Abstract
The appearance of modern sources and detectors of terahertz radiation (1011–1013 Hz) stimulated the rapid development of practical applications for radiation in this frequency range. Therefore, the question of the safety of terahertz radiation for living objects was sharply raised. In this review, we present an analysis of research on this issue published from 2010 to the present. A brief description of the most significant works performed before 2010 is given. Particular attention is paid to the sources of terahertz radiation used in the studies and the results of experimental work on the study of possible bioeffects when such radiation is applied to both individual cell lines and microorganisms and animals generally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
CONTENTS
Introduction
1. Types of terahertz radiation sources
2. Effect of terahertz radiation on cell lines
2.1. Human blood cells
2.2. Human epithelial cells
2.3. Stem cells
2.4. Animal cells and hybrid cell lines
3. Effect of terahertz radiation on complex biological systems: microorganisms and animals
4. Effect of low-intensity radiation in the millimeter range (0.129–0.150 THz) on animals and humans
5. Discussion
Conclusions
References
INTRODUCTION
Human beings are exposed to electromagnetic (EM) radiation every day; this is the price they must pay for the vast amount of technology surrounding them. The issue of the effect of EM radiation on humans arose simultaneously with the inception of these technologies. The terahertz range occupies a significant area of the EM spectrum, from 0.1 to 10 THz, and lies between the infrared and microwave ranges, which correspond to a wavelength range of 30–3000 µm and a photon energy of 0.4–41 meV.
Over the past quarter of a century, the issue of the lack of sources and receivers of terahertz radiation has been solved, and, at the moment, the scope of its application is vast: from production control and scanners in security systems to communication and imaging in medicine [1–3]. The latter is especially interesting, because it has a number of practical applications, in particular, for the diagnosis of breast cancer [4], melanoma [5], and carcinoma [6]. The number of studies of the effect of terahertz radiation on biological objects has grown rapidly, especially over the last 10–15 years. In contrast to ionizing radiation (the ionization of biological molecules requires energy of the order of 1 eV [7]), which leads to the formation of active free radicals, the question of the danger of terahertz radiation is still open.
One of the first large-scale research projects designed to study the possible effects of terahertz radiation on biomolecules and cells and the fundamental mechanisms of its interaction with biological objects was the Tera-Hertz radiation in Biological Research, Investigation on Diagnostics and study of potential Genotoxic Effects (THz-BRIDGE) project [8]. The project was carried out between 2001 and 2004. It united ten scientific research institutes from different countries. In addition to biomolecules (DNA bases), the main subjects of research were human lymphocytes, epithelial cell cultures, and liposomes. According to the results of the project, it was concluded that no biological effects of terahertz radiation were observed in many cases, but, under specific conditions, the effect of radiation led to a change in the permeability of the liposome membrane and could also cause genotoxic and epigenetic effects in lymphocytes. The importance of further studies in this field to find the exact relationship between the radiation dose and the observed effect is noted.
It should be noted that currently available experimental data on the presence or absence of the effect of terahertz radiation on living organisms, tissues, and cells are quite scattered and sometimes contradictory. In view of the wide range of experimental irradiation parameters reported in publications, as well as the differences in the types of biological objects studied and the evaluation methods, it is difficult to derive any definite patterns and give them a theoretical justification. The main results of the studies conducted between the completion of the THz-BRIDGE project and March 2011 were detailed in [7, 9].
The purpose of this review is to summarize and systematize the results of experimental studies on the possible bioeffects of terahertz radiation on living biological systems that were published from 2011 to early 2018 and to describe briefly the results of the most significant studies performed before 2011. Structured data on the types of biological objects, the observed effects of terahertz radiation, the used radiation sources, and the irradiation parameters (frequency, exposure time, and power density) are presented. If there was no information about the last parameter in the work, this value was calculated based on the values of the source power and the size of the irradiated region.
1. TYPES OF TERAHERTZ RADIATION SOURCES
The interaction of terahertz radiation with biological objects is determined by two main factors: the composition and properties of the object to be affected (in particular, the refractive index, the absorption coefficient, etc.) and the teraherz radiation parameters of the effect (duration of irradiation, intensity, spatial profile, spot size, average power, and radiation spectrum).
Although the first sources of terahertz radiation in the form of a black body appeared in the mid-1920s (Globar, registered in 1925), the impact on a biological object was first carried out with the use of a klystron [10] and an infrared laser [11] in 1968 and 1970, respectively.
Table 1 presents the models and characteristics of sources used in the last decade to study the effect of terahertz radiation on biological objects. If data are available, the maximum parameters of the source are indicated in the table. Tables 2–6 give the real values of the parameters of terahertz radiation used in the experiment. More detailed information on the sources of terahertz radiation and their evolution can be found in some review papers [12–14].
Lasers generating radiation in the far infrared range can be attributed to the first category of terahertz radiation sources. A powerful CO2 pump laser is used as a rule in these systems; it excites vibrational levels in gas molecules, e.g., methanol. The frequency of transitions between its levels is in the terahertz range. Such laser systems ensure the generation of coherent, monochromatic continuous radiation with the possibility of discrete frequency tuning up to 10 THz and are characterized by a sufficiently high value of the average output power (~100 mW). The disadvantages of these systems are their cumbersomeness and high cost. Despite this, they have been used in a large number of studies over the past 8 years [15–25].
The second category includes all kinds of electronic devices that make it possible to obtain narrowband continuous radiation of medium power in a frequency range below 1 THz. Typically, such systems consist of frequency synthesizers (or generators) and frequency multipliers. These systems are reliable, compact, and operate at room temperature. Examples of their use for they study of the effect of terahertz radiation on blood cells, epithelial cells, and hybrid cell lines can be found in [26–28], respectively.
Sources of terahertz radiation that operate on the principle of electron acceleration include backward-wave tubes and free-electron lasers. At the heart of these systems, which are different from each other at first glance, is the same principle of electron slowing in the EM field. In backward-wave oscillator (BWOs), combs are used as a deceleration system, and wigglers are used in accelerators. BWOs enable the generation of narrowband (1–10 MHz) frequency-tunable terahertz radiation. The maximum achievable frequency is ~1.5 THz. Although these devices are capable of generating radiation with a relatively high average power (~100 mW) in the low-frequency range of the terahertz spectrum, the radiation power decreases to several milliwatts with increasing frequency (the dependence is inversely proportional to f 2–f 3). The fact that a BWO, as a rule, is optimized for a specific spectral range can be attributed to its disadvantages. In some cases, the frequency tuning range can reach an octave. BWOs were used in studies of fibroblasts [15] and rat glioma cells [29].
Free-electron lasers consist of two main elements: an electron accelerator producing a beam of relativistic electrons and a wiggler for undulating an electron beam. Modulation of the electron beam causes electron oscillations and the emission of bright terahertz radiation. Lasers on free electrons offer frequency tuning of the radiation and can work in both continuous and pulsed modes. To date, a compact free-electron laser located at the ENEA research center (Italy) [30–33] and the Novosibirsk Free Electron Laser (NovoFEL) of the Institute of Nuclear Physics, Siberian Branch of the Russian Academy of Sciences, have been used in studies of the effect of terahertz radiation on living biological systems [34–38]. While most of the studies in the former case were completed before 2010 [30–32] (with the exception of [33] in 2015), the experiments at the NovoFEL setup date back to 2010–2016. It should also be noted that, while radiation in the range of 120–130 GHz was used at the ENEA facility, the NovoFEL experiments made it possible to obtain new data in the frequency range of 1.5–2.3 THz. The advantages of free-electron lasers are a high output power of the radiation and a wide range of frequency tuning. At the same time, such systems are cumbersome, difficult to operate, and expensive, besides they require an entire staff of personnel to operate. In addition to free-electron lasers, a study of the effect of terahertz radiation on biological objects was carried out at the Daresbury Laboratory (United Kingdom) with the ALICE linear accelerator [39]. Since the dimensions of a bunch of electrons (duration ~2 ps) are comparable with the wavelength of terahertz radiation, their radiation can be considered coherent, and the intensity of such coherent radiation increases in proportion to the square of the charge of the electron bunch. The typical charge of a bunch of electrons on the accelerator is 60 pC. This device was used to irradiate cells with pulses of broadband radiation with a frequency of up to 0.5 THz.
Although the methods of optical rectification and photoconductivity, which were developed back in the 1980s, made it possible to perform a direct transformation of the radiation of multimode femtosecond lasers into terahertz lasers [40, 41], the first work on their use in biology was reported in 2003. The optical rectification effect consists of the appearance of nonlinear polarization in a medium upon the passing of an intense laser pulse through it, the shape of which repeats the shape of the envelope of the optical pulse. This pulse, or more precisely, its time derivative (that is, current surge), is a rather effective source of terahertz radiation: the conversion efficiency reaches ~3% at the moment, and the record energy of the broadband (0.1–10 THz), single-cycle, picosecond pulse is 0.9 mJ [42]. The considered sources are widely used due to their simplicity and the possibility of generating high-power broadband terahertz radiation. This type of source was used in a number of works to irradiate the skin of a mouse [43], an artificial skin sample [44–46], the sensory ganglion neurites of chick embryos, mouse thymocytes and splenocytes [47–49], and male and female Drosophila [50–52].
Photomixers and photoconductive antennas are a very promising sources of terahertz radiation and are widely used. Their advantages include compactness and the possibility of generating both broadband and frequency-tunable radiation at room temperature without the need for high-power optical radiation sources. At the heart of the device is a semiconductor element with lithographically drawn conducting electrodes with a certain gap, to which a constant voltage is applied. The structure is called a photoconductive antenna when it is irradiated with femtosecond laser pulses and a photomixer in the case of irradiation with continuous lasers emitting at close frequencies [53]. However, such antennas have a relatively low efficiency of converting the radiation of femtosecond lasers or close-in-frequency continuous laser sources (in the case of photomixing) into pulsed or continuous terahertz radiation, respectively. Using photomixers, it is possible to create a tunable frequency (0.1–3 THz) source, the power of which in the range above 1 THz is limited to 1 µW [54]. A similar source was used in [55] to study the genotoxic effects of terahertz radiation on human peripheral blood leukocytes. Photomixers generate narrowband frequency-tunable radiation in the range of 0.01–1.5 THz. Modern units are equipped with a fiber input and often an antenna, ensuring the generation of continuous terahertz radiation of ~10 mW power at a frequency of 0.1 THz and ~10 µW at a frequency of 1 THz. A photomixer based on an uni-traveling-carrier photodiode, UTC-PD (~0.07–0.3 THz, ~10 µW at a frequency of 0.1 THz and ~3 µW at a frequency of 0.3 THz) was used in [56] to irradiate human skin fibroblasts.
The plasma that is formed in a gaseous medium as a result of optical breakdown is another type of source of terahertz radiation used to affect biological objects. Terahertz radiation is generated by focusing two femtosecond laser pulses at the fundamental frequency and the second harmonic frequency, which coincide in time and space (two-frequency pulse). Second-harmonic radiation modulates the photoionization rate, thereby increasing the electron concentration, and the effect of a two-frequency pulse results in a nonzero photoelectron drift rate, which is equivalent to the emission of a photocurrent that generates terahertz radiation [57]. The advantages of such a source are the simplicity of implementation, high values of peak power and intensity, and the broad spectrum of radiation. The disadvantages include a low conversion factor, a conical spatial distribution of intensity, and some difficulties with radiation collimation. Such a source (spectrum of 0.5–6.5 THz, power rate in the pulse of 7.8 kW/cm2) was used to irradiate blood cells [55] and mesenchymal stem cells (spectrum of 0.5–20 THz, power density in the pulse of 2 mW/cm2) [19, 20].
A source in the form of a planar array of emitters made on a printed circuit board [58] should be mentioned separately. The cell culture object (HCE-T) seeded on the bottom of a Petri dish is placed directly on the board. Spreading along the substrate, radiation at 0.12 THz frequency due to leaks has an effect on the biological object; the power density reaches 5 mW/cm2.
On the basis of Gunn diodes invented in 1962, sources were created at 10 GHz and higher (up to 1.7 THz by frequency multiplication). The power of such generators can vary from ~100–300 mW at frequencies close to 0.1 THz to microwatt magnitudes near 1.7 THz. In particular, the generator based on the Gunn diode was the basis of the KVCh-O2 apparatus developed by Russian scientists and used in numerous studies of the effect of radiation at a frequency of 0.129 THz (corresponding to the molecular absorption spectrum and atmospheric oxygen radiation) [59].
2. EFFECT OF TERAHERTZ RADIATION ON CELL LINES
Most experimental work studies the effect of terahertz radiation on human cells, in particular, blood cells and epithelial cells. Particular attention is paid to the effects of terahertz radiation on stem cells, and the stem cells of both humans and mice were considered as model objects [19, 20]. Some works are devoted to the effect of terahertz radiation on animal cells, in particular, isolated neurons of the mollusk Lymnaea stagnalis cultured outside the body [36] and rat C6 glioma cells [29].
The results of the study of the effects of terahertz radiation on particular cell lines with the specified parameters of radiation, irradiation regimes, and methods used to evaluate the effect of terahertz radiation are presented below.
2.1. Human Blood Cells
The effect of terahertz radiation and, in particular, its genotoxicity, on human blood cells (lymphocytes and peripheral blood leukocytes) was actively studied within the framework of the THz-BRIDGE project. The results are presented in [26, 30, 31, 61] and summarized in [9].
Lymphocytes play an essential role in the protective processes of the body. In addition, they can be easily obtained from peripheral blood. Therefore, lymphocytes were actively used to study the possible bioeffects caused by terahertz radiation. In [30, 31], a free-electron laser operating at frequencies of 0.12 and 0.13 THz was used as the radiation source. Irradiation of lymphocytes taken from nine donors for 20 min with radiation with an average power of 1 mW (power rate of 0.05 mW/cm2) and 0.6 mW (power rate of 0.03 mW/cm2) did not reveal any possible genotoxic effect (the frequency of micronuclei and the cell proliferation were estimated by calculating the proliferation index) [30]. Nevertheless, it is worth noting the small size of the used statistical sample (especially for experiments at a frequency of 0.13 THz), which does not allow us to make an unambiguous conclusion about the total absence of terahertz radiation effects at these parameters.
Similar studies were performed in [31] with peripheral blood leukocytes (samples of whole blood were studied, not samples divided into blood fractions) after their irradiation with radiation at frequencies of 0.12 and 0.13 THz. At the same time, neither damage to chromosomes nor changes in cell proliferation were recorded. The DNA comet assay, which provides information on primary DNA damage (which could not be detected with a micronucleus test in the case of DNA repair), also revealed no statistically significant differences between cells exposed to irradiation and control cells.
In contrast to the aforementioned studies, the existence of genomic instability as a result of exposure to continuous radiation with a frequency of 0.1 THz and a power density of 0.031 mW/cm2 is suggested in [26]. An increase in levels of aneuploidy (changes in the set of chromosomes) and asynchronous replication of the centromeres of certain chromosomes as a function of the time of irradiation was revealed.
The radiation of a gas laser at 81 µm (3.7 THz) was used in [62] to affect human erythrocytes and lymphocytes of human peripheral blood (the irradiation time was 30, 60, and 90 min). Unfortunately, the authors gave only the value of the radiation power (20 mW) in the paper without specifying the spot size, which makes it impossible to calculate the average radiation power density. It was shown that irradiation for 60 min did not affect spontaneous hemolysis of erythrocytes, but it reduced the stability of their cell membrane to hypo-osmotic stress and promoted the release of hemoglobin from erythrocytes. A decrease in the viability of some lymphocytes as a result of the exposure was noted (exact statistics are not given in percentages), and the enhancement of both spontaneous and stimulated cell division of the remaining lymphocytes was more pronounced, with longer exposure times (90 min). The authors also suggested that monocytes can have a significant effect on the lymphocyte response to submillimeter radiation and, based on the obtained data, it was possible to create diagnostic tests to identify the deficiency of the immune system and the presymptomatic stage of various diseases.
A study of the genotoxicity of terahertz radiation on peripheral blood leukocytes (taken from eight donors) but in a much wider frequency range (0.1–6.5 THz) was performed in [55]. The DNA-comet assay was used to assess the damage to and change in cellular DNA. Broadband pulsed terahertz radiation was generated by laser optical breakdown in the air (pulse repetition frequency of 1 kHz, duration of ~1 ps, and average power density of 0.008 mW/cm2). In addition, two other sources of terahertz radiation were used: photoconductive antennas generating radiation in the frequency range of 0.1–2 THz (75 MHz, duration 1 ps, average power density 0.125 mW/cm2) and 0.1–1 THz (82 MHz, duration 2 ps, average power density 0.2 mW/cm2), respectively. Irradiation of the cells for 20 min did not lead to direct DNA damage in any of the three experiments. It should nevertheless be understood that the terahertz radiation in these experiments was characterized by a low power rate, and the effect of radiation with higher intensity can potentially lead to entirely different results.
Data on the effect of radiation with a frequency of 2.52 THz on the cells of the Jurkat line (the line of human leukemic T-lymphocytes) are presented in [18, 21, 23]. In [18], the radiation of a gas laser at a frequency of 2.52 THz with a power density of 227 mW/cm2 was used to irradiate Jurkat cells for a predetermined time (5, 10, 20, 30, and 40 min). Evaluation of the viability of cells by the MTT test (colorimetric test to assess the metabolic activity of cells) 24 h after irradiation showed that, at exposure times of more than 20 min, the number of dead cells exponentially increased with exposure time. Thus, when the cells are irradiated for approximately 30 min, about 40% of the cells die, and irradiation for 40 min causes the death of almost 80% of the cells. Flow cytometry data also confirmed that cell death (by both apoptosis and necrosis) is observed at irradiation times of 30 and 40 min. The quantification was slightly different from the MTT test, and the number of dead cells was 62% for an irradiation time of 40 min (32% of the cells showed signs of early apoptosis and 30% showed signs of necrosis).
The radiation of a gas laser with a frequency of 2.52 THz but with a much higher power density than in [18] (636 mW/cm2), was also used to affect the Jurkat cells [17, 21, 23, 25]. The exposure time varied from 30 to 50 min in different works. According to experimental measurements and computer simulations, such an irradiation regime resulted in an increase in temperature on average by 6°C. After cellular irradiation, the expression of mRNA and microRNA expression was analyzed. The obtained data were compared with the data on mRNA and microRNA expression in control cells stored under hyperthermia conditions (control cells in the plate were placed in a water bath at 44°C for 40 min). It was shown that exposure to terahertz radiation results in a change in the expression of 532 mRNA (672 in the control sample) and 66 microRNA (53 in the control sample). The expression of some genes increased linearly with increasing irradiation time [17, 25]. The authors analyzed the signaling pathways that are activated in response to terahertz radiation and hyperthermia; an exhaustive list of data on the analysis of the total transcriptional response of mRNA and microRNA is presented in [23]. It was found that 18 key signal pathways associated with exposure to radiation were specific (unique) and differed from the 13 signaling pathways associated with cell hyperthermia, resulting in the assumption that terahertz radiation could cause biochemical and cellular lapses different from the response stimulated standard hyperthermia. It is concluded that terahertz radiation can potentially be a convenient, noncontact tool for selective control of particular genes and cellular processes.
2.2. Human Epithelial Cells
In studies of the effect of terahertz radiation, keratinocytes (cells of the outer layer of the skin (epidermis)) [15] and fibroblasts (cells of the middle layer of the skin (dermis)) [15, 16, 18] were selected as model epithelial cells. In addition, the study of terahertz radiation bioeffects was carried out on corneal epithelial cells (HCE-T) [39, 58] and retinal pigment epithelial cells (ARPE-19) [39] of the human eye. In [44] the authors studied the effect on a 3D sample of artificial human skin that recreated a typical tissue structure and consisted of epidermal keratinocytes and dermal fibroblasts rather than the effect on a cell line in a monolayer.
Studies of the effects of terahertz radiation on epithelial cells were carried out even within the THz-BRIDGE project. In particular, primary keratinocytes were exposed to radiation in the frequency range of 0.1–3 THz for 10–30 min from two different sources: one with a repetition rate of 80 MHz (pulse duration 20–30 ps, 0.2–3 THz) and an average output power of ~1 µW and another with a repetition rate of 250 kHz (0.1–2.7 THz) and an average output power of ~1 mW [63]. Due to the wide range of focal spot diameters of terahertz radiation (from 130 µm to 3.7 mm) and the insufficient description of the parameters of the effect, it is difficult to calculate the value of the average power density unambiguously. In review works the following values are reported: 10 [7] and 2.5 [9] mW/cm2. The activity or viability of cells and their ability for subsequent differentiation were assessed for 8 days after the terahertz effect. The resazurin reduction assay, which was used to assess cell viability, did not reveal any differences between irradiated and nonirradiated cells. Their ability to differentiate also remained normal throughout the observation period.
Dermal fibroblasts (HDFs) were exposed to short-term exposure (irradiation time 15 s, 1 and 2 min) to radiation with a frequency of 2.52 THz at a power density of 227 mW/cm2 [18]. Cellular analysis with a confocal scanning microscope revealed the presence of a positive signal (i.e., the staining of dead cells with fluorescent dyes with propidium iodide (PI) and annexin (FITC-AV)) when the cells were irradiated with terahertz radiation for more than 12 s. At shorter times, there was no fluorescence, which indicated cell viability. However, the authors assumed that the studied samples were in a small layer of the medium and that the drying of the medium under exposure to terahertz radiation could provoke cell death. The expression of genes that are activated by six primary cellular pathways for response to stress was tested 4 h after the cell irradiation. A slight increase in the expression of several genes for the irradiation time of both 1 min and 2 min was observed. Presumably, the main genes that respond to radiation are proinflammatory cytokines.
In [16, 24], dermal fibroblasts were exposed to terahertz radiation of the same frequency (2.52 THz) but a smaller power density (84.8 mW/cm2) and for a longer time (5–80 min). Cell viability after irradiation was assessed by the MTT test. In addition, the quantitative method of polymerase chain reaction (PCR) was used to analyze possible protein damage (the expression of heat shock proteins (HSPs), the CCNE2 gene, and other genes associated with DNA repair was estimated). The obtained data were compared with the cellular response to elevated temperature (hyperthermia) and ultraviolet radiation. For a given irradiation regime with terahertz radiation, the temperature increase was on average 3°C. The percentage of viable cells (>90%) and the level of expression of heat shock proteins (a 2.5- to 3.2-fold increase in expression) are similar in the experimental group subjected to irradiation and the group kept under higher temperature (40°C). In these two groups, there was no increased expression of genes associated with DNA repair (in contrast to the experimental group of cells exposed to ultraviolet radiation, where there was a 40-fold increase in the expression of cyclin CCNE2). In conclusion, the authors assumed that radiation with a frequency of 2.52 THz under the selected regimes and parameters of irradiation has mainly thermal effects on human dermal fibroblasts, comparable to the cellular and molecular effects that arise under conditions of hyperthermic stress. The possibility of direct damage to intracellular proteins exposed to terahertz radiation has not been confirmed.
The effect of long-term exposure (2–24 h) of radiation with a frequency of 0.106 THz on dermal fibroblasts and keratinocytes (HaCaT) was studied in [64]. DNA migration (the DNA-comet assay) was evaluated to detect single- and double-stranded DNA breaks as a result of cell irradiation. DNA damage at the chromosome level was assessed by a micronucleus test. For both fibroblasts and keratinocytes, the comet assay and the micronucleus test did not reveal statistically significant changes corresponding to DNA chain breaks or chromosome damage in cells irradiated for both 2 and 8 h by terahertz radiation with mean power densities of 0.04, 0.39, or 0.88 mW/cm2. Similarly, there was no evidence of a change in proliferation of irradiated cells as compared to control cells. An increase in the average power density up to 2 mW/cm2 and the irradiation time up to 24 h also did not lead to a change in the micronucleus frequency and proliferation indices in cells of these types.
In order to assess the genotoxicity of terahertz radiation at frequencies of 0.38 and 2.52 THz (with a power density of up to 0.88 mW/cm2 when HDF and HaCaT are irradiated for 2 or 8 h), similar methods were used in [15]: DNA-comet assay, calculation of the proliferation index, and the micronuclear test. No DNA damage (strand rupture or chromosome damage) or changes in proliferation were detected in any of the numerous experimental groups.
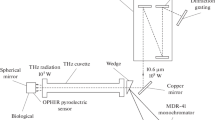
The effect of continuous terahertz radiation (at frequencies 1.4, 2.52, and 3.11 THz) on human keratinocytes of the HEK001 line upon irradiation for 20 min was examined in [22]. Calculation of the radiation power density according to the parameters specified in the article (the power of the source is 20 mW, the size of the focusing spot is 3.5 mm) gives a value of ~207 mW/cm2, which does not correspond to the power density values declared by the authors of 44.2 and 52 mW/cm2. Cell viability was evaluated by the MTT test 4 h after irradiation and did not reveal any changes in comparison with control cells not exposed to terahertz radiation. At the same time, analysis of the mRNA expression revealed a significant number of over- and under expressed genes: 451, 448, and 583 when exposed to terahertz radiation at 1.4, 2.52, and 3.11 THz, respectively. No changes in the expression of the so-called hyperthermal genes were observed in any of the experimental groups. Most of the over- and under expressed genes were unique for each particular frequency regime of exposure, as well as the key signaling pathways. Thus, the authors suggested that the biochemical and cellular responses may differ when exposed to terahertz radiation of different frequencies.
A study of cell morphology and the proliferation of corneal epithelial cells (HCE-T) and retinal pigment epithelium (ARPE-19) of the human eye after exposure to potent terahertz radiation was carried out in [39]. A linear accelerator ALICE (United Kingdom) served as the source of broadband coherent terahertz radiation (with frequency up to ~0.5 THz). The radiation was characterized by a high peak and low average power, which accordingly increased the probability of its “delivery” through a highly absorbing aquatic environment to the studied biological objects while minimizing the possible thermal effects. Analysis of the morphology and proliferation of ARPE-19 cells after 3 and 6 h of irradiation with terahertz radiation with an average power density of 0.14–0.18 mW/cm2 revealed no differences from the control cells. Similarly, the absence of cytotoxicity and changes in morphology and proliferation were obtained for HCE-T cells irradiated for 4 h and 5 h 20 min with terahertz radiation with an average power density of 0.32–0.37 mW/cm2. The longer exposure (for 24 h) of radiation with a frequency of 0.12 THz and a power density of 5 mW/cm2 on human corneal epithelial cells (HCE-T) also did not cause changes in the morphology or frequency of micronucleus formation, nor did it lead to an increase in expression of heat shock proteins (Hsp27, Hsp70, Hsp90α) [58].
A comprehensive study of the possible bioeffects of terahertz radiation on human fetal fibroblasts (HFFF2) using a full arsenal of modern methods of analysis was carried out in [33]. The source of radiation in the frequency range 0.1–0.15 THz was a compact free-electron laser. The exposure was carried out for 20 min with radiation at a power density of 0.40 mW/cm2. It should be noted that most of the used methods did not reveal any changes in the exposed cells as compared to the control cells. The comet assay applied 2 and 24 h after the exposure revealed no DNA damage as compared to the control cells, just as Western blotting did not reveal changes in the expression of specific proteins (heat shock proteins, cytoskeleton proteins, proteins involved in apoptosis, etc.). Similarly, analysis of the phosphorylated form of histone H2AX, conducted 30 min, 2 h, and 24 h after exposure, revealed no difference between irradiated cells and control cells. In the analysis of cell morphology1 h after exposure, some differences were detected from the control cells, which turned out to be temporary and were not detected in reexamination after 48 h. At the same time, the micronuclear test showed a significant increase in micronuclei in the experimental groups as compared to the control ones. It was found that terahertz radiation could cause chromosome loss, i.e., induce aneuploidy. Increased polymerization of actin (which is involved in the morphogenesis and orientation of the fission spindle), as shown by ultrastructural analysis on an electron microscope, is considered by the authors to be a possible cause of the observed loss of chromosomes.
The fetal fibroblasts examined by the authors [33] are a convenient model, because these cells are more sensitive to genotoxic factors than the fibroblasts obtained from an adult organism. Nevertheless, the probability of terahertz radiation exposure to fetal cells is incredibly small, because the depth of radiation penetration in tissues, as a rule, does not exceed several millimeters. At the same time, the probability of exposure of adult skin (dermal fibroblasts, in particular) to terahertz radiation is quite high due to the vast spread of terahertz technologies (e.g., among the military, security control personnel, patients undergoing terahertz treatment). Therefore, a logical continuation of the work [33] was a recently published study [65], in which dermal fibroblasts of an adult human were selected as the object of terahertz effect. The radiation source, irradiation regime (frequency range 0.1–0.15 THz, irradiation time 20 min, average power density 0.40 mW/cm2), and methods for diagnostics of terahertz radiation bioeffects were exactly similar to those described in [33]. The analysis did not reveal the induction of DNA damage as a result of radiation. However, a significant increase in the frequency of centromere-positive micronuclei and cases of nondisjunction of chromosomes was detected, which indicated the induction of aneuploidy. Thus, it was demonstrated that exposure to terahertz radiation can affect the integrity of the genome by means of aneugenic effects (chromosome lag in anaphase), rather than by direct rupture of DNA strands.
Significant results were obtained in [44–46] under the action of intense pulses of terahertz radiation of picosecond duration on samples of artificial skin. Data for immunochemical analysis of histone H2AX, the phosphorylation of which is one of the most characteristic cellular responses to DNA double-strand break, confirmed the presence of DNA damage due to terahertz radiation. Irradiation of the samples was carried out for 10 min at two different values of the energy of terahertz pulses: 1 and 0.1 µJ. This corresponds to an average power density of 57 mW/cm2 in the former case and 5.7 mW/cm2 in the latter case. Significant induction of H2AX phosphorylation was detected in both cases. To determine the possible effectiveness of the repair of DNA damaged by terahertz effect, an analysis of the expression level of specific proteins was performed with Western blotting. An increase in the expression of tumor suppressor proteins and DNA repair (p53, p21, p16, p27, and KU70) was found, which indicates simultaneous activation of repair mechanisms for injuries under the terahertz effect. In general, the effect of terahertz pulses with an energy of 1 µJ and 0.1 µJ for 10 min on artificial skin samples led to a change in the expression of 442 genes and 397 genes, respectively, as noted in [46], and 622 and 345 genes respectively according to data [45]. Most of the genes having a change in expression observed in both the former and latter modes of irradiation belong to the epidermal differentiation complex in the chromosomal region 1q21. Increased expression of these genes is often observed in diseases such as psoriasis and skin cancer. Under exposure to terahertz radiation, the levels of expression of these genes, conversely, decreased, which allowed the authors to presume the potential use of terahertz radiation for therapeutic purposes for the directed regulation of the expression of a multitude of genes responsible for inflammatory and oncological skin diseases.
Research on the effect of terahertz radiation on human skin fibroblasts is still being carried out today. In addition to a recently published work [65], [56] studies the nonthermal effects of terahertz radiation on human skin fibroblasts. A distinctive feature of the study was the use of low-power continuous radiation (~10 µW at 0.1 THz, 3 µW at 0.3 THz) for long-term cell irradiation (for 3, 70, and 94 h). A uni-traveling-carrier photodiode (UTC-PD) was used as the source of terahertz radiation, generating radiation in the range of 0.07–0.3 THz with the possibility of wide frequency tuning with a step of 1 GHz. An evaluation of cell proliferation by a method based on the measurement of electrical impedance did not reveal differences between cells exposed to radiation for 94 h and control cells. A colorimetric method based on new tetrazolium compounds also showed neither change in cell activity as compared to the control nor cytotoxicity of the radiation when exposed, respectively, for 70 and 3 h.
2.3. Stem Cells
To date, there are only a few studies that have examined the effects of terahertz radiation on stem cells, with both human [34, 39] and mice [19, 20] stem cells selected as model cells.
It is known that embryonic stem cells are susceptible to various physical and chemical stimuli. In this case, the most likely response to such effects may be a change in cell differentiation. Thus, the differentiation of embryonic stem cells hES07 was estimated (by morphology and staining with Oct4 and Nanog markers) after exposure to broadband coherent terahertz radiation (frequency up to 0.5 THz, average power density 0.14 mW/cm2) for 3 h [39]. No differences between the cells of the experimental and control groups were revealed. There were also no changes in the cellular adhesion and cytoskeleton structure of cells attached to the substrate after their irradiation with terahertz radiation (with an average power density of 0.02–0.04 mW/cm2) for 2 or 4 h as compared to the control group. Cell proliferation (estimated by staining with bromodeoxyuridine (BrdU)) after 2 h of exposure to radiation with a similar power density also did not differ from the nonirradiated control cells.
An elaborate study of the effect of narrowband terahertz radiation (2.3 THz) on hESM01 human embryonic stem cells was carried out [34]. The exposure time was 1 h, and the average radiation power density was 0.14 W/cm2. Morphological analysis (after 16 and 20 h after exposure to radiation) and cytogenetic analysis of structural chromosome aberrations revealed no deviations from the control cells, nor was there any spontaneous cell differentiation or cell cycle arrest. The presence of phosphorylated histone H2AX was analyzed 2 h after terahertz exposure and did not confirm the formation of double-stranded DNA breaks. At the same time, data were obtained on the change in the expression of 73 genes as a result of irradiation of cells compared to nonirradiated cells. However, in percentage terms, the change in expression was observed in less than 1% of the total number of analyzed genes.
More pronounced effects were revealed in a series of works [19, 20, 60] under the action of terahertz radiation on the mesenchymal stem mouse cells. Two sources of radiation were used: pulsed broadband radiation (0.1–20 THz with a maximum intensity of 10 THz) and continuous radiation (2.52 THz). Morphological analysis with the help of light microscopy showed the presence of inclusions similar to lipid bodies after cell irradiation with broadband terahertz radiation with a power density of the order of 1 mW/cm2. Upon irradiation for 9 h, there were much more such inclusions than during the 2 h of cell exposure of terahertz radiation. Thus, it was shown that the appearance of morphological changes depends on the frequency of radiation and the time of exposure. Analysis of the gene expression revealed the activation and suppression of some genes as a result of terahertz radiation. Activation of heat shock proteins (Hsp90, Hsp105, and CRP) under the effect of broadband radiation for 9 h was not detected; this was confirmed by nonthermal mechanisms of terahertz radiation effect, which caused a change in gene expression.
2.4. Animal Cells and Hybrid Cell Lines
An experimental analysis of the effect of terahertz radiation on the transmembrane transport in cellular systems on the example of isolated neurons of the mollusk Lymnaea Stagnalis cultivated outside of the body was carried out in [36]. The cells were exposed to the terahertz radiation of a free-electron laser with wavelengths of 130 µm (2.3 THz) and 150 µm (2 THz). The average power density varied in the range of 0.5–20 mW/cm2; the irradiation time was 10 s. The functional state of the cells after exposure was assessed by the membrane potential of the neurons, while the viability and integrity of the membrane were determined with Trypan Blue and a BCECF-AM fluorescent dye. It was found that radiation with a frequency of 2.3 THz (in contrast to radiation with a frequency of 2 THz) can cause reversible violations of the barrier properties of the neuron membrane. The authors suggested that radiation in the terahertz range can cause a discontinuity of the phospholipid bilayer (the basis of cell membranes) with the formation of structural defects similar to through hydrophilic pores.
The cytotoxic effect with relatively short-term exposure (1–3 min) to continuous terahertz radiation (0.12–0.18 THz) with a power density of 3.2 mW/cm2 on rat glioma cells was detected in [29]. The authors evaluated the change in the membrane potential of mitochondria by flow cytometry. For this purpose, two fluorescent dyes were used: TMRM, accumulating mainly on the inner mitochondrial membrane, and a DNA-binding dye DRAQ7. It was shown that, as a result of cell exposure to terahertz radiation for 1 min, the number of apoptotic cells increases by 1.5 times as compared with the initial value; under irradiation for 5 min, this index increases by 2.4 times. Thus, the work demonstrated a dose-dependent cytotoxic effect.
Hintzsche et al. [28] used the AL cell line to study effects under the action of radiation with a frequency of 0.106 THz, because this line was previously widely used in studying fission spindle disturbances upon exposure to ionizing radiation. The cells of this line essentially contain a standard set of chromosomes of Chinese hamster ovary-K1 cells and one 11th human chromosome. After analyzing 6365 mitotic cells, the authors concluded that the exposure of AL cells to terahertz radiation with a power density of 0.043–4.3 mW/cm2 leads to statistically significant violations of the fission spindle in anaphase and telophase. At the same time, in contrast to the detected functional changes in chromosomes in irradiated cells, no changes in the structural organization of chromosomes were detected.
In contrast to the studies evaluating the potential adverse effect of terahertz radiation on living biosystems, the effect of stimulated cell growth of the sensory ganglion neurites of chick embryos under the action of broadband pulsed terahertz radiation (0.05–2 THz, pulse duration 2.5 ps, irradiation time 3 min) was observed. The radiation power density varied in the range of 0.5–60 µW/cm2. It is shown that the stimulating effect is maximally manifested at a relatively low power density and is dose-dependent. However, the power density values at which this effect was observed differed in [47] and [49] and amounted to 0.5 and 5 µW/cm2, respectively.
The experimental setup described in [47] and [49] was also used in [48] to study the effect of terahertz radiation on thymocytes and splenocytes of the CBA mouse. The cell viability after irradiation was estimated by the fluorescence intensity of special dyes that bind to DNA. In addition, comparative analysis of the cell (irradiated and unirradiated) distribution on the phases of the cell cycle was carried out. With the selected parameters of terahertz radiation exposure (power density up to 10 µW/cm2, irradiation time 1 min), cell viability and cell phase distribution did not differ in control cells and cells exposed to terahertz radiation. Similarly, no changes were observed in A-549 (human lung carcinoma cells), BT-20 (mammary adenocarcinoma cells), or COLO 320 HSR (sigmoid colon carcinoma cells) cell lines or in cell suspension cultures HMC-1, U937, and HL-60.
3. EFFECT OF TERAHERTZ RADIATION ON COMPLEX BIOLOGICAL SYSTEMS: MICROORGANISMS AND ANIMALS
In most studies, cells of different cell lines were used as model objects to study the effect of terahertz radiation. Nevertheless, there are a number of studies in which the work was carried out directly on living organisms. The studies on this topic carried out before 2010 are described in [9] (in particular, the effect of radiation with a frequency of 3.6 THz at a power density of ~5 mW/cm2 and irradiation time of τ = 5 and 30 min on the behavioral characteristics of male C57Bl/6J mice [66], the effect of radiation with a frequency of 0.15 THz on changes in the functional activity of platelets in white rats [67], and the intensity of lipid peroxidation processes and antioxidant properties of blood in white rats under stress [68]); therefore, the results published after 2011 are presented in this section.
In [50–52], broadband (0.1–2.2 THz) pulsed (duration ~1 ps) terahertz radiation was used for a 3‑min exposure of females of the fruit fly Drosophila melanogaster that were preliminarily placed under stress conditions (lack of food and limited space). The purpose of the first work was to study the sensitivity of mature and immatureoocytes to the effects of terahertz radiation and the effect on the number and dynamics of development of F1 progeny [50]. It was found that only mature eggs are sensitive to radiation, which is manifested by a change in the dynamics of development of male individuals in the offspring in comparison with the control and a decrease in the number of males that reach the imago stage (adult stage of insect development). The dynamics of the development of female individuals in the offspring at the same time was comparable to control. An absence of changes in comparison with the control was also observed for the offspring of both male and female flies, which were affected when the eggs were immature. Later works [51, 52] studied the viability and life expectancy of both male and female individuals that underwent terahertz radiation exposure under stress conditions. It was found that, under stressful conditions, this effect significantly increased their viability in the second half of the adult cycle. The period of acute mortality characteristic for flies of this species came 9 days later than in flies from the control group. For males, a positive effect on viability was observed from exposure to terahertz radiation during a monotonous decline in the number of individuals, and an adverse effect was observed with a relatively stable number of individuals. Thus, sex differences were demonstrated in the response (sensitivity) to the action of terahertz radiation. The authors proposed the presence of indirect terahertz radiation exposure to gene expression and signaling pathways responsible for the viability and lifespan of flies. The long-term effects of terahertz radiation (in particular, on the dynamics of maturation of individuals in the first generation), as well as the presence or absence of effects, with respect to the degree of maturity of the fly oocytes were not detected at the time of irradiation .
In [37], bacteria Escherichia coli (E. coli) with biosensor constructs (pKatG-gfp) were exposed to radiation with a frequency of 1.5, 2, and 2.3 THz and a power density of 1.4 W/cm2. It was found that exposure to these wavelengths for 15 min induces a stress-sensitive promoter of the catalase gene for a long time after irradiation (GFP expression was observed in more than eight cell generations). When irradiated for 10 min, the expression is unstable, and it is completely absent at an exposure time of 5 min. The E. coli/ pCopA-GFP and E. coli/pEmrR-GFP genosensors were also later examined [69]. It was found that the systems for controlling oxidative stress and maintaining the homeostasis of transition metals were among the first to respond to the nonthermal effect of terahertz radiation, while the control system of the resistance of E. coli cells to the presence of antibiotics did not react to radiation. In this case, the induction of the genosensor response has a threshold character according to the absorbed dose and is weakly dependent on the wavelength of terahertz radiation.
In studies of the same scientific group dating back to 2016 [35] in which bacteria Escherichia coli and Salmonella typhimurium were selected as a model, there was no mutagenic or genotoxic effect of radiation with a frequency of 2.3 THz and a power density of 1.4 W/cm2 at similar irradiation times, i.e., 5, 10, and 15 min. The Ames test was used to evaluate the mutagenicity potential of the radiation, the SOS chromotest was used to estimate genotoxicity. The authors reported cell growth (1.3 times higher than for the control) as a result of exposure to radiation for 15 min and an increase in the activity of two enzymes (Alkaline phosphatase and β-Galactosidase) [35]. Thus, despite the absence of genotoxicity and mutagenicity, and even the possible reduction of the effects of known mutagens (for example, 4-NQO) due to exposure, the effect of terahertz radiation on cellular metabolism and DNA–protein interactions is assumed.
Archaea Halorubrum saccharovorum was another microorganism for the study of the effect terahertz radiation with a frequency of 2.3 THz [38]; a free-electron laser setup (Novosibirsk) was used, the same as in [35, 37]. Unlike the previously discussed E. coli, which is an obligate anaerobe that inhabits the intestines of mammals, archaea H. saccharovorum is a free-living extremophile. The authors identified the proteins that altered the expression level as a result of nonthermal exposure to terahertz radiation with a power density of 0.8 W/cm2 for 5 h. Sixteen protein fractions with a more than 1.5-fold difference in protein concentration were detected. Comparison of the results of the proteome response analysis to the effect of radiation showed that the reaction of archeal cells was due to the regulation of protein synthesis and membrane processes, while a systemic response is observed in E. coli cells and was associated primarily with oxidative stress. The authors attributed such different reactions of the two microorganisms to the effect of terahertz radiation by the difference in the ecological niches they occupy and their means of adapting to them.
The results of experimental studies performed in vivo by direct effect of terahertz radiation on the skin of mice (male C57BL/6J and BALB/c nude lines) are presented in [43]. Specific areas of the skin were exposed to broadband (up to ~2.5 THz) terahertz radiation of femtosecond duration (310 fs) with an average power density of 0.32 µW/cm2 for 1 h. Complex bioinformation and functional analysis was carried out to identify changes in gene expression. The expression of 149 genes (up-regulation was observed in 82 genes, and down-regulation was seen in 67 genes) was different from the expression of the control sample genes. It was found that the body’s response to the nonthermal action of terahertz radiation was not similar to responses to burns or the effect of UV or neutron radiation, but it was similar to the response to the wound process: activation of the transforming growth factor TGF-β was observed. It was shown that the regular exposure of terahertz radiation to a wound model in vivo for 10 days resulted in a slowing the healing processes as compared to control samples that were not exposed.
4. EFFECT OF LOW-INTENSITY RADIATION IN THE MILLIMETER RANGE (0.129–0.150 THz) ON ANIMALS AND HUMANS
A separate line of research actively developed by Russian scientists is associated with the use of low-intensity radiation in the millimeter range, which includes part of the terahertz range. The frequency spectra of radiation and absorption of the most important active cellular metabolites (in particular, NO, O2, etc.) are mainly concentrated in the low-frequency part of the terahertz range (~0.12–0.15 THz). The control of reactivity with the help of radiation of this frequency range enables regulation of the metabolism in the biosphere. In studies on this subject, exposure was applied to either animals (rats) or, for therapeutic purposes, directly to humans for the treatment of various diseases.
Kirichuk et al. [70, 71] obtained data on the effect of terahertz radiation (on the frequencies of the molecular spectrum of radiation and absorption of nitric oxide ~0.15 THz) on the functional activity of the thyroid gland and the parameters of gas and electrolyte blood composition of white rats in a state of acute immobilization stress. Under the effect of radiation (the power density of radiation falling on a skin area of animals of approximately 3 cm2 in size was 0.2 mW/cm2) for 30 min applied under stress conditions, the functional activity of the thyroid gland was fully normalized and the parameters of gas and electrolyte composition of blood were completely restored. Thirty minutes of exposure to radiation at a frequency of 0.129 THz (the frequency of the molecular spectrum of radiation and absorption of atmospheric oxygen) with a power density of 0.1 mW/cm2 has a positive effect on the recovery of disturbances in the state of immobilization stress in the hemocoagulation and fibrinolytic activity of blood [59]. It is not possible to cover all the works of this research team within the framework of this review; more detailed data can be found in [72].
The spectrum of diseases for which the methods of terahertz therapy has been proposed for treatment and prevention is quite broad and includes venous thrombosis in traumatological patients (with fractures of the lower limb), deforming arthrosis, alcoholic polyneuropathy, angina pectoris, various eye diseases, etc. For example, the use of terahertz therapy (an Orbita medical device) significantly increased visual acuity and eliminated functional scotoma in pediatric patients (3–9 years old) and increased visual acuity and electrophysiology indicators (rhythm and overall ERG) in patients with age-related macular degeneration [73, 74]. The exposure was focused on biologically active points of the skin with radiation at a frequency of 0.129 THz (data on the radiation power of 100 µW are given only in [73], the total irradiation time for biologically active points averaged 6–15 min), which corresponds to the frequency of the molecular spectrum of radiation and absorption of atmospheric oxygen.
5. DISCUSSION
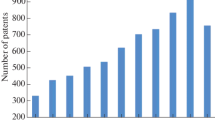
In total, more than 50 papers on the effect of terahertz radiation on biological objects are analyzed in this review. Most of them were published between 2011 and 2018 and were not covered in reviews [7, 9]. Figure 1 shows the publication activity since the first works on this subject appeared (in the 1960s) to the present. The data on the number of publications for 1960–2009, which are presented in [9], are supplemented with new data on the works published in the period from 2010 to early 2018. The emergence of new effective sources and receivers of terahertz radiation and the subsequent widespread use of terahertz technologies in a variety of spheres of human activity stimulated interest in the safety of this type of radiation. It is seen in the diagram that the overwhelming majority of publications fall in the last two decades, and, as compared to 2000–2009, the number of publications in 2010–2018 has grown by more than a half.
The arsenal of terahertz radiation sources used to study its effect on biological objects is incredibly broad: from gas lasers and BWOs to sources based on laser radiation and free-electron lasers. Gas lasers and free-electron lasers are widely used in the studies (Fig. 2a). Thus, the most considerable amount of research is based on sources falling in the frequency range of 1–4 THz (Fig. 2b).
Distribution of publications on (a) the types of terahertz radiation sources and (b) the ranges of operating frequencies: (1) gas laser, (2) free-electron laser, (3) accelerator, (4) BWO, (5) optical rectification of laser radiation, (6) photoconductive antenna/photomixer, (7) HF generator, (8) optical breakdown in the gas medium, and (9) Gunn diode.
This accounts for the most significant share of sources operating in the frequency range of 1–4 THz (Fig. 2b). It should be noted that the spectrum of broadband sources of terahertz radiation (based on optical rectification and optical breakdown) is much broader and can reach tens of terahertz.
The ranges of 0.1–0.149, 0.15–0.999, and 1–4 THz in Fig. 2b include data on narrowband sources, the operating frequency of which falls into a given range, while broadband radiation sources are allocated to a separate, eponymous sector.
Figure 3 shows the data on the characteristic parameters of the effect, in particular, the radiation power density and the irradiation time. As a rule, the average power density exceeds 100 mW/cm2 when free-electron lasers are used in studies. Gas lasers also make a significant contribution to the range of values of both 2.1–200 and 201–2000 mW/cm2. A fifth (20%) of studies with a value of I < 0.2 mW/cm2 account for either sources based on the optical rectification method (low pulse frequency) or photoconductive antennas/photomixers. The distribution of publications on the values of the irradiation times of the studied biological objects by terahertz radiation is close to uniform (Fig. 3b): in 28% of the studies, the impact was relatively short-term (up to 9 min); in 40%, the irradiation lasted 10–60 min; 28% of the objects were exposed to prolonged exposure (1–24 h).
It should be noted that, as a result of the continuous increase in the number of publications in the last two decades, considerable experience has been accumulated to date in the formulation of experiments on the effect of terahertz radiation on living biological systems that take into account the characteristics of this type of radiation. According to [7], the absorption coefficients of water and skin cells are commensurable, and the depth of the penetration of terahertz radiation into the tissue is several hundreds of micrometers in the low-frequency range and decreases to ~50 µm with increasing frequency. With this in mind, the researchers were faced with the task of assuring the reliable delivery of terahertz radiation to the studied objects in aqueous media with strong absorption in the terahertz range and accurate control of the radiation dose. To solve this problem, special agarose substrates with a minimum absorption in a given frequency range [55] and flow cells [38] were proposed; they ensure a laminar flow of a liquid layer several tens of micrometers thick perpendicular to the direction of radiation propagation. In the study of the effects of long-term exposure to terahertz radiation on cells (within a few hours), specialized systems for delivering radiation directly to the incubator were developed; the optimal conditions for cell maintenance were set [15, 16, 18, 22, 26, 58]. In the discussed experimental studies, terahertz radiation affected living objects located at different levels of organization: cellular, subcellular, tissue, and even organismic. Figure 4 shows the distribution of works by the types of objects undergoing the effect of terahertz radiation.
The main types of cells exposed to terahertz radiation are blood cells, epithelial cells, and stem cells. Summarizing the available data, it can be said that, in most of the early studies (mainly until 2008) conducted on blood cells, no adverse effects were reported from the action of terahertz radiation. Nevertheless, later studies obtained data showing that the aneuploidy level increased under certain parameters of exposure to terahertz radiation [26], the cell viability was reduced [18, 62], and the cell membrane became less resistant to specific external factors [62]. At the same time, the DNA comet assay used to assess damage and change in cellular DNA confirmed that DNA was damaged as a result of terahertz effect in only one of three studies [31, 55]. An interesting result of [21, 23] was the conclusion that the response of the cells of the Jurkat line to terahertz radiation differed from the response stimulated by standard hyperthermia. It was also noted that terahertz radiation could potentially be a convenient noncontact instrument for the selective control of specific genes and cellular processes.
The study of the effect of terahertz radiation on epithelial cells is also of great interest. Taking into account the strong absorption of radiation by water, the depth of its penetration of the tissues of the organism is limited mainly by the outer and middle layers of the skin. Keratinocytes, dermal fibroblasts, corneal epithelial cells, and retinal pigment epithelial cells were examined as model cell lines. In some cases, terahertz radiation had only thermal effects on dermal fibroblasts [16] that were comparable to the cellular and molecular effects that occur under hyperthermic stress conditions. In [39, 58, 63, 64], there were no statistically significant changes in key indicators (morphology, proliferation, etc.) in irradiated cells as compared to nonirradiated cells; also, there was no direct DNA damage [15, 33, 64]. The results of some studies confirmed the presence of potentially dangerous effects from terahertz radiation on epithelial cells; in particular, a significant number of up- and downregulated genes [22] and induction of aneuploidy [33, 65] after irradiation were detected. After terahertz radiation, intense induction of the picosecond duration of the artificial skin samples [44] and significant induction of phosphorylation of H2AX were detected, which indicated possible DNA damage. Simultaneous activation of the mechanisms of damage repair was also revealed.
Although embryonic stem cells are considered very sensitive to various physical and chemical stimuli, the effect of terahertz radiation does not lead to significant changes in the proliferation, differentiation, cell adhesion, or cytoskeleton structures in them as compared to nonirradiated cells [34, 39]. Analysis of the gene expression showed an expression change in less than 1% of the total number of analyzed genes. At the same time, the morphological analysis performed after the exposure of mesenchymal stem mouse cells to terahertz radiation [19, 20, 60] revealed differences between the irradiated (the presence of inclusions, similar to the lipid bodies) and nonirradiated cells, the appearance of which depended on the radiation frequency and impact time. Analysis of the gene expression revealed the activation and suppression of certain genes as a result of terahertz exposure, and it was shown that the mechanism of this exposure, which lead to a change in gene expression, was nonthermal.
In addition to the mesenchymal stem mouse cells, the effect of terahertz radiation on other animal cells was also studied. For example, it was found that radiation with a frequency of 2.3 THz can cause reversible disruption of the barrier properties of the neuron membrane of mollusk Lymnaea stagnalis [36]. A cytotoxic effect with relatively short-term exposure (1–3 min) to continuous terahertz radiation (0.12–0.18 THz) with a power density of 3.2 mW/cm2 on rat glioma cells was detected in [29]. The effect of radiation with a frequency of 0.106 THz and a power density in the range of 0.043–4.3 mW/cm2 on the hybrid cell line AL does not cause structural changes in the organization of the chromosome but leads to functional changes (they caused a statistically significant breach in anaphase spindle and telophase [28]).
There are some works that studied the effect of terahertz radiation directly on microorganisms and animals: from bacteria and archaea to fruit flies and mice. The sensitivity of mature oocytes of flies Drosophila melanogaster to broadband (0.1–2.2 THz) pulsed terahertz radiation was observed [50], and sex differences in response to radiation (increased viability in the later adult half cycle was observed only in females after irradiation) were found [51]. The induction of the stress-sensitive promoter of the catalase gene in response to terahertz radiation in bacterium E. coli with genosensory constructs (pKatG-gfp) was shown in [37]. The systems that reacted first to terahertz exposure were analyzed, and differences were shown in the responses of the cells of archаea Halorubrum saccharovorum and bacteria E. coli [69, 38]. There is a lack of genotoxicity and mutagenicity for radiation with a frequency of 2.3 THz and a power density of 1.4 W/cm2 in the bacteria Escherichia coli and Salmonella typhimurium, while terahertz radiation can affect the cell metabolism and DNA–protein interactions [35]. A change in the expression of 149 genes was detected as a result of the direct action of broadband terahertz radiation on mouse skin (in vivo), and it was shown that the response of the organism to nonthermal effects was similar to the response to the presence of a wound process [43].
CONCLUSIONS
Information on the biological effects of terahertz radiation is of the utmost importance for the assessment of the potential harm to human health and the development of modern safety standards. Although the issue of terahertz radiation safety has been actively studied for the last two decades, it is still not possible to make an unambiguous conclusion as to whether this type of radiation represents a hazard or to determine the threshold dose at which negative effects appear.
Nevertheless, one can not detract from the fact that, by now, the world scientific community has succeeded in obtaining a significant amount of data on the possible effects of terahertz radiation. It is often possible to identify not only the potential danger from its impact but also to receive the opposite results, in particular, to observe the stimulation of cell growth of neurites of sensory ganglia or the therapeutic effect of the radiation of the low-power, low-frequency region (0.129–0.150 THz) in the terahertz range. The potential for the use of powerful pulses of terahertz radiation to normalize the expression of genes associated with inflammatory and oncologic skin diseases is of particular interest.
Based on the results of the analysis of the research, it is impossible to make an unambiguous conclusion due to the wide range of exposure parameters. It should also be noted that the share of studies in which effects were observed as a result of exposure increased as compared to the studies performed before 2011. This is partly due to the use of more powerful sources of terahertz radiation and, in part, to the integrated application of more accurate and modern methods for the diagnosis of the presence/absence of the effect of exposure.
The development of technology and the emergence of new powerful sources of terahertz radiation stimulate a new round of research on the effects of radiation on biological objects of different levels. We believe that studies involving sources with high peak power and low average power are of particular interest, since this ensures the delivery of radiation to the studied biological objects through the aqueous medium and minimizes the possible thermal effects of the impact.
REFERENCES
Brun, M.A., Formanek, F., Yasuda, A., Sekine, M., Ando, N., and Eishii, Y., Phys. Med. Biol., 2010, vol. 55, p. 4615.
Davies, A.G., Linfield, E.H., and Johnston, M.B., Phys. Med. Biol., 2002, vol. 47, p. 3679.
Siegel, P.H., IEEE Trans. Terahertz Sci. Technol., 2013, vol. 3, p. 229.
Ashworth, P.C., Pickwell-MacPherson, E., Provenzano, E., Pinder, S.E., Purushotham, A.D., Pepper, M., et al., Opt. Express, 2009, vol. 17, p. 12444.
Fitzgerald, A.J., Berry, E., Zinovev, N.N., Walker, G.C., Smith, M.A., and Chamberlain, J.M., Phys. Med. Biol., 2002, vol. 47, p. 67.
Woodward, R.M., Wallace, V.P., Cole, B.E., Pye, R.J., Arnone, D.D., Linfield, E.H., et al., in Proc. SPIE, Cohn, G.E., Ed., 2002, p. 160.
Wilmink, G.J. and Grundt, J.E., J. Infrared, Millimeter, Terahertz Waves, 2011, vol. 32, p. 1074.
THzBRIDGE Report, 2004. http://www.frascati.enea.it/ THz-BRIDGE/reports/Project_Summary_final.pdf.
Hintzsche, H. and Stopper, H., Crit. Rev. Environ. Sci. Technol., 2012, vol. 42, p. 2408.
Webb, S.J. and Dodds, D.D., Nature, 1968, vol. 218, p. 374.
Zalyubovskaya, N.P., Kiseliov, R.I., Valitov, R.A., Kitchenko, A.V., and Teslenko-Ponomarenko, E.F., in Problemy ehksperimental’noi i klinicheskoi radiologii (Problems in Experimental and Clinical Radiology), Kiev, 1970, vol. 6, p. 202.
Hafez, H.A., Chai, X., Ibrahim, A., Mondal, S., Férachou, D., Ropagnol, X., et al., J. Opt. (Bristol, U. K.), 2016, vol. 18, 093004.
Yin, X. Ng, B.W.-H., and Abbott, D., Terahertz Imaging for Biomedical Applications, New York: Springer, 2012.
Agranat, M.B., Il’ina, I.V., and Sitnikov, D.S., High Temp., 2017, vol. 55, no. 6, p. 922.
Hintzsche, H., Jastrow, C., Heinen, B., Baaske, K., Kleine-Ostmann, T., Schwerdtfeger, M., et al., Radiat. Res., 2013, vol. 179, p. 38.
Wilmink, G.J., Rivest, B.D., Roth, C.C., Ibey, B.L., Payne, J.A., Cundin, L.X., et al., Lasers Surg. Med., 2011, vol. 43, p. 152.
Grundt, J.E., Cerna, C., Roth, C.C., Ibey, B.L., Lipscomb, D., Echchgadda, I., et al., in Proc. Int. Conf. Infrared, Millimeter, and Terahertz Waves, Houston, TX, 2011.
Wilmink, G.J., Ibey, B.L., Roth, C.L., Vincelette, R.L., Rivest, B.D., Horn, C.B., et al., Proc. SPIE, 2010, vol. 7562, 75620K.
Alexandrov, B.S., Rasmussen, K.O., Bishop, A.R., Usheva, A., Alexandrov, L.B., Chong, S., et al., Opt. Express, 2011, vol. 2, p. 2679.
Alexandrov, B.S., Phipps, L.M., Alexandrov, L.B., Booshehri, L.G., Erat, A., Zabolotny, J., et al., Sci. Rep., 2013, vol. 3, 1184.
Echchgadda, I., Grundt, J.E., Cerna, C.Z., Roth, C.C., Ibey, B.L., and Wilmink, G.J., in Proc. Int. Conf. Infrared, Millimeter, and Terahertz Waves, IRMMW-THz, Tucson, TX, 2014.
Echchgadda, I., Cerna, C.Z., Sloan, M.A., Elam, D.P., Ibey, B.L., Fort, J., et al., Proc. SPIE, 2015, vol. 9321, 93210Q.
Echchgadda, I., Grundt, J.E., Cerna, C.Z., Roth, C.C., Payne, J.A., Ibey, B.L., et al., IEEE Trans. Terahertz Sci. Technol., 2016, vol. 6, p. 54.
Wilmink, G.J., Rivest, B.D., Ibey, B.L., Roth, C.L., Bernhard, J., and Roach, W.P., Proc. SPIE, 2010, vol. 7562, 75620L.
Wilmink, G.J., Grundt, J.E., Cerna, C., Roth, C.C., Kuipers, M.A., Lipscomb, D., et al., in Proc. Int. Conf. Infrared, Millimeter, and Terahertz Waves, IRMMW-THz, Houston, TX, 2011.
Korenstein-Ilan, A., Barbul, A., Hasin, P., Eliran, A., Gover, A., and Korenstein, R., Radiat. Res., 2008, vol. 170, p. 224.
Bourne, N., Clothier, R.H., D’Arienzo, M., and Harrison, P., ATLA, Altern. Lab. Anim., 2008, vol. 36, p. 667.
Hintzsche, H., Jastrow, C., Kleine-Ostmann, T., Stopper, H., Schmid, E., and Schrader, T., Radiat. Res., 2011, vol. 175, p. 569.
Borovkova, M., Serebriakova, M., Fedorov, V., Sedykh, E., Vaks, V., Lichutin, A., et al., Opt. Express, 2017, vol. 8, p. 273.
Scarfi, M.R., Romano, M., Di Pietro, R., Zeni, O., Doria, A., Gallerano, G.P., et al., J. Biol. Phys., 2003, vol. 29, p. 171.
Zeni, O., Gallerano, G.P., Perrotta, A., Romano, M., Sannino, A., Sarti, M., et al., Health Phys., 2007, vol. 92, p. 349.
Ramundo-Orlando, A., Gallerano, G.P., Stano, P., Doria, A., Giovenale, E., Messina, G., et al., Bioelectromagnetics, 2007, vol. 28, p. 587.
Amicis, A., De Sanctis, S., De Cristofaro, S., Di Franchini, V., Lista, F., Regalbuto, E., et al., Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2015, vol. 793, p. 150.
Bogomazova, A.N., Vassina, E.M., Goryachkovskaya, T.N., Popik, V.M., Sokolov, A.S., Kolchanov, N.A., et al., Sci. Rep., 2015, vol. 5, 7749.
Sergeeva, S., Demidova, E., Sinitsyna, O., Goryachkovskaya, T., Bryanskaya, A., Semenov, A., et al., Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2016, vol. 803-804, p. 34.
Ol’shevskaya, Yu.S., Kozlov, A.S., Petrov, A.K., Zapara, T.A., and Ratushnyak, A.S., Vestn. Novosib. Gos. Univ., Ser. Fiz., 2010, vol. 5, p. 177.
Demidova, E.V., Goryachkovskaya, T.N., Malup, T.K., Bannikova, S.V., Semenov, A.I., Vinokurov, N.A., et al., Bioelectromagnetics, 2013, vol. 34, p. 15.
Goryachkovskaya, T.N., Konstantinova, S.H., Meshcheriakova, I.A., Bannikova, S.V., Demidov, E.A., Bryanskaya, A.V., et al., Vavilov J. Genet. Breed., 2016, vol. 20, p. 869.
Williams, R., Schofield, A., Holder, G., Downes, J., Edgar, D., Harrison, P., et al., Phys. Med. Biol., 2013, vol. 58, p. 373.
van Exter, M., Fattinger, C., and Grischkowsky, D., Appl. Phys. Lett., 1989, vol. 55, p. 337.
Cantor, A.J., Cheo, P., Foster, M., and Newman, L., IEEE J. Quantum Electron., 1981, vol. 17, p. 477.
Vicario, C., Ovchinnikov, A.V., Ashitkov, S.I., Agranat, M.B., Fortov, V.E., and Hauri, C.P., Opt. Lett., 2014, vol. 39, p. 6632.
Kim, K.T., Park, J., Jo, S.J., Jung, S., Kwon, O.S., Gallerano, G.P., et al., Sci. Rep., 2013, vol. 3, 2296.
Titova, L.V., Ayesheshim, A.K., Golubov, A., Fogen, D., Rodriguez-Juarez, R., Hegmann, F.A., et al., Opt. Express, 2013, vol. 4, p. 559.
Titova, L.V., Ayesheshim, A.K., Golubov, A., Rodríguez-Juarez, R., Kovalchuk, A., Hegmann, F.A., et al., Proc. SPIE, 2013, vol. 8585, 85850Q.
Titova, L.V., Ayesheshim, A.K., Golubov, A., Rodriguez-Juarez, R., Woycicki, R., Hegmann, F.A., et al., Sci. Rep., 2013, vol. 3, 2363.
Tsurkan, M.V., Smolyanskaya, O.A., Bespalov, V.G., Penniyainen, V.A., Kipenko, A.V., Lopatina, E.V., et al., Proc. SPIE, 2012, vol. 8261, 82610S.
Duka, M.V., Nesgovorova, Y.S., Smolyanskaya, O.A., Bespalov, V.G., Kudryavtsev, I.V., Polevshchikov, A.V., et al., J. Opt. Technol., 2013, vol. 80, p. 655.
Tsurkan, M.V., Smolyanskaya, O.A., and Bryantseva, N.G., Nauchn.-Tekh. Vestn. Inf. Tekhnol., Mekh. Opt., 2013, vol. 1, p. 4184.
Fedorov, V.I., Weisman, N.Y., Nemova, E.F., Mamrashev, A.A., and Nikolaev, N.A., Biophysics (Moscow, Russ. Fed.), 2013, vol. 58, no. 6, p. 820.
Weisman, N.Y., Fedorov, V.I., and Nemova, E.F., Contemp. Probl. Ecol., 2015, vol. 8, p. 237.
Fedorov, V.I., Weisman, N.Y., Nemova, E.F., and Nikolaev, N.A., Biophysics, 2014, vol. 59, p. 458.
Pine, A.S., Suenram, R.D., Brown, E.R., and McIntosh, K.A., J. Mol. Spectrosc., 1996, vol. 175, p. 37.
Lampin, J. and Peytavit, E., Proc. SPIE, 2010, vol. 7763, 77630A1.
Angeluts, A.A., Gapeyev, A.B., Esaulkov, M.N., Kosareva, O.G., Matyunin, S.N., Nazarov, M.M., et al., Quantum Electron., 2014, vol. 44, p. 247.
Yaekashiwa, N., Otsuki, S., Hayashi, S., and Kawase, K., Radiat. Res., 2018, vol. 59, p. 116.
Borodin, A.V., Esaulkov, M.N., Kuritsyn, I.I., Kotelnikov, I.A., and Shkurinov, A.P., J. Opt. Soc. Am. B, 2012, vol. 29, p. 1911.
Koyama, S., Narita, E., Shimizu, Y., Shiina, T., Taki, M., Shinohara, N., et al., Int. J. Environ. Res. Public Health, 2016, vol. 13, p. 793.
Kirichuk, V.F. and Tsymbal, A.A., Biomed. Eng., 2010, vol. 44, no. 1, p. 11.
Bock, J., Fukuyo, Y., Kang, S., Lisa, PhippsM., Alexandrov, L.B., Rasmussen, K.O., et al., PLoS One, 2010, vol. 5, p. 8.
Doria, A., Gallerano, G.P., Giovenale, E., Messina, G., Lai, A., Ramundo-Orlando, A., et al., Infrared Phys. Technol., 2004, vol. 45, p. 339.
Fedorov, V.I., Shevela, E.Ya., Klement’ev, V.M., and Khamoyan, A.G., Al’manakh Klin. Med., 2008, vol. 17, p. 102.
Clothier, R.H. and Bourne, N., J. Biol. Phys., 2003, vol. 29, p. 179.
Hintzsche, H., Jastrow, C., Kleine-Ostmann, T., Kärst, U., Schrader, T., and Stopper, H., PLoS One, 2012, vol. 7, p. 1.
Franchini, V., De Sanctis, S., Marinaccio, J., De Amicis, A., Coluzzi, E., Di Cristofaro, S., et al., Environ. Mol. Mutagen., 2018, vol. 00, p. 1.
Bondar, N.P., Kovalenko, I.L., Avgustinovich, D.F., Khamoyan, A.G., and Kudryavtseva, N.N., Bull. Exp. Biol. Med., 2008, vol. 145, p. 401.
Kirichuk, V.F., Ivanov, A.N., Antipova, O.N., Krenitskii, A.P., Maiborodin, A.V., and Tupikin, V.D., Bull. Exp. Biol. Med., 2008, vol. 145, no. 1, p. 75.
Kirichuk, V.F. and Tsymbal, A.A., Bull. Exp. Biol. Med., 2009, vol. 148, no. 2, p. 200.
Demidova, E.V., Goryachkovskaya, T.N., Mescherya-kova, I.A., Malup, T.K., Semenov, A.I., Vinokurov, N.A., et al., IEEE Trans. Terahertz Sci. Technol., 2016, vol. 6, p. 435.
Kirichuk, V.F. and Tsymbal, A.A., Vestn. Ross. Akad. Med. Nauk, 2010, vol. 4, p. 37.
Kirichuk, V.F. and Tsymbal, A.A., Bull. Exp. Biol. Med., 2010, vol. 145, no. 2, p. 191.
Kirichuk, V.F. and Tsymbal, A.A., Zakonomernosti i mekhanizmy biologicheskogo deistviya elektromagnitnykh voln teragertsevogo diapazona (Regularities and Mechanisms of Biological Division of Electromagnetic Waves of Terahertz Range), Saratov: Saratov. Gos. Med. Univ., 2015.
Eremenko, K.U.E., Aleksandrova, N.N., and Kirichuk, V.F., Fyodorov J. Ophthalmic Surg., 2017, p. 61.
Eremenko, K.Yu., Aleksandrova, N.N., and Kirichuk, V.F., Tochka Zreniya. Vostok–Zapad, 2017, p. 121.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Il’ina, I.V., Sitnikov, D.S. & Agranat, M.B. State-of-the-Art of Studies of the Effect of Terahertz Radiation on Living Biological Systems. High Temp 56, 789–810 (2018). https://doi.org/10.1134/S0018151X18050127
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018151X18050127