. It is worth nothing that liposome bilayer is about 4 nm thick and that the peak electric field applied through the 0.13 THz radiation was up to 2700 V/cm, about 2 orders of magnitude lower than naturally developed across lipid bilayers. Since a clear role of the peak electric field in eliciting the observed effect resulted, with a window effect around 2.6-2.7 kV/cm (Fig. 5), a possible rectification of THz pulse by liposomes has been hypothesized, which in turn could lead to change in the lipid membrane functionality.
By using the same source of THz radiation (a Compact THz Free Electron Laser), other studies have been carried out on cellular systems to evaluate possible genotoxic effects induced by THz radiation. The evaluation of genotoxic effects is indeed one of the short-term targets to assess the potential health risk following human exposure to chemical and physical agents, since DNA damage of somatic cells can lead to development of cancer. Scarfì et al. [42] have reported no effect on the levels of micronuclei (MN) in binucleated cells after lymphocytes were exposed to 0.12 THz radiation for 20 min. In this experiment, picosecond THz pulses illuminated the target at an average power level of 1 mW and an integrated energy incidence (dose) of 0.45 J/cm2. The end point searched in this case is indicative of damage incurred directly by the DNA [43]. On the contrary, Korestein et al. [44] have suggested the existence of genomic instability (Fig. 6), reflected by an increased levels of aneuploidy (abnormal chromosome number) and an increased levels of asynchronous replication of centromeres, in lymphocytes exposed to continuous wave 0.1 THz radiation (0.031 mW/cm2). In this case the exposure were carried out for a longer time (ca. 2 h) than above done. Further the observed aneuploidy appeared to be chromosome-specific in agreement with other studies on cellular response induced by chemical or physical stimuli [45]. The authors suggested, as possible mechanism underlying these effects, that THz radiation is able to excite low-frequency collective vibrational modes of biomolecules [45]. Recently the coherent collective vibration models of molecule (e.g., DNA and proton) have been theoretical analysed, indicating that the interaction between these biological systems and THz radiation can produce resonance excitation effect capable to explain mutagenesis effects [46].
Effects of continuous wave 0.1 THz radiation on genomic stability. Dependence of aneuploidy levels of chromosomes 11, 17, 1 and 10 on exposure duration. A significant change in total aneuploidy level of chromosomes 11 and 17 is present by increasing the exposure time to 2 h. (From Fig. 3 of A. Korestein-Ilan, et al.,- Terahertz radiation increases genomic instability in human lymphocytes- Rad. Res. 170:224-234- © 2008 by Radiation Research Society).
Clearly a significant amount of experimental work is still required in order to fully understand the interactions between THz radiation and biological systems.
References
P. H. Siegel, IEEE Trans MW Theory and Tech 52, 2438–2447 (2004).
R. Woodward, Preclinica 2, 328–335 (2004).
D. L. Woolard, E. R. Brown, M. Pepper, and M. Kemp, Proceedings IEEE 93, 1722–1743 (2005).
D. F. Plusquellic, K. Siegrist, E. J. Heilweil, and O. Esenturk, Chem Phys Chem 8, 2412–2431 (2007).
B. Ferguson and X. C. Zhang, Nature Materials 1, 26–33 (2002).
P. H. Siegel, IEEE MTT 50, 910–918 (2002).
M. A. Belkin, F. Capasso, A. Belyanin, D. L. Sivco, A. Y. Cho, D. C. Oakley, C. J. Vineis, and G. W. Turner, Nature Photonics 1, 288–292 (2007).
Report of a DOE-NSF-NIH Workshop on opportunities in THz science, Arlington, VA ed. by M.S. Sherwin, C.A. Schumettenmaer and P.H. Bucksbaum Chapter 6, pp. 57-62 (2004).
N. S. Scrutton, M. J. Sutcliffe, and P. L. Dutton. J. Royal Soc. Interface pp. 1-5 (2006).
L. Masgrau, A. Roujeinikova, L. Johannissen, P. Hothi, J. Basran, K. E. Ranaghan, A. J. Mulholland, M. J. Sutcliffe, N. S. Scrutton, and D. Leys, Science 312, 237–241 (2006).
D. Leys, J. Basran, F. Talfournier, M. J. Sutcliffe, and N. S. Scrutton, Nat Struct Biol 10, 219–225 (2003).
M. J. Sutcliffe, L. Masgrau, A. Roujeinikova, L. O. Johannissen, P. Hothi, J. Basran, K. E. Ranaghan, A. J. Mulholland, D. Leys, and N. S. Scrutton, Philos Trans R Soc Lond B Biol Sci 361 (1472), 1375–1386 (2006).
M. Walther, B. Fisher, M. Schall, H. Helm, and P. U. Jepsen, Chem Phys Lett 332, 389–395 (2000).
T. J. Jones, B. Rainsford, D. Fisher, and T. Abbott, Vibr Spectrosc 41, 144–154 (2006).
Y. G. Qu, H. Chen, X. C. Qin, L. Wang, L. B. Li, and T. Y. Kuang, Sci China Ser C-Life Sci 50, 50–355 (2007).
R. Balu, H. Zhang, E. Zukowski, J. Y. Chen, A. G. Markelz, and S. K. Gregurick, Biophysical Journal 94, 3217–3226 (2008).
G. I. Groma, J. Hebling, I. Z. Kozma, G. Varo, J. Hauer, J. Kuhl, and E. Riedle, PNAS 105, 6888–6893 (2008).
A. Matei, N. Drichko, B. Gompf, and M. Dressel, Chemical Physics 316, 61–71 (2005).
S. Ye, Y. He, and A. G. Markelz, App Phys Let 90, 243901 (2007).
Y.-I. Chen, J. R. Knab, J. Cerne, and A. G. Markelz, Physical Review E 72, 040901 (2005).
M. Brucherseifer, M. Nagel, P. H. Bolivar, H. Kurtz, A. Bosserhoff, and R. Buttner, Applied Physics Letters 24, 4049–4051 (2000).
M. Nagel, P. Haring Bolivar, M. Brucherseifer, and H. Kurz, Appl Phys Lett 80, 154 (2002).
J.-B. Masson, M.-P. Sauviat, J.-L. Martin, and G. Gallot, Proc. Natl. Acad. Sci. U.S.A. 103, 4808 (2006).
J. Xu, K. W. Plaxco, S. J. Allen, and J. E. Bjarnason, ER Brown Appl Phys Lett 90, 031908 (2007).
S. K. Pal and A. H. Zewail, Chem Rev 104, 2099–2123 (2004).
U. Heugen, G. Schwabb, E. Brundermann, M. Hyden, X. Yu, D. M. Leitner, and M. Havenith, PNAS 103, 12301–12306 (2006).
M. Gruebele and M. Havenith, Angew Chemie Int. 47, 6486–6490 (2008).
A. G. Pakhomov, Y. Akye, O. N. Pakhomova, B. E. Stuck, and M. R. Murphy, Bioelectromagnetics 19, 393–413 (1998).
V. I. Fedorov, S. S. Popova, and A. N. Pisarchik, Int J Infrared and Millimeter Waves 24, 1235–1253 (2003).
K. Mileva, B. Georgieva, and N. Radicheva, Acta Physiol Pharmacol Bulg 27, 89–100 (2003).
V. M. Brovkovich, N. B. Kurilo, and V. L. Barishpolts, Radiobiologia 31, 268–271 (1991).
A. A. Kataev, A. A. Alexandrov, L. L. Tikhonova, and G. N. Berestovsky, Biofizika 38, 446–462 (1993).
V. I. Geletyuk, V. N. Kazachenko, N. K. Chemeris, and E. E. Fesenko, FEBS Lett 359, 85–88 (1995).
S. I. Alekseev and M. C. Ziskin, Bioelectromagnetics 20, 24–33 (1999).
A. S. Koryagin, A. A. Yastrebova, V. N. Krylov, and A. V. Kornauchov, Millimetrovye Volny v Biologii i Medicine 2, 8–10 (2000).
A. G. Shein and R. N. Nikulin, Biomedicinskie Technologii i Radioelektronika 4, 4–10 (2004).
B. G. Yemets and M. B. Yemets, in Proc. IEEE 4th Int. Kharkov Symp. Phys. Eng. Microwaves, Millim, Submillim., Waves, Kharkov, Ukraine, vol.2, pp. 946-948 (2001).
G. Yu, S. Liu, W. Wang, in Proc. XXVII International Conference on Infrared and Millimeter Waves, Conference Digest, W2.4 IEEEpp. 215-216 (2002).
A. Beneduci, in M. E. Bernstain (Ed), Bioelectrochemistry Research Developments, Novascience Publishers Inc, New York, pp. 35-80 (2008).
A. Ramundo-Orlando, G. P. Gallerano, P. Stano, A. Doria, E. Giovenale, G. Messina, M. Cappelli, M. D’Arienzo, and I. Spassovsky, Bioelectromagnetics 22, 303–313 (2007).
O. Zeni, G. P. Gallerano, A. Perrotta, M. Romanò, A. Sannino, M. Sarti, M. D’Arienzo, A. Doria, E. Giovenale, A. Lai, G. Messina, and M. R. Scarfì, Health Physics 92, 349–357 (2007).
M. R. Scarfì, M. Romano, and R. Di Pietro, J Biol Phys 29, 171–177 (2003).
A. Korestein-Ilan, A. Barbul, P. Hasin, A. Eliran, A. Gover, and R. Korestein, Rad Res 170, 224–234 (2008).
R. Globus, D. L. Woolard, and T. Krhomova, J Biol Phys 29, 89–100 (2003).
Z. Dai, C. Zhang, S. Wang, and L. Zhou, Proc SPIE 6534, 65340X (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramundo Orlando, A., Gallerano, G.P. Terahertz Radiation Effects and Biological Applications. J Infrared Milli Terahz Waves 30, 1308–1318 (2009). https://doi.org/10.1007/s10762-009-9561-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10762-009-9561-z