Abstract
This review discusses the current state of research concerning the effects of terahertz (THz) radiation on living cells in the context of biosafety of THz radiation for the human organism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
ELECTROMAGNETIC RADIATION OF THE TERAHERTZ RANGE
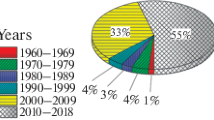
The terahertz (THz) range of the electromagnetic spectrum lies between the infrared (IR) and microwave ranges and covers the frequency range from 0.1 to 10.0 THz, which corresponds to the wavelength band from 30 μm to 3 mm [1–4]. In the recent decades, the use of the THz range has been extensively increasing thanks to advances in the methods of generating and detecting THz radiation [5–7]. Currently, THz technologies are a rapidly developing field, as illustrated by the growing number of patent applications analyzed in [8]. The number of patents granted between 2006 and 2015 increased threefold. A certain decline in the patent activity that occurred in 2016 was not discussed by the authors. In our opinion, this may be related to a temporary saturation with technological solutions in the THz range of frequencies (Fig. 1).
Dynamics of annual numbers of patent applications from 2006 to 2016 [8].
The interest to THz radiation and its growing implementation in different areas of human activity are due to several of its features important for practical applications [9–13]:
—THz radiation is nonionizing and does not damage biological molecules, because the photon energy (0.04–0.004 eV) is low in comparison to the energy of ionization that could cause atomic or molecular dissociation;
—it penetrates well through many dielectric materials, such as wood, paper, textiles, plastics, and ceramics;
—it is efficiently absorbed by polar compounds, including water;
—it is absorbed differently by various biological tissues;
—its energy corresponds to the energy of hydrogen bonds and Van-der-Waals forces of intermolecular interactions;
—in the form of molecular crystals, different molecules exhibit THz absorption spectra with characteristic sets of resonance frequencies;
—in contrast to visible and IR light, THz radiation exhibits Rayleigh scattering (1/λ4) in opaque and finely dispersed media, in nano- and microporous systems, as well as in biological tissues, so that the effects of Mie scattering can be disregarded; interactions of THz radiation with these media can be described in terms of effective medium theory and different models of dielectric response.
These properties of THz radiation make it a promising tool that might be applied for the purposes of security surveillance [14, 15], new-generation communications [16], as well as medical diagnostics and therapy [17, 18].
PROBLEMS OF USING THz RADIATION IN MEDICAL DIAGNOSIS: THz DOSIMETRY
Biomedical applications of THz radiation largely concern the field of noninvasive, minimally invasive, and intraoperational diagnostics. Thanks to the simplicity of measurements, human skin tissues, as well as different types of malignant skin tumors, were one of the first biological tissues to be investigated in the THz spectral range in vivo and in vitro [19–26]. The use of THz imaging was described for assessment of tissue damage after burns [27], for control of wound healing without removing the bandages and plaster [28], for detection of caries [29], and for noninvasive diagnosis of diabetes [30, 31]. It was found that healthy tissues and malignant neoplasms of different nosological forms and location exhibit different optical features in the THz range [2, 13, 32, 33]. Diagnostic use of THz radiation was also described in ophthalmology [34].
Active development and implementation of THz‑range technologies in everyday practice leads to a growing exposure of human population to this type of radiation, which raises concerns about potential associated health risks, considering our insufficient understanding of the induced biological effects. In this context, it is important to find out how the response of living objects depends on physical parameters of THz radiation and to determine the safety limits of its use [35]. The currently existing sanitary standards concern only the spectrum range from 300 kHz to 300 GHz [36, 37]. In Russia, the safety limit for power flux density (PFD), or intensity, of electromagnetic radiation with frequencies below 300 GHz is set at 200 µW/cm2 per hour of exposure for the working personnel [36] and 10 µW/cm2 for the general population (round-the-clock exposure) [38]. According to the guidelines of the International Commission on Non-Ionizing Radiation Protection (ICNIRP), the general safety limit for the intensity of radiation in the frequency range of 2 to 300 GHz is 1 mW/cm2 for 6 min of exposure [39]. This safety limit is based on verified thermal effects, which were shown to be caused by exposure in this frequency range. For frequencies higher than 300 GHz, there exist no conventional limits for exposure of the general population. Limitations are used only for laser radiation, where the safety limits lie between 1 to 100 mW/cm2 depending on the laser radiation type [40]. Although there exist extrapolations of the data for the neighboring ranges of the electromagnetic spectrum, they are inappropriate to set evidence-based norms [35, 39].
It is important to notice that permissible exposure limits cannot be determined without understanding the mechanisms by which THz radiation interacts with biological objects. To date, there exist two most popular hypotheses:
—the first one assumes that the effects of THz radiation are due to heating of exposed objects as a result of its strong absorption by water, which is mainly observed for continuous-wave radiation sources [10, 41–43];
—the second hypothesis considers nonthermal mechanisms of interactions between THz waves and biological systems. For instance, in spite of its low energy and therefore a low probability of breaking chemical bonds, THz radiation can induce linear or nonlinear resonance effects in DNA. Under certain conditions, this can significantly alter the molecular dynamics and locally disrupt hydrogen bonds between DNA strands, modifying gene expression [44, 45]. This notion is particularly relevant for exposure to high-energy pulsed THz radiation [46]. Whereas the average power of picosecond THz pulses is usually rather low (of the µW or mW order), its peak values may be as high as 1 MW and more [47], which is enough for THz radiation to penetrate the cytoplasmic and nuclear membranes [48, 49].
The nonthermal character of radiation effects on biological systems is explained using the theory proposed by H. Fröhlich: in the 1970–1980s, he hypothesized that THz radiation contributes significantly to formation of special coherent states, so-called Fröhlich condensates, in biological matter [50, 51]. Their presence does not necessarily imply that they can interact with the radiation as free oscillators in case of frequency resonance. According to Fröhlich, radiation acts as a trigger that switches the kinetics of biochemical processes in living organisms [51].To explain the effect of radiation using this approach, it is insufficient to identify oscillations with frequencies close to the frequencies of radiation that is being studied. It is essential to investigate the kinetics of the entire network of processes involved in the interaction between electromagnetic radiation and biological objects [52, 53].
To date, several review articles have described effects of THz radiation on all levels of biological organization, from affecting the conformation of biopolymers (proteins and DNA) [2, 4, 10, 54–57] to inducing organism-level responses [10, 55, 56]. The latter can be illustrated by the following examples. Exposure of mice to THz laser radiation with a frequency of 3.68 THz and intensity of 40 mW/cm2 for 30 min had a negative effect on the behavior of the animals, causing the avoidance response, displaced motor activity, and anxiety, which persisted at least 24 h after irradiation [58]. In drosophila flies, exposure to THz radiation (frequency range, 0.1–2.2 THz; pulse duration, 1 ps; peak power, 8.5 mW; pulse frequency, 76 MHz) for 30 min had an effect on the following parameters:
—the lifespan of adult individuals and the first-generation offspring;
—the period of reaching maturity in the first-generation offspring;
—the proportion between the numbers of male and female individuals.
These observations indicate changes in system-level traits. The underlying processes may involve epigenetic regulation and different intercellular signaling pathways [59–61].
In the problem of investigating THz radiation and implementing it for the purposes of diagnostics and therapy, several aspects can be identified:
—safety of using THz radiation in diagnostic procedures, which requires development of the standards to determine the safety limits for using THz radiation sources in medical diagnostic systems for an appropriate period of time (e.g., 10–20 min); this research can be conveniently performed in cells and cell cultures;
—possible involvement of THz radiation in the programming of cell growth and development, its effects on functional state and proliferation of cells, and cell–cell interactions, which may be significant for creation of engineered tissue constructs; these experiments should rule out any damaging effects on the genetic apparatus of the cells;
—the problem of influence on the functioning of complex multicellular organisms, investigation of long-term consequences of this exposure.
In the present review, we focus mainly on the biological effects of THz radiation on the cell level. This is an issue of central importance aimed at targeted identification of specific cell responses, which might be masked within a systemic response of a multicellular organism.
EFFECTS OF THz RADIATION ON BLOOD CELLS
To date, a substantial body of data has been accumulated concerning the optical properties of blood and its components in the THz range [13, 62, 63]. It should be noted that no changes in the spectral and morphological characteristics of blood cells were observed in the course of spectroscopy on standard pulsed THz units (frequency range, 0.1–3.2 THz; average power, 100 nW; exposure, 1–5 min) [5, 62, 63].
At the same time, there have been publications describing effect of THz radiation on blood cells. It was found that exposure of erythrocyte suspension to monochromatic radiation of a backward-wave tube with adjustable output frequency (frequency, 0.18 to 0.33 THz; intensity, 3 mW/cm2; duration, 180 min) decreased their osmotic resistance, which was assessed by hemoglobin release from erythrocytes [64]. In erythrocyte suspension exposed to continuous-wave THz radiation (frequency, 3.68 THz; intensity, 40 mW/cm2; duration, 60 min), hemoglobin release after addition of water (1 : 2) increased 24 times in comparison to unexposed erythrocytes [65]. Viability of erythrocytes exposed to broad-band THz radiation (frequency range, 0.1–1.75 THz; duration, 60 min) decreased more than in control cells placed in solutions containing 0.54 to 0.48% NaCl [66]. Cell viability was assessed by staining with tryptan blue, a dye that penetrates damaged cell membranes. A study using the Novosibirsk free-electron laser (FEL) as a THz source (peak power, up to 1 MW; pulse frequency, 5.6 MHz) showed that exposure of erythrocytes to radiation with wavelengths of 130–146 µm and a mean intensity of 10 W/cm2 for 5 s did not induce detectable changes in cell morphology or number of aggregated erythrocytes. When the period of exposure was increased to 10–15 s, the number of erythrocytes within aggregates decreased. The authors supposed that the observed effect was due to strong ultrasound waves passing the exposed medium at the frequency of laser pulses, 5.6 MHz [67]. In control experiments, erythrocytes were heated to body temperature and exposed to ultrasound, which did not cause any similar effects. Exposure times of longer than 25 s caused cell lysis. All these facts indicate that exposure to THz radiation can affect permeability of erythrocyte membranes.
Study [68] was performed to determine biologically safe limits for the energy THz radiation in a number of pulsed THz systems, which involved evaluation of damaging effects on DNA of blood leukocytes using the DNA comet assay [69]. Temperature variations were calculated for the selected irradiation modes. It was shown that exposure of blood leukocytes to pulse THz radiation (frequency range, 0.1–6.5 THz; duration, 20 min) did not cause DNA damage for intensities of up to 200 µW/cm2, while the temperature of the exposed specimen increased by less than 1°C. For comparison, other studies [70, 71] showed that exposure of blood specimens obtained from healthy donors to pulsed THz radiation (frequency range, 0.12–0.13 THz; mean intensity, 30–250 µW/cm2; duration, 20 min) did not induce genetic changes in blood leukocytes and did not affect cell cycle kinetics, whereas THz radiation with higher intensity of up to 2 mW/cm2 could induce DNA damage [72]. Exposure to continuous-wave THz radiation (with a frequency of 3.68 THz and intensity of 40 mW/cm2, which significantly exceeded the permissible limits) for 30 min decreased the number of viable cells, and after 90 min of exposure their number dropped nearly twofold [65]. At the same time, exposure of human blood lymphocytes to continuous THz radiation (frequency, 0.1 THz; intensity, 31 µW/cm2; duration, 120 and 1440 min (24 h)) caused an increase in aneuploidy of chromosomes 11 and 17 during cell division, which implies genomic instability and can lead to malignant transformation [73].
Exposure of a human T-cell culture to THz radiation (frequency, 2.52 THz; intensity, 636 mW/cm2; duration, 30–50 min) was accompanied with an increase in temperature of specimens by 3°C [74]. Therefore, the effects of THz radiation were compared to those of heating to the corresponding temperature. It was found that THz radiation activated 75% of genes, including those that encode proteins of the cell membrane and components of intracellular signal transmission pathways, whereas heating alone activated only 55% of genes. The same authors observed that the expression of genes encoding heat shock proteins, transcriptional regulators, cell growth factors, and proinflammatory cytokines was elevated 240 min after irradiation [75]. It was concluded that THz radiation can affect gene expression, and this effect was independent from the increase in temperature induced by irradiation [76].
Thus, experiments on exposure of blood cells to THz radiation showed that it increased permeability of the cell membrane, affected cell morphology, proliferation, and aggregation, and had genotoxic and cytotoxic effects (Table 1). As it follows from Table 1, these effects of THz radiation were observed for PFD levels that exceed the accepted permissible limits or for significant exposure times.
EFFECTS OF THz RADIATION ON SKIN CELLS
The report of the committee on potential health effects of exposure electromagnetic fields pointed out that, considering the expected increase in the use of THz technologies in the 21st century, effects of THz radiation on the skin (low-intensity long-term exposure) and the cornea (high-intensity short-term exposure) should be a priority research direction [35]. In compliance with these recommendations, a large portion of the corresponding research is performed in fibroblast cells. For instance, in study [77], cultures of human skin fibroblasts were exposed to continuous-wave THz radiation (frequency, 2.52 THz; intensity, 84.8 mW/cm2; duration, 5–80 min), while cells heated to 40°C and cells exposed to UV radiation (wavelength, 254 nm; power, 38 W; duration, 3 min) served as controls. It was found that irradiation for 5, 10, or 20 min did not diminish the numbers of viable cells. For exposure periods of 40 to 80 min, their numbers slightly decreased (by less than 10%); at the same time, cell proliferation was enhanced, and DNA transcription levels remained unchanged. Exposure of 240 min upregulated the expression of heat shock protein genes, with a dynamics that was different from the pattern observed for the thermal control [78]. At the same time, THz radiation did not affect the expression of marker genes of DNA damage caused by exposure to UV radiation [77, 78]. It was shown that increasing the intensity of THz radiation to 227 mW/cm2 induced activation of stress response genes. The study also included theoretical estimation and experimental measurements of temperature in the course of irradiation. The authors related the observed effects to the increase in cell temperature by 3°C [79]. Exposure of human fibroblasts to continuous-wave THz radiation (frequency, 0.14 THz; intensity, 20–200 mW/cm2; duration, 20 min) did not cause significant differences in cell proliferation, migratory activity, and NO production [80]. This work did not investigate the markers of DNA damage.
A whole range of genetic and cytological techniques was employed to investigate the effects of pulsed THz radiation (frequency range, 0.1–0.15 THz; mean intensity, 0.4 mW/cm2; duration, 20 min) on a culture of HFFF2 human fibroblasts; experiments were performed at 16°C. According to theoretical estimates, the temperature increase during irradiation was 0.3°C. The authors did not observe induced DNA damage as assessed using the DNA comet assay, histone H2AX phosphorylation, or telomere length modulation, nor did they detect apoptosis or changes in specific signaling proteins. These results suggest that effects induced by THz radiation may rather be aneugenic than clastogenic, and could probably result in chromosome loss. Furthermore, enhanced actin polymerization, which was observed by ultrastructural analysis after THz irradiation, supports the hypothesis that abnormal assembly of the mitotic spindle may lead to the observed impairment of chromosome disjunction during cell division. Taking into account that chromosomal rearrangements and aneuploidy are well-established signs of malignant transformation, the authors underline that, under conditions of growing use of THz radiation, understanding of its effects on the genome will be of central significance [81].
Yaekashiva et al. investigated effects of THz radiation on a culture of human skin NB1RBG fibroblasts using a continuous-wave source tunable in the frequency range of 70–300 GHz [82]. Irradiation was performed in a special temperature-controlled chamber (37 ± 0.2°C); the radiation intensity was 1.27 μW/cm2 at 0.1 THz and 0.38 μW/cm2 at 0.3 THz; the time of exposure ranged from 180 to 5640 min (94 h). No changes in cell proliferation and activity, nor any signs of cytotoxicity were detected in these experiments.
In another study, human keratinocyte cultures were exposed to continuous-wave THz radiation at the frequencies of 1.4, 2.52, and 3.11 THz for 20 min; the radiation intensity was constant and constituted 44.2 mW/cm2 [83]. mRNA analysis performed after 240 min showed that the patterns of gene expression were specific for each frequency. The authors concluded that exposure of cell cultures to different THz frequencies can induce unique biochemical and cellular responses. This suggests that the use of THz radiation as a tool stimulating specific cell properties requires careful selection of frequency [83].
In studies [84–86], human epidermal keratinocytes and dermal fibroblasts within artificial multilayer human skin tissue were exposed to pulsed THz radiation (frequency range, 0.1–2.5 THz; intensity, 5.7 and 57 mW/cm2; duration, 10 min). For comparison, cells were also exposed to pulsed UV light (wavelength, 400 nm; duration, 2 min). It was found that THz radiation selectively suppressed the expression of genes associated with psoriasis, atopic dermatitis, and other inflammatory skin diseases, as well the genes of proteins involved in apoptosis [84]. For the genes associated with carcinogenesis, it was shown that THz radiation downregulated the expression of genes promoting proliferation of tumor cells, tumor growth, and metastasis, and at the same time stimulated the expression of tumor suppressor proteins [84–86]. Importantly, activity of the same proteins had different dynamics in UV-irradiated cells and in those exposed to THz radiation. Based on these results, the authors suggested that THz radiation may be used in therapeutic purposes to normalize the functioning of genes involved in skin neoplasms and inflammatory diseases.
Keratinocytes of mouse dorsal skin were exposed in vivo to pulsed THz radiation (frequency range, 0.1–2.6 THz; intensity, 0.32 mW/cm2; duration, 60 min) [87]. In 1440 min (24 h) after irradiation, 149 genes were found to be activated; they were involved, in particular, in tissue growth and regeneration, organogenesis, and cell migration. Furthermore, the pattern of gene expression in cell exposed to THz radiation was different from the patterns characteristic for exposure to UV or neutron radiation [87]. The authors supposed that THz irradiation decreased hydration of the skin, which modified the activity of intracellular signaling pathways.
Data concerning the effects of THz radiation on skin cells are summarized in Table 2. It can be noted that, in contrast to what was observed for blood cells, there is no definite relationship between the effects of THz radiation and the power of the THz source. This may be partially due to the difference of approaches used to assess these effects. In particular, methods evaluating proliferation, migration, and functional activity of the cells [80, 82] are less sensitive than molecular biological techniques that can be used to detect cellular DNA damage, as well as to analyze the expression of individual genes and the synthesis of the corresponding proteins [78, 79, 83–87]. At the same time, in most studies that descried some effects of THz radiation, the power of sources was significantly above safety limits.
EFFECTS OF THz RADIATION ON NERVE CELLS
Effects of continuous-wave laser THz radiation (frequencies, 0.71, 1.63, 2.45, 2.56, 3.68, and 4.28 THz; intensity, 2–20 mW/cm2; duration, 60 min) were investigated in isolated neurons of the supraesophageal ganglion of great pond snail Lymnaea stagnalis [88]. Significant effect were observed at 3.68 and 0.71 THz (Table 3). Exposure to 0.71 THz radiation changed adhesive properties of cell membranes and disrupted contacts between the neurons and the support. Exposure to 3.68-THz radiation caused structural changes in the somatic membranes, axons, and growth cones. This was a delayed effect that developed within 2400–3000 min (40–50 h) after irradiation. During this period, pigment granules redistributed, and the membrane became heterogeneous. Next, there appeared abnormal randomly directed sprout-like structures, whereas classical neurite growth was not observed [88]. The same experiment showed that radiation-induced responses of cell membranes were not the same at different stages of cell growth. The effect described above was observed in cells at the initial stage of regeneration of neural network (before the formation of neural sprouts). In neurons with developed sprouts that were in the course of network formation, the observed effects were different: damages to neurite growth cones and arrest of their growth, which impaired the formation of neuronal connections [88, 89] (Fig. 2).
Effect of continuous-wave radiation with a frequency of 3.68 THz on isolated neurons at the stage of neural network formation: (a) an example of network formation; (b) thickenings in the neurite growth zone and arrest of their growth after exposure to THz radiation. Exposure caused damages to neurite growth cones and arrest of their further growth, impairing formation of neuronal connections. Scale bar, 25 μm.
The same biological object, isolated neurons of L. stagnalis, was used to investigate the effects of high-intensity pulsed THz radiation generated by FEL (pulse duration, 30–100 ps; pulse frequency, 5.6–11.2 MHz). Exposure to 2.3-THz radiation with a mean intensity of 30 mW/cm2 for 60 s caused a gradual decrease of the membrane potential accompanied with morphological damage to the membrane and intracellular structures and followed by cell death within 120 min (2 h) after exposure. When the mean intensity was decreased 10-fold to 3 mW/cm2, cell death occurred within 180 min (3 h) after exposure. With a further decrease in the mean intensity (to 0.3 mW/cm2), the number of viable cells stabilized in 120 min (2 h) after exposure. However, no viable cells could be detected in 60 min (1 h) after 60-s exposure to radiation with a frequency of 2.14 THz, even when the mean intensity was low (0.3 mW/cm2) [89].
Modifications of barrier properties of neural membranes were investigated using exposure to FEL radiation (frequency, 2.3 THz; mean intensity, from 0.5 to 20 mW/cm2). It was found that irradiation caused dose-dependent damage to nonspecific permeability of the cell membrane, as observed by appearance of the vital dye trypan blue in the cytoplasm. The dye was present only in some parts of the cells and unevenly distributed in the cytoplasm. The authors supposed that THz radiation induced formation of pass-through hydrophilic pores, which could be the only possible way for the dye to penetrate the cell. The effect was reversible: in 24 h after irradiation, the membrane potential and functional responses of these cells did not differ from normal [90]. Irradiation at the 2.0-THz frequency with the same exposure parameters did not induce significant changes in most exposed cells. Only a few individual neurons were uniformly stained and had a decreased or zero membrane potential, but their numbers did not differ from those observed in control experiments. For all irradiation regimens used, the temperature of the medium in the working chamber with neurons was monitored, and no significant variations were detected during the exposure [90].
The restoration of damaged membranes was assessed using the BCECF-AM dye (7′-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester), which can penetrate undamaged cell membranes and is converted into the fluorescent form, BCECF, by intracellular esterases of live neurons. Fluorescence was detected in unstained cells and in some cells that had captured trypan blue, suggesting that their membrane can be restored after damage to keep negatively charged fluorescent probes within the cell [90].
To test the hypothesis that irradiation induces formation of hydrophilic lipid pores and identify the mechanisms of this process, prior to laser irradiation, the saline surrounding the neurons was supplemented with lucifer yellow, a dye that does not penetrate intact membranes, and with antioxidants. It was found that the dye uptake by the cells significantly decreased in the presence of histochrome, a phenolic antioxidant. This may indicate that hydrophilic pores in the cell membrane are generated in free-radical processes that can be blocked by antioxidants [91]. It was found that THz radiation can reversibly damage the barrier properties of the cell membrane and induce targeted delivery of biologically active compounds, while antioxidants can modulate this process and provide protection from adverse effects of electromagnetic radiation in the THz range [91].
After exposure of a glial cell culture to continuous-wave radiation (frequency, 0.12–0.18 THz; intensity, 3.2 mW/cm2; duration, 1 min), the number of cells at the early stage of apoptosis increased 1.5 times; when the exposure was prolonged to 5 min, the number of such cells increased 2.4-fold [92]. The estimated increase in the temperature of the specimens was no more than 0.1°C. This results apparently supports the notion that THz radiation can represent a biological hazard [93].
Thus, it was found that THz radiation had selective effects on nerve cells depending on the frequency (Table 3). For instance, exposure to low-frequency continuous-wave THz radiation modified the adhesive properties of cell membranes and induced apoptosis. Radiation with a frequency of 3.68 THz affected the formation of neural networks; in this case, its intensity exceeded the maximal permissible level [35–39]. At the same time, FEL radiation with a high peak intensity and low mean intensity (0.3 mW/cm2) caused either reversible membrane permeability at the frequency 2.3 THz or cell death at the frequency of 2 THz.
EFFECTS OF THz RADIATION ON STEM CELLS
It was shown that exposure to ultrabroad-band pulsed THz radiation (frequency range, 1–30 THz with a maximum at 10 THz; mean intensity, 1–3 mW/cm2; duration, 540 min, or 9 h) promoted differentiation of mouse mesenchymal stem cells to adipocytes. In contrast, short-term exposure to continuous-wave THz radiation (frequency, 2.52 THz; intensity, 1–3 mW/cm2; duration, 120 min, or 2 h) helped to maintain pluripotency [94–96]. Irradiation was performed at 26–27°C, and expression of heat shock proteins did not increase, which implied a nonthermal nature of the observed effects. The authors supposed that upregulation of gene expression was determined by the structure of their promoters that can form specific structures facilitating transcription when exposed to THz radiation. The authors concluded that the use of THz radiation as a stimulus capable of modulating gene expression and cell programming may be of great practical significance for regenerative medicine [96].
Exposure of a human embryonic stem cell culture to a pulsed THz source (central frequency, 2.3 THz; mean intensity, 0.4 W/cm2; duration, 60 min (1 h); temperature, 36.5–37.5°C) enhanced the transcription of 1% of genes involved in the functioning of mitochondria [97]. These results indirectly support the conclusions of [96]: sensitivity of genes to THz radiation depends on the properties of their promoters. At the same time, the authors did not observe genotoxic effects of THz radiation or any effects on cell morphology and the mitotic index.
MECHANISMS THAT UNDERLIE THE EFFECTS OF THz RADIATION
To sum up, the data discussed above indicate that THz radiation can have a multitude of effects on animal cells, which involves modification of the properties of cell membranes, pore formation, modulation of cell viability and proliferation. The following mechanisms might be involved in cell responses to THz radiation:
—changes in the conformation of membrane-bound proteins triggering intracellular regulatory cascades that affect the genetic apparatus of the cell and its enzyme system, as well as permeability of the cell membrane for different compounds;
—changes in the conformation of membrane-bound proteins responding to external regulatory signals;
—changes in the conformation of membrane-bound proteins acting as pumps or transport channels for uptake or release of various compounds;
—redistribution of the electric charge on the cell membrane;
—induction of resonance oscillations of macromolecules that make up the cell membrane, and the cytoskeleton as a whole.
Some authors observed cytotoxicity of THz radiation and its effect on the genetic apparatus of the cell, which were of a rather specific character and depended on the parameters of the radiation source and on the design of the experiment [98, 99]. However, there currently exists no scientific consensus as to whether THz radiation has a damaging effect on biological objects of different complexity [100–102]. This is primarily related to the fact that there exist no standardized experimental procedures, the diversity of available THz sources is insufficient, and there are not enough tools to enable accurate assessment of the initial functional state of the biological object in question. Accordingly, for adequate evaluation of specific biological effects of THz radiation, each of the above aspects should be taken into account. Variations in these parameters may be of crucial importance, to the extent that opposite effects may be observed in experiments with a minor difference of a certain parameter. In the analysis of biological responses, the use of particular techniques with different sensitivity can also directly affect the outcome of the experiment (for instance, the method may produce a negative result because of insufficient sensitivity). As a result of limitations arising because of these factors, specific responses to THz radiation are often difficult to detect, the experiments themselves are sometimes insufficiently reproducible, and comparison of data from different experiments may be incorrect.
REFERENCES
Y.-S. Lee, Principles of Terahertz Science and Technology (Springer, USA, 2009). https://doi.org/10.1007/978-0-387-09540-0
X. Yang, X. Xiang Zhao, K. Yang, Y. Liu, Y. Liu, W. Fu, and Y. Luo, Trends Biotechnol. 34, 810 (2016). https://doi.org/10.1016/j.tibtech.2016.04.008
V. I. Fedorov, Biomed. Radioelektron., No. 2, 17 (2011).
A. A. Angeluts, A. V. Balakin, M. G. Evdokimov, M. N. Esaulkov, M. M. Nazarov, I. A. Ozheredov, D. A. Sapozhnikov, P. M. Solyankin, O. P. Cherkasova, and A. P. Shkurinov, Quantum Electron. 44, 614 (2014).
A. E. Yachmenev, D. V. Lavrukhin, I. A. Glinskiy, N. V. Zenchenko, Y. G. Goncharov, I. E. Spektor, R. A. Khabibullin, T. Otsuji, and D. S. Ponomarev, Opt. Eng. 59, 061608 (2019). https://doi.org/10.1117/1.OE.59.6.061608
Komandin G, V. Anzin, V. Ulitko, A. Gavdush, A. Mukhin, Y. Goncharov, O. Porodinkov, and I. Spektor, Opt. Eng. 59, 061603 (2019). https://doi.org/10.1117/1.OE.59.6.061603
N. Chernomyrdin, V. Zhelnov, A. Kucheryavenko, I. Dolganova, G. Katyba, V. Karasik, I. Reshetov, and K. Zaytsev, Opt. Eng. 59, 061605 (2019). https://doi.org/10.1117/1.OE.59.6.061605
D. A. Usanov, N. V. Romanova, and E. A. Saldina, Ekon. Nauki 3, 189 (2017). https://doi.org/10.22394/2410-132X-2017-3-3-189-202
O. V. Betskii, A. S. Koz’min, and Yu. G. Yaremenko, Biomed. Radioelektron., No. 3, 48 (2008).
G. J. Wilmink, and J. E. Grundt, J. Infrared, Millim., Terahertz Waves 32, 1074 (2011). https://doi.org/10.1007/s10762-011-9794-5
M. M. Nazarov, A. P. Shkurinov, E. A. Kuleshov, and V. V. Tuchin, Quantum Electron. 38, 647 (2008). https://doi.org/10.1070/QE2008v038n07ABEH013851
O. V. Betskii, A. P. Krenitskii, A. V. Maiborodin, and V. D. Tupikin, Biomed. Radioelektron., No. 12, 3 (2003).
O. A. Smolyanskaya, N. V. Chernomyrdin, A. A. Konovko, K. I. Zaytsev, I. A. Ozheredov, O. P. Cherkasova, M. M. Nazarov, J. P. Guillet, S. A. Kozlov, Yu. V. Kistenev, J. L. Coutaz, P. Mounaix, V. L. Vaks, J. H. Son, H. Cheon, et al., Prog. Quantum Electron. 62, 1 (2018). https://doi.org/10.1016/j.pquantelec.2018.10.001
J. Federici, B. Schulkin, F. Huang, D. Gary, R. Barat, F. Oliveira, and D. Zimdars, Semicond. Sci. Technol., No. 20, 266 (2005). https://doi.org/10.1088/0268-1242/20/7/018
H. Guerboukha, K. Nallappan, and M. Skorobogatiy, Adv. Opt. Photon. 10, 843 (2018). https://doi.org/10.1364/AOP.10.000843
R. Piesiewicz, M. Jacob, M. Koch, J. Schoebel, and T. Kürner, IEEE J. Sel. Top. Quantum Electron. 14, 421 (2008). https://doi.org/10.1109/JSTQE.2007.910984
V. I. Fedorov, Biophysics 62, 324 (2017). https://doi.org/10.1134/S0006350917020075
A. A. Svistunov, A. A. Tsymbal, P. F. Litvitskii, and I. A. Budnik, Vestn. RAMN 72, 365 (2017). https://doi.org/10.15690/vramn817
V. P. Wallace, P. F. Taday, A. J. Fitzgerald, R. M. Woodward, J. Cluff, R. J. Pye, and D. D. Arnone, Faraday Discuss. 126, 255 (2004). https://doi.org/10.1039/B309357N
K. I. Zaytsev, K. G. Kudrin, V. E. Karasik, I. V. Reshetov, and S. O. Yurchenko, Appl. Phys. Lett. 106, 053702 (2015). https://doi.org/10.1063/1.4907350
K. I. Zaytsev, A. A. Gavdush, N. V. Chernomyrdin, and S. O. Yurchenko, IEEE Trans. Terahertz Sci. Technol. 5, 817 (2015). https://doi.org/10.1109/TTHZ.2015.2460677
E. Pickwell and V. P. Wallace, J. Phys. D: Appl. Phys. 39, R301 (2006). https://doi.org/10.1088/0022-3727/39/17/R01
R. M. Woodward, B. E. Cole, V. P. Wallace, R. J. Pye, D. D. Arnone, E. H. Linfield, and M. Pepper, Phys. Med. Biol. 47, 3853 (2002). https://doi.org/10.1088/0031-9155/47/21/325
I. Echchgadda, J. A. Grundt, M. Tarango, B. L. Ibey, T. D. Tongue, M. Liang, H. Xin, and G. J. Wilmink, J. Biomed. Opt. 18, 120503 (2013). https://doi.org/10.1117/1.JBO.18.12.120503
K. I. Zaitsev, N. V. Chernomyrdin, K. G. Kudrin, I. V. Reshetov, and S. O. Yurchenko, Opt. Spectrosc. 119, 404 (2015). https://doi.org/10.1134/S0030400X1509026X
K. I. Zaytsev, K. G. Kudrin, S. A. Koroleva, I. N. Fokina, S. I. Volodarskaya, E. V. Novitskaya, A. N. Perov, V. E. Karasik, and S. O. Yurchenko, J. Phys.: Conf. Ser. 486, 012014 (2014). https://doi.org/10.1088/1742-6596/486/1/012014
D. Mittleman, THz Imaging, inSensing with THz Radiation (Springer, Berlin, 2003), p. 117. https://doi.org/10.1007/978-3-540-45601-8
R. Woodward, V. Wallace, B. Cole, R. Pye, D. Arnone, E. Linfield, and M. Pepper, Proc. SPIE 4625, 160 (2002). https://doi.org/10.1117/12.469785
E. Pickwell, V. P. Wallace, B. E. Cole, S. Ali, C. Longbottom, R. J. Lynch, and M. Pepper, Caries Res. 41, 49 (2007). https://doi.org/10.1159/000096105
O. Cherkasova, M. Nazarov, and A. Shkurinov, Opt. Quantum. Electron. 48, 1 (2016). https://doi.org/10.1007/s11082-016-0490-5
G. G. Hernandez-Cardoso, S. C. Rojas-Landeros, M. Alfaro-Gomez, A. I. Hernandez-Serrano, I. Salas-Gutierrez, E. Lemus-Bedolla, A. R. Castillo-Guzman, H. L. Lopez-Lemus, and E. Castro-Camus, Sci. Rep. 7, 42124 (2017). https://doi.org/10.1038/srep42124
Q. Sun, Y. He, K. Liu, S. Fan, E. P. J. Parrott, and E. Pickwell-MacPherson, Quant. Imaging Med. Surg. 7, 345 (2017). https://doi.org/10.21037/qims.2017.06.02
A. A. Gavdush, N. V. Chernomyrdin, K. M. Malakhov, S. I. T. Beshplav, I. N. Dolganova, A. V. Kosyrkova, P. V. Nikitin, G. R. Musina, G. M. Katyba, I. V. Reshetov, O. P. Cherkasova, G. A. Komandin, V. E. Karasik, A. A. Potapov, V. V. Tuchin, and K. I. Zaytsev, J. Biomed. Opt. 24, 027001 (2019). https://doi.org/10.1117/1.JBO.24.2.027001
I. Ozheredov, M. Prokopchuk, M. Mischenko, T. Safonova, P. Solyankin, A. Larichev, A. Angeluts, A. Balakin, and A. Shkurinov, Laser Phys. Lett. 15, 055601 (2018). https://doi.org/10.1088/1612-202X/aaac76
Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) Opinion on Potential health effects of exposure to electromagnetic fields (EMF), Health effects of EMF (2015).
On Approval of the Sanitary (Sanitary and Epidemiological) Rules and Norms (SanPiN) 2.2.4.3359-16, Sanitary and Epidemiological Requirements for the Physical Factors at Workplaces, Decree No. 81 (2016). http://docs.cntd.ru/document/420362948.
IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz. IEEE Std C95.1 (2005).
Yu. B. Kudryashov, Yu. F. Perov, and A. B. Rubin, Radiation Biophysics: Radio Frequency and Microwave Electromagnetic Radiation (Fizmatlit, Moscow, 2008) [in Russian].
Health Phys. 74, 494 (1998).
T. Kleine-Ostmann, C. Jastrow, K. Baaske, B. Heinen, M. Schwerdtfeger, U. Karst, H. Hintzsche, H. Stopper, M. Koch, and T. Schrader, IEEE Trans. Terahertz Sci. Technol. 4, 12 (2014). https://doi.org/10.1109/TTHZ.2013.2293115
A. Ramundo-Orlando and G. P. Gallerano, J. Infrared, Millim. Terahertz Waves 30, 1308 (2009). https://doi.org/10.1007/s10762-009-9561-z
E. Berry, G. C. Walker, A. J. Fitzgerald, N. N. Zinov’ev, M. Chamberlain, S. W. Smye, R. E. Miles, and M. A. Smith, J. Laser Appl. 15, 192 (2003). https://doi.org/10.2351/1.1585079
T. T. L. Kristensen, W. Withayachumnankul, P. U. Jepsen, and D. Abbott, Opt. Express 18, 4727 (2010). https://doi.org/10.1364/OE.18.004727
B. S. Alexandrov, V. Gelev, A. R. Bishop, A. Usheva, and K. O. Rasmussen, Phys. Lett. A 374, 1214 (2010). https://doi.org/10.1016/j.physleta.2009.12.077
S. M. Chitanvis, J. Polym. Sci., Part B 44, 2740 (2006). https://doi.org/10.1002/polb.20910
L. B. Alexandrov, K. O. Rasmussen, A. R. Bishop, and B. S. Alexandrov, Sci. Rep. 7, 9731 (2017). https://doi.org/10.1038/s41598-017-09537-y
G. N. Kulipanov, Y. Y. Choporova, B. A. Knyazev, V. M. Popik, A. N. Skrinsky, and N. A. Vinokurov, IEEE Trans. Terahertz Sci. Technol. 5, 798 (2015). https://doi.org/10.1109/TTHZ.2015.2453121
L. Titova, F. A. Hegmann, and O. Kovalchuk, in Terahertz Biomedical Science and Technology, Ed. by Joo-Hiuk Son (CRC, Taylor and Francis Group, 2014), p. 241. https://doi.org/10.1201/b17060-16
P. Weightman, Phys. Biol. 9, 053001 (2012). https://doi.org/10.1088/1478-3975/9/5/053001
V. I. Fedorov, S. S. Popova, and A. N. Pisarchik, Int. J. Infrared Millim. Waves 24, 1235 (2003). https://doi.org/10.1023/A:1024801304083
H. Frohlich, Adv. Electron. Electron Phys. 53, 85 (1980). https://doi.org/10.1016/S0065-2539(08)60259-0
J. Preto, Chaos 26, 123116 (2016). https://doi.org/10.1063/1.4971963
P. Weightman, Proc. SPIE 8941, 89411F (2014). https://doi.org/10.1117/12.2057397
O. P. Cherkasova, V. I. Fedorov, E. F. Nemova, and A. S. Pogodin, Opt. Spectrosc. 107, 534 (2009). https://doi.org/10.1134/S0030400X09100063
S. Romanenko, R. Begley, A. R. Harvey, L. Hool, and V. P. Wallace, J. R. Soc. Interface 14, 20170585 (2017). https://doi.org/10.1098/rsif.2017.0585
H. Hintzsche and H. Stopper, Crit. Rev. Environ. Sci. Technol. 42, 2408 (2012).
S. Yamazaki, M. Harata, T. Idehara, K. Konagaya, G. Yokoyama, H. Hoshina, and Y. Ogawa, Sci. Rep. 8, 9990 (2018). https://doi.org/10.1038/s41598-018-28245-9
N. P. Bondar, I. L. Kovalenko, D. F. Avgustinovich, A. G. Khamoyan, and N. N. Kudryavtseva, Bull. Exp. Biol. Med. 145, 401 (2008). https://doi.org/10.1007/s10517-008-0102-x
V. I. Fedorov, N. Ya. Weisman, E. F. Nemova, and N. A. Nikolaev, Biophysics 59, 458 (2014). https://doi.org/10.1134/S0006350914030063
V. I. Fedorov and N. Ya. Weisman, Biophysics 60, 835 (2015). https://doi.org/10.1134/S0006350915050048
V. I. Fedorov and N. Ya. Weisman, Biophysics 62, 460 (2017). https://doi.org/10.1134/S0006350917030046
O. P. Cherkasova, M. M. Nazarov, A. A. Angeluts, and A. P. Shkurinov, Opt. Spectrosc. 120, 50 (2016). https://doi.org/10.1134/S0030400X16010069
O. P. Cherkasova, M. M. Nazarov, and A. P. Shkurinov, EPJ Web of Conf. 195 (2018). https://doi.org/10.1051/epjconf/201819510003
S. A. Il’ina, G. F. Bakaushina, V. I. Gaiduk, A. M. Khrapko, and N. B. Zinov’eva, Biofizika 24, 513 (1979).
V. I. Fedorov, A. G. Khamoyan, E. Ya. Shevela, and E. R. Chernykh, Proc. SPIE 6734, 673404 (2007). https://doi.org/10.1117/12.753111
V. I. Fedorov, V. A. Vechkanov, and O. V. Papafilova, Biomed. Radioelektron. 5, 39 (2014).
A. F. Munzarova, E. L. Zelentsov, and A. S. Kozlov, Vestn NGU, Ser. Fiz. 8, 117 (2013).
A. A. Angeluts, A. B. Gapeev, M. N. Esaulkov, O. G. Kosareva, S. N. Matyunin, M. M. Nazarov, T. N. Pashovkin, P. M. Solyankin, O. P. Cherkasova, and A. P. Shkurinov, Quantum Electron. 44, 247 (2014). https://doi.org/10.1070/QE2014v044n03ABEH015337
A. B. Gapeyev, N. A. Romanova, and N. K. Chemeris, Biophysics 56, 672 (2011). https://doi.org/10.1134/S0006350911040087
O. Zeni, G. P. Gallerano, A. Perrotta, M. Romano, A. Sannino, M. Sarti, M. D. Arienzo, A. Doria, E. Giovenale, A. Lai, G. Messina, and M. R. Scarf, Health Phys. 92, 349 (2007). https://doi.org/10.1097/01.HP.0000251248.23991.35
M. R. Scarfi, M. Romano, Di R. Pietro, O. Zeni, A. Doria, G. P. Gallerano, E. Giovenale, G. Messina, A. Lai, G. Campurra, D. Coniglio, and M. D’Arienzo, J. Biol. Phys. 29, 171 (2003). https://doi.org/10.1023/A:1024440708943
A. Doria, G. P. Gallerano, E. Giovenale, G. Messina, A. Lai, A. Ramundo-Orlando, V. Sposato, M. D. Arienzo, A. Perrotta, M. Romano, M. Sarti, M. R. Scarfi, I. Spassovsky, and O. Zeni, Infrared Phys. Technol. 45, 339 (2004). https://doi.org/10.1016/j.infrared.2004.01.014
A. Korenstein-Ilan, A. Barbul, P. Hasin, A. Eliran, A. Gover, and R. Korenstein, Radiat Res. 170, 224 (2008). https://doi.org/10.1667/RR0944.1
G. J. Wilmink, J. I. Grundt, C. Cerna, C. C. Roth, M. A. Kuipers, D. Lipscomb, I. Echchgadda, and B. L. Ibey, in Proceedings of the Infrared, Millimeter and Terahertz Waves Conference (IRMMW-THz),2011, Paper No. 12442107. https://doi.org/10.1109/irmmw-THz.2011.6104966
J. E. Grundt, C. Cerna, C. C. Roth, B. L. Ibey, D. Lipscomb, I. Echchgadda, and G. J. Wilmink, in Proceedings of the Infrared, Millimeter and Terahertz Waves Conference IRMMW-THz,2011, Paper No. 12442108. https://doi.org/10.1109/irmmw-THz.2011.6104967
I. Echchgadda, J. E. Grundt, C. Z. Cerna, C. C. Roth, B. L. Ibey, and G. J. Wilmink, in Proceedings of the Infrared, Millimeter, and Terahertz Waves Conference IRMMW-THz,2014, Paper No. 14770846. https://doi.org/10.1109/IRMMW-THz.2014.6956140
G. J. Wilmink, B. D. Rivest, B. L. Ibey, C. L. Roth, J. Bernhard, and W. P. Roach, Proc. SPIE 7562, 75620L (2010). https://doi.org/10.1117/12.844916
G. J. Wilmink, B. D. Rivest, C. C. Roth, B. L. Ibey, J. A. Payne, L. X. Cundin, J. E. Grundt, X. Peralta, D. G. Mixon, and W. P. Roach, Lasers Surg. Med. 43, 152 (2011). https://doi.org/10.1002/lsm.20960
G. J. Wilmink, B. L. Ibey, C. L. Roth, R. L. Vincelette, B. D. Rivest, C. B. Horn, J. Bernhard, D. Roberson, and W. P. Roach, Proc. SPIE 7562, 75620K (2010). https://doi.org/10.1117/12.844917
O. Cherkasova, M. Surovtseva, A. Lykov, O. Kazakov, A. Kabakov, O. Poveshchenko, A. Poveshchenko, D. Serdyukov, S. Kuznetsov, and A. Letyagin, AIP Conf. Proc. 2098, 020004 (2019). https://doi.org/10.1063/1.5098148
A. de Amicis, S. D. Sanctis, S. D. Cristofaro, V. Fran-chini, F. Lista, E. Regalbuto, E. Giovenale, G. P. Gallerano, P. Nenzi, R. Bei, M. Fantini, M. Benvenuto, L. Masuelli, E. Coluzzi, C. Cicia, and A. Sgura, Mutat. Res. Genet. Toxicol. Environ. Mutagen. 793, 150 (2015). https://doi.org/10.1016/j.mrgentox.2015.06.003
N. Yaekashiwa, S. Otsuki, S. Hayashi, and K. Kawase, J. Rad. Res. 59, 116 (2018). https://doi.org/10.1093/jrr/rrx075
I. Echchgadda, C. Z. Cernab, M. A. Sloanb, D. P. Elamc, and B. L. Ibeya, Proc. SPIE 9321 (2015). https://doi.org/10.1117/12.2082542
L. V. Titova, A. K. Ayesheshim, A. Golubov, R. Rodriguez-Juarez, R. Woycicki, F. A. Hegmann, and O. Kovalchuk, Sci. Rep. 3, 1 (2013). https://doi.org/10.1038/srep02363
L. V. Titova, A. K. Ayesheshima, A. Golubovb, R. Rodriguez-Juarez, A. Kovalchuk, F. A. Hegmanna, and O. Kovalchuk, Proc. SPIE 8585, 85850Q (2013). https://doi.org/10.1117/12.2004998
L. V. Titova, A. K. Ayesheshim, A. Golubov, D. Fogen, R. Rodriguez-Juarez, F. A. Hegmann, and O. Kovalchuk, Biomed. Opt. Express 4, 559 (2013). https://doi.org/10.1364/BOE.4.000559
K. T. Kim, J. Park, S. J. Jo, S. Jung, O. S. Kwon, G. P. Gallerano, W. Y. Park, and G. S. Park, Sci. Rep. 3, 2296 (2013). https://doi.org/10.1038/srep02296
J. S. Olshevskaya, A. S. Ratushnyak, A. K. Petrov, A. S. Kozlov, and T. A. Zapar, in Proceedings of the International Conference in Computational Technologies in Electrical and Electronics Engineering EEE Region 8 SIBIRCON 2008, July 21–25,2008 (Novosib. Nauch. Tsentr, Novosibirsk, 2008), p. 210. https://doi.org/10.1109/SIBIRCON.2008.4602607
Yu. S. Ol’shevskaya, A. S. Kozlov, A. K. Petrov, T. A. Zapara, and A. S. Ratushnyak, Zh. Vyssh. Nervn. Deyat. 59, 353 (2009).
Yu. S. Ol’shevskaya, A. S. Kozlov, A. K. Petrov, T. A. Zapara, and A. S. Ratushnyak, Vestn. NGU, Ser. Fiz. 5, 177 (2010).
T. A. Zapara, S. P. Treskova, and A. S. Ratushniak, J. Surf. Invest.: X-ray, Synchrotr. Neutron Tech. 9, 869 (2015). https://doi.org/10.1134/S1027451015050195
M. Borovkova, M. Serebriakova, V. Fedorov, E. Sedykh, V. Vaks, A. Lichutin, A. Salnikova, and M. Khodzitsky, Biomed. Opt. Express 8, 273 (2017). https://doi.org/10.1364/BOE.8.000273
V. I. Fedorov, Biomed. Radioelectron. 1, 34 (2014).
J. Bock, Y. Fukuyo, S. Kang, M. L. Phipps, L. B. Alexandrov, K. O. Rasmussen, A. R. Bishop, E. D. Rosen, J. S. Martinez, H. T. Chen, G. Rodriguez, B. S. Alexandrov, and A. Usheva, PLoS One 5, e15806 (2010). https://doi.org/10.1371/journal.pone.0015806
B. S. Alexandrov, K. O. Rasmussen, A. R. Bishop, A. Usheva, L. B. Alexandrov, S. Chong, Y. Dagon, L. G. Booshehri, C. H. Mielke, M. L. Phipps, J. S. Martinez, H. T. Chen, and G. Rodriguez, Biomed. Opt. Express 2, 2679 (2011). https://doi.org/10.1364/BOE.2.002679
B. S. Alexandrov, M. L. Phipps, L. B. Alexandrov, L. G. Booshehri, A. Erat, J. Zabolotny, C. H. Mielke, H. T. Chen, G. Rodriguez, K. O. Rasmussen, J. S. Martinez, A. R. Bishop, and A. Usheva, Sci. Rep. 3, 1 (2013). .https://doi.org/10.1038/srep01184
A. N. Bogomazova, E. M. Vassina, T. N. Goryachkovskaya, V. M. Popik, A. S. Sokolov, N. A. Kolchanov, M. A. Lagarkova, S. L. Kiselev, and S. E. Peltek, Sci. Rep. 5, 1 (2015). https://doi.org/10.1038/srep07749
V. I. Fedorov, D. S. Serdyukov, O. P. Cherkasova, S. S. Popova, and E. F. Nemova, J. Opt. Technol. 84, 509 (2017). https://doi.org/10.1364/JOT.84.000509
K. I. Zaytsev, I. N. Dolganova, N. V. Chernomyrdin, G. M. Katyba, A. A. Gavdush, O. P. Cherkasova, G. A. Komandin, M. A. Shchedrina, A. N. Khodan, D. S. Ponomarev, I. V. Reshetov, V. E. Karasik, M. Skorobogatiy, V. N. Kurlov, and V. V. Tuchin, J. Opt. 22, 013001 (2020). https://doi.org/10.1088/2040-8986/ab4dc3
M.-O. Mattsson, O. Zeni, and M. Simko, J. Infrared Millim. Teraherz Waves (2018). https://doi.org/10.1007/s10762-018-0483-5
I. Ilina, D. S. Sitnikov, and M. B. Agranat, High Temp. 56, 789 (2018). https://doi.org/10.1134/S0018151X18050127
D. S. Sitnikov, I. V. Ilina, and A. A. Pronkin, Opt. Eng. 59, 061613 (2020). https://doi.org/10.1117/1.OE.59.6.061613
Funding
This work was supported by the Russian Science Foundation, grant no. 18-12-00328.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement on the Welfare of Animals
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Translated by D. Timchenko
Rights and permissions
About this article
Cite this article
Cherkasova, O.P., Serdyukov, D.S., Ratushnyak, A.S. et al. Effects of Terahertz Radiation on Living Cells: a Review. Opt. Spectrosc. 128, 855–866 (2020). https://doi.org/10.1134/S0030400X20060041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0030400X20060041