Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by inflammation in the joints. Although methotrexate (MX) is the first-line treatment, side effects are common. This study aimed to investigate the effects of quercetin (QT) and/or MX on inflammation and systemic toxicity in a rat model of RA. Male Wistar rats were divided into control (C), RA, QT, MX, and QT + MX groups (n=6). The RA induction consisted of three intra-articular injections of methylated bovine serum albumin (1×/week) in the temporomandibular joint (TMJ). QT (25 mg/kg) and/or MX (0.75 mg) administration occurred by oral gavage daily. We performed mechanical hyperalgesia in TMJ, leukocyte recruitment in synovial fluid, histopathology, and immunohistochemistry (TNF-α, IL-17, and IL-10) in synovial membrane and toxicity parameters. The RA showed a reduction in the nociceptive threshold (p<0.001), increase in leukocyte recruitment in synovial fluid (p<0.001), intense inflammatory infiltrate (p<0.001), and intense immunoexpression of TNF-α, IL-17, and IL-10 in the synovial membrane (p<0.001) compared to C (p<0.001). QT and/or MX therapy reduced inflammatory parameters (p<0.001). However, downregulation of IL-10 was observed only in the groups that received MX (p<0.001). Leukocytosis was seen in RA (p<0.05), but QT and/or MX reversed it (p<0.05). MX was associated with pathological changes in the liver and higher levels of transaminases when compared to the other groups (p<0.05). QT co-administered with MX reversed this hepatotoxicity (p<0.05). There were no alterations in the kidney between the groups (p>0.05). QT has potential to support MX therapy, showing anti-inflammatory and hepatoprotective effects in this model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease, characterized by inflammation, pain, and dysfunction in multiple joints [1]. Although the joints of the hands, wrists, and feet are the most affected in RA, the temporomandibular joint (TMJ) may be involved [2]. However, studies that assess the RA manifestations in the orofacial region are scarce [3].
The treatment of RA varies according to the stage, activity, and severity of the disease and rarely achieves complete remission [1]. Disease-modifying antirheumatic drugs (DMARDs) have antiproliferative, immunosuppressive, and anti-inflammatory activity, controlling the progression of RA [4]. Currently, methotrexate (MX) is the first-line treatment in RA progression [5]. Despite the good therapeutic effect, the long-term administration of MX may cause relevant side effects, such as drug resistance, leukopenia, liver, and kidney damage [6, 7].
Therefore, alternative therapies and natural compounds with potential to intensify the action of MX and reduce its side effects have been encouraged in RA [1, 8]. Quercetin (QT/3,3′, 4′, 5,7-pentahydroxyfavone) is a polyphenic flavonoid widely found in fruits and vegetables, such as apple, red grape, onion, and broccoli, and in nutritional supplement with antioxidant and anti-inflammatory properties [9, 10]. This substance is safe, well tolerated, and have been used to treat or prevent diverse chronic conditions including periodontitis, obesity, diabetes, cancer, and rheumatic diseases [11,12,13,14,15].
Evidences indicate QT is an applicable therapy in RA, which promotes apoptosis of synoviocytes in cell culture, downregulates pro-inflammatory mediators and modulates transcription factor signaling pathways in preclinical studies, ameliorates clinical symptoms, and reduces pro-inflammatory cytokines in plasma levels of RA patients [16,17,18,19]. However, it has not been reported that this flavonoid enhances the therapeutic effect of MX and reduces its side effects.
In this way, the present study aimed to investigate the effects of QT alone or combined with MX on inflammation and systemic toxicity in a rat model of RA.
MATERIALS AND METHODS
Ethics Statement
The experimental procedures were approved by the Ethical Committee on Animal Use (CEUA) of the Federal University of Ceará, Brazil (no. 148/17), according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications no 8023, revised 1978) and the ARRIVE guidelines, UK. The animals were maintained in cages (3 rats per cage) in a quiet room with controlled temperature (22oC), light/dark cycle 12/12 h, and easy access to water and food.
Animals
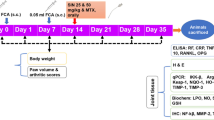
Thirty male Wistar rats (7–8 weeks old; 180–220 g), from the Central Animal Facility of the Federal University of Ceará, were randomly divided into five experimental groups (n=6): control (C), animals with RA (RA), animals with RA and treated with QT (QT), animals with RA and treated with MX (MX), animals with RA and treated with QT and MX (QT + MX).
Induction of Experimental Rheumatoid Arthritis
The antigen-induced RA model was chosen because it develops an immune-mediated arthritis with morphological characteristics similar to that of humans. Initially, the animals were sensitized to the antigen with a subcutaneous injection in the back containing 500 μg of methylated bovine serum albumin (mBSA; Sigma-Aldrich, St. Louis, USA) diluted in an emulsion with 100 μl of phosphate-buffered saline (PBS) and 100 μl of complete Freund’s adjuvant (CFA; Sigma-Aldrich, St. Louis, USA). Subcutaneous booster injections containing 500 μg of mBSA, 100 μl of PBS, and 100 μl of incomplete Freund’s adjuvant (IFA; Sigma-Aldrich, St. Louis, USA) were administered on the 7th and 14th days after the first immunization.
Twenty-one days after the sensitization phase, the animals received intra-articular challenges with mBSA. After intraperitoneal anesthesia with ketamine 2% (80 mg/kg) and xylazine 10% (10 mg/kg), 10 μg of mBSA dissolved in 10 μl of PBS was administered in the supra-disc space of the left TMJ. Intra-articular reinforcement challenges were administered on 28th and 35th days. The animals were euthanized 24 h after the third intra-articular challenge by a high dose of 10% ketamine and 2% xylazine. The control group received TMJ injections with PBS [20].
Drug Administration Protocol
A pilot study was performed to provide the best anti-inflammatory dose of QT. Thus, QT 25, 50, or 100 mg/kg was administered by oral gavage daily in rats with RA in TMJ [21]. It was observed that the groups treated with QT in different doses did not show statistical difference in the inflammatory parameters (Supplement Material: SM Fig. 1, SM Fig. 2 and SM Table). Thus, the lowest QT dose was chosen to the following analyses.
QT (25 mg/kg; purity≥98%; Sigma-Aldrich, St. Louis, USA) and/or MX (0.75 mg; Tecnomet, Zodiac, São Paulo, Brazil) was performed by oral gavage daily from the 24th h after the first intra-articular challenge until the last day of induction of RA (D22-D35) [15]. Control group and RA group received saline solution.
Mechanical Hyperalgesia Assessment
Mechanical hyperalgesia was assessed in the TMJ by an electronic analgesimeter (Eletronic Von Frey Digital, Insight, São Paulo, Brazil). The animals were conditioned to the nociceptive response assessment test for 5 days and after this period were kept for 20 min in plastic boxes, and then the device was applied to the left TMJ region, until a reflex response from the animal was obtained (head removal movement) [22]. The application was repeated three times, recording the intensity of force in grams (g). The average of the recorded values was made 6 h after the intra-articular challenges on 21st, 28th, and 35th days by a blinded and calibrated examiner (A.C.F.C.).
Leukocyte Recruitment in Synovial Fluid
After euthanasia, the skin and muscles around the left TMJ were dissected, and the joint cavity was washed and aspirated with 50 μl of phosphate-buffered saline (PBS) added to 10 mM of ethylenediamine tetra acetic acid (EDTA). The washing procedure was repeated, and the synovial fluid was collected. For total leukocyte count, 20 μl synovial fluid was diluted in 380 μl of Turk’s solution, and a treatment-blinded examiner (L.M.S.) determined the number of leukocytes using a Neubauer chamber (BRAND®, Sigma-Aldrich, St. Louis, USA) [23].
Histopathological Analysis of the Synovial Membrane
The TMJs collected were fixed in 10% buffered formaldehyde for 48 h and demineralized in 4% ethylenediamine tetra acetic acid (EDTA). After inclusion in paraffin, the samples were cut into 5-μm sections, mounted on slides, and stained with hematoxylin-eosin (HE). An experienced and treatment-blinded pathologist (K.M.A.P.) analyzed them under a light microscope (Leica DM 2000, Wetzlar, Germany) and applied scores by a semi-quantitative analysis for inflammatory infiltrate in the synovial membrane (0 = absent, 1 = mild, 2 = moderate, 3 = severe) [24].
Immunohistochemical Analysis of the Synovial Membrane
The streptavidin-biotin-peroxidase method was used in tissue sections (3 μm) embedded in paraffin in microscope slides coated with poly-l-lysine. After deparaffinization, rehydration, and antigenic recovery with citrate buffer (pH 6.0; 30 min; 85°C), the slides were subjected to blockade peroxidase with 3% hydrogen peroxide diluted in PBS for 30 min. After washing in PBS, the samples were incubated overnight with the primary rabbit anti-tumor necrosis factor (TNF)-α (Abcam, Cambridge, UK), primary rabbit anti-interleukin (IL)-17 (Santa Cruz Biotechnology, Texas, USA), and primary rabbit anti-IL-10 (Abcam, Cambridge, UK), using a 1:100, 1:100 and 1:150 dilution, respectively.
Envision System Plus-HRP (Dako, Santa Clara, USA) or the ImmunoCruz ABC Kit (Santa Cruz Biotechnology, Texas, USA) were used for secondary antibody incubation. Immunostaining was stained with 3,3-diaminobenzidine (DAB) (Dako, Santa Clara, USA) and counterstained with Harris hematoxylin. Then, the cuts were dehydrated in a graded series of alcohol, diaphanized in xylol, and mounted with Enthellam (Merck KGaA, Darmstadt, Germany) and a coverslip [25].
Five fields (×400) under an optical microscope (Leica DM 2000, Wetzlar, Germany) were selected at random for cell counts exhibiting cytoplasmic and/or nuclear positivity (brown staining) for TNF-α, IL-17, and IL-10 in the synovial membrane. Then, a treatment-blinded examiner (A.C.F.C.) obtained an average of the count value of these sections [20].
Toxicity Parameters Analysis
The peripheral blood samples were collected from the caudal vein for the total leukocyte count and biochemical analysis of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The leukocyte recruitment in the peripheral blood followed the same protocol as the synovial fluid counted by a treatment-blinded examiner (A.C.F.C.). For biochemical analysis of transaminases, the peripheral blood was centrifuged (1800 G; 10 min), and the plasma was collected, stored in a −80oC freezer, and analyzed by a specific kit according to the manufacturer’s guidelines (Labtest, Lagoa Santa, Brazil) [26].
After the euthanasia, liver and kidney were removed and fixed in buffered formaldehyde (10%) for 48 h. After this period, histological slides (HE; 5μm) were obtained. The histopathological analysis was performed by an experienced and treatment-blinded pathologist (K.M.A.P.) under an optical microscope (Leica DM 2000, Wetzlar, Germany). Scores were applied for inflammatory infiltrate (0 = absent, 1 = mild, 2 = moderate, 3 = intense), edema (0 = absent, 1 = mild, 2 = moderate, 3 = severe), and hemorrhagic lesion (0 = absent, 1 = mild, 2 = moderate, 3 = severe) [27].
Statistical Analysis
Quantitative data were presented as mean ± standard error of the mean (SEM), analyzed by Shapiro-Wilk normality test and compared by analysis of variance (ANOVA)-one-way, followed by Tukey post hoc test (parametric data). Semi-quantitative data were presented as median (maximum and minimum), analyzed by Shapiro-Wilk normality test, and compared by Kruskal-Wallis test, followed by Dunn post hoc test (nonparametric data). The software GraphPad Prism 7 (La Jolla, USA) was used to perform the statistical analysis and to create the artwork. Differences were considered as statistically significant at p<0.05.
RESULTS
Mechanical Hyperalgesia Assessment
Mechanical hyperalgesia in the TMJ region is a parameter used to assess the development of inflammatory diseases in the joint. In the present study, it was observed that during the induction of RA in the TMJ, the untreated arthritic animals showed a significant reduction in the nociceptive threshold from the 6th h after the first, second, and third intra-articular challenge when compared to C (p<0.0001) (Fig. 1a–c). At the 6th h after the second intra-articular challenge, the arthritic animals treated with QT and/or MX showed a significant increase in the nociceptive threshold when compared to untreated arthritic animals (p<0.0001) (Fig. 1b). At the 6th h after the third intra-articular challenge, it was observed that there was no statistical difference in the nociceptive threshold between the animals that received QT co-administered with MX (QT + MX) and the control animals (p>0.05) (Fig. 1c).
a Analysis of mechanical hyperalgesia in the left TMJ on 6th h after the 1st intra-articular challenge of experimental groups. b Analysis of mechanical hyperalgesia in the left TMJ on 6th h after the 2nd intra-articular challenge of experimental groups. c Analysis of mechanical hyperalgesia in the left TMJ on 6th hour after the 3rd intra-articular challenge of experimental groups. Each bar represents the mean ± SEM (n=6). p<0.0001; ANOVA-one-way; Tukey. Same letters indicate no statistical significance. C control animals, RA animals with RA in TMJ, QT animals with RA in TMJ and treated with QT, MX animals with RA in TMJ and treated with MX, QT + MX animals with RA in TMJ and treated with QT and MX.
Leukocyte Recruitment in Synovial Fluid
There was a significant increase in the leukocyte recruitment in the synovial fluid of untreated arthritic animals when compared to control animals (p<0.0001). Arthritic animals treated with QT and/or MX showed a significant reduction in this cell migration when compared to untreated arthritic animals (p<0.0001). There was no statistical difference between the treated groups and the C group (p>0.05) (Fig. 2).
Leukocyte recruitment in synovial fluid of experimental groups. Each bar represents the mean ± SEM (n=6). p<0.0001; ANOVA-one-way; Tukey. Same letters indicate no statistical significance. C control animals, RA animals with RA in TMJ, QT animals with RA in TMJ and treated with QT, MX animals with RA in TMJ and treated with MX, QT + MX animals with RA in TMJ and treated with QT and MX.
Histopathological Analysis of the Synovial Membrane
In histopathological analysis, the RA group presented an intense mononuclear inflammatory infiltrate in the synovial membrane with pannus formation (Table 1; Fig. 3c, d). All treatment groups (QT, MX, QT + MX) showed significant reduction in the inflammatory infiltrate when compared to the RA group (p<0.0001), and there was no statistical difference between them and C group (p>0.05) (Table 1; Fig. 3e–j).
Photomicrographs of the TMJ of experimental groups. a Normal TMJ overview. b Normal TMJ synovial membrane. c TMJ of RA group overview, showing pannus formation (P). d Synovial membrane of RA group, showing intense mononuclear inflammatory infiltrate (MII) and hemorrhage (H). e TMJ of QT group overview. f Synovial membrane of QT group, with moderate inflammatory infiltrate. g TMJ of MX group overview. h Synovial membrane of MX group, with moderate inflammatory infiltrate. i TMJ of QT + MX group overview. j Synovial membrane of QT + MX group, with mild inflammatory infiltrate. ×40 and ×200 magnification. C control animals, RA animals with RA in TMJ, QT animals with RA in TMJ and treated with QT, MX animals with RA in TMJ and treated with MX, QT + MX animals with RA in TMJ and treated with QT and MX.
Immunohistochemical Analysis of the Synovial Membrane
The untreated arthritic animals showed a significant increase in the immunoexpression of TNF-α, IL-17 and IL-10 of the synovial membrane when compared to control animals (p<0.0001). Arthritic animals treated with QT and/or MX showed a significant reduction in TNF-α and IL-17 immunoexpression (p<0.0001). However, the QT + MX group showed the best response in TNF-α, with no difference in relation to the C group (p>0.05). QT did not modify the immunoexpression of IL-10 in arthritic animals (p>0.05). The MX administration reduced the IL-10 immunoexpression in RA and QT groups (p<0.0001), and it was not observed a statistical difference with the C group (p>0.05) (Fig. 4a–r).
Photomicrographs and evaluation of the immunoexpression of inflammatory markers in synovial membrane of experimental groups. a–f TNF-α. g–l IL-17. m–r IL-10. ×400 magnification. Each bar represents the mean ± SEM (n=6). p<0.0001; ANOVA-one-way; Tukey. Same letters indicate no statistical significance. C control animals, RA animals with RA in TMJ, QT animals with RA in TMJ and treated with QT, MX animals with RA in TMJ and treated with MX, QT+MX animals with RA in TMJ and treated with QT and MX.
Toxicity Parameters Analysis
The untreated arthritic animals presented leukocytosis when compared to the C group (p<0.0001). QT and/or MX treatment showed a significant reduction of leukocyte recruitment in peripheral blood when compared to untreated arthritic animals (p<0.0001). There was no statistical difference between the treated groups when compared to the C group in this parameter (p>0.05) (Fig. 5a)
a Leukocyte recruitment in peripheral blood of experimental groups. b Plasma ALT level of experimental groups. c Plasma AST level of experimental groups. Each bar represents the mean ± SEM (n=6). p<0.0001; ANOVA-one-way; Tukey. Same letters indicate no statistical significance. C control animals; RA animals with RA in TMJ; QT animals with RA in TMJ and treated with QT; MX animals with RA in TMJ and treated with MX; QT + MX animals with RA in TMJ and treated with QT and MX.
In biochemical analysis of transaminases, it was observed that the RA and the use of isolated MX were associated with higher levels of ALT and AST when compared to the C group (p<0.0001). The use of QT reduced the ALT and AST levels of these groups, demonstrating a hepatoprotective tendency of this flavonoid (p<0.0001) (Fig. 5b, c).
In the histopathological analysis of the liver, the arthritic animals that received only MX had the worst parameters for inflammatory infiltrate, edema, and hemorrhagic injury (p<0.05). QT co-administered with MX (QT + MX) reduced inflammatory infiltrate, edema, and hemorrhagic injury (p<0.05). The RA, QT, and QT + MX groups showed no statistical difference in the morphological parameters assessed when compared to control animals (p>0.05) (Table 2; Supplement Material: SM Fig. 3).
In the histopathological analysis of the kidney, there were no significant morphological alterations for inflammatory infiltrate, edema, and hemorrhagic between the experimental groups (p>0.05) (Table 2; Supplement Material: SM Fig. 3).
DISCUSSION
Our results suggest that QT reduces inflammation and shows hepatoprotective effect in an experimental model of RA in TMJ. Therefore, QT may be useful to support the MX therapy in RA, reducing its side effects.
In the present study, the possible mechanism for reversing the hyperalgesic state in TMJ region of arthritic animals with QT and/or MX therapy was the reduction of joint inflammation [28]. Pain is a relevant symptom in RA and affects the quality of life of patients [19]. In the orofacial region, pain perception occurs through activation of the trigeminal nociceptive pathway [29]. In processes involving inflammatory pain, this activation begins by sensitizing the free nerve endings by inflammatory mediators, resulting in positive functional regulation of nociceptive receptors, responsible for hyperalgesia [30]. The pharmacological management of peripheral inflammatory pain is important to prevent, to block, or to reverse the hyperalgesic state [31].
Synovitis in RA is characterized by an intense cellular infiltration in the synovium and synovial membrane [32]. Thus, RA patients have high levels of leukocytes, such as macrophages, monocytes, plasmocytes and dendritic cells, and high levels of pro-inflammatory mediators, such as IL-1β, IL-6, IL-17, and TNF-α in the synovial fluid and tissues [33]. Our investigation corroborates this evidence when untreated arthritic animals showed an increase in leukocyte recruitment in the synovial fluid and synovial membrane with pannus formation. An interesting outcome was that QT and/or MX therapy reduced this joint inflammation.
The imbalance of cytokine production is common in RA, with excess of pro-inflammatory mediators compared with anti-inflammatory mediators [5]. Thus, we investigated the immunoexpression of TNF-α, IL-17, and IL-10 immunoexpression that show relevance in the RA pathogenesis. TNF-α plays a central role in the development and maintenance of RA, directly contributing to synovial inflammation and tissue degradation by increasing the production of chemokines, adhesion molecules, and other effector cells [34]. IL-17, on the other hand, acts locally on synoviocytes and osteoblasts, contributing to synovitis and joint destruction mediated by metalloproteinases and other effector molecules [34, 35]. The effect of IL-17A is enhanced by TNF-α and causes upregulation of other pro-inflammatory cytokines, such as IL-6 and IL-8, promoting an invasive synoviocyte phenotype [36]. In fact, the use of TNF-α and IL-17 inhibitors is considered a well-established therapy for RA, revealing the importance of these mediators in this pathological condition [37].
IL-10 is a key cytokine in autoimmune disorders, playing immunoregulatory, anti-inflammatory, and/or pro-inflammatory roles in the pathogenesis of RA [38, 39]. Generally, IL-10 is located in the lining layer of the synovial membrane, where monocyte migration occurs, inhibiting pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α [40]. Thus, the IL-10 deficiency is associated with an exacerbation of the inflammatory response, resulting in tissue damage [41]. Our study observed an increase in the immunoexpression of IL-10 in the synovial membrane in the RA group compared to the C group. This is possibly an attempt to repair the local lesion through a compensatory mechanism for downregulation in the pro-inflammatory cytokines. This finding corroborates a previous study that found higher serum levels of IL-10 in RA patients when compared to healthy volunteers and that it correlated with the disease activity [38].
In the present study, QT and/or MX therapy reduced the immunoexpression of TNF-α and IL-17 in arthritic animals, in agreement with other studies in the literature [42]. However, the reversal of TNF-α immunoexpression was more effective in the group with the co-administered drugs, suggesting a synergistic effect. MX has an immunosuppressive action, reducing neutrophil chemotaxis and anti- and pro-inflammatory cytokines [4, 15, 43]. On the other hand, QT reduces inflammation in RA through the modulation of immune cells; inflammatory mediators, such as IL-1β, IL-6, IL-17, and TNF-α; and matrix metalloproteinases [15]. Besides that, previous studies revealed that this flavonoid increases the levels of pro-inflammatory cytokines, like IL-10, although the mechanism is unclear [44]. However, the immunoexpression of IL-10 in the arthritic animals was not modified by QT in this investigation, but only with MX administration.
RA patients treated with MX show a good clinical response, but side effects are common with the long-term administration, requiring blood count monitoring [7]. In addition, it is estimated that 30–50% of patients treated with MX experience reduced or no response with continued use [45]. Despite leukopenia and nephrotoxicity being common during MX therapy, the therapeutic protocol of this study was not able to promote these alterations, probably due to the short period of drug administration. Only the untreated arthritic animals showed changes in peripheral blood: leukocytosis. The increase of the seric leukocytes was also observed in RA patients, during periods of disease activity, due to chronic systemic inflammation [46]. In addition, the MX therapy and the untreated arthritic animals were associated to the hepatic morphological alterations and to the increase of liver transaminases levels. Our results showed that QT reduces the hepatotoxicity associated with RA and MX therapy, suggesting a hepatoprotective effect.
RA is a predominantly articular disease, although extra-articular manifestations can be observed, such as cutaneous, cardiac, pulmonary, and ocular [47]. Liver damage is not recognized as an extra-articular manifestation of RA. However, patients with RA often experience hepatic alterations, such as increase of ALT and AST by laboratory tests and histopathology alterations by biopsy [48]. The cause of hepatic damage in RA is controversial. The authors suggest that these injuries are secondary to the administration of potentially hepatotoxic drugs, such as MX [49]. Other evidence shows that the liver of patients with autoimmune diseases is more susceptible to damage, as this organ plays a significant role in modulating the immune system in response to chronic inflammatory disorder [50].
In agreement with our results, recent studies suggest the hepatoprotective potential of QT [51]. These authors show that this flavonoid acts in modulating oxidative stress mechanisms, apoptosis, inflammation, and signaling pathways in cell culture and in different animal models of liver injury, such as drugs, toxins, or alcohol induced [51, 52]. However, there are no findings about the use of QT in hepatotoxicity induced by MX therapy in RA.
The limitations of this study include the use of an animal model and the short therapeutic protocol of MX that was not able to develop leukopenia or nephrotoxicity in the animals. In addition, although QT has shown positive effects in this experimental model, it is known that the use in naturally occurring form has questionable relevance due to poor bioavailability. Further studies should be encouraged to investigate the use of QT bioconjugates.
CONCLUSIONS
Our research suggested that QT is a potential adjunctive therapy for MX, presenting anti-inflammatory and hepatoprotective effects in mBSA-induced RA in TMJ of rats. Clinical investigations are required to support these findings.
References
Aletaha, D., J.S. Smolen, and J. S. 2018. Diagnosis and management of rheumatoid arthritis: a review. Jama 320: 1360–1372. https://doi.org/10.1001/jama.2018.13103.
Lin, C.Y., C.H. Chung, H.Y. Chu, L.C. Chen, K.H. Tu, C.H. Tsao, et al. 2017. Prevalence of Temporomandibular Disorders in Rheumatoid Arthritis and Associated Risk Factors: A Nationwide Study in Taiwan. Journal of Oral & Facial Pain & Headache 31: e29–e36. https://doi.org/10.11607/ofph.1917.
González-Chávez, S.A., C. Pacheco-Tena, T. de Jesús Caraveo-Frescas, C.M. Quiñonez-Flores, G. Reyes-Cordero, and R.M. Campos-Torres. 2020. Oral health and orofacial function in patients with rheumatoid arthritis. Rheumatology International 40: 445–453. https://doi.org/10.1007/s00296-019-04440-3.
Friedman, B., and B. Cronstein. 2019. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine 86: 301–307. https://doi.org/10.1016/j.jbspin.2018.07.004.
Alam, J., I. Jantan, and S.N.A. Bukhari. 2017. Rheumatoid arthritis: recent advances on its etiology, role of cytokines and pharmacotherapy. Biomedicine & Pharmacotherapy 92: 615–633. https://doi.org/10.1016/j.biopha.2017.05.055.
Yu, J., and P. Zhou. 2020. The advances of methotrexate resistance in rheumatoid arthritis. Inflammopharmacology 4: 1–11. https://doi.org/10.1007/s10787-020-00741-3.
Wang, W., H. Zhou, and L. Liu. 2018. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. European Journal of Medicinal Chemistry 158: 502–516. https://doi.org/10.1016/j.ejmech.2018.09.027.
Basu, A., J. Schell, and R.H. Scofield. 2018. Dietary fruits and arthritis. Food & Function 9: 70–77. https://doi.org/10.1039/c7fo01435j.
Gardi, C., K. Bauerova, B. Stringa, V. Kuncirova, L. Slovak, S. Ponist, F. Drafi, L. Bezakova, I. Tedesco, A. Acquaviva, S. Bilotto, and G.L. Russo. 2015. Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Archives of Biochemistry and Biophysics 583: 150–157. https://doi.org/10.1016/j.abb.2015.08.008.
Salehi, B., L. Machin, L. Monzote, J. Sharifi-Rad, S.M. Ezzat, M.A. Salem, et al. 2020. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega. https://doi.org/10.1021/acsomega.0c01818.
Andres, S., S. Pevny, R. Ziegenhagen, N. Bakhiya, B. Schäfer, K.I. Hirsch-Ernst, and A. Lampen. 2018. Safety aspects of the use of quercetin as a dietary supplement. Molecular Nutrition & Food Research 62: 1700447. https://doi.org/10.1002/mnfr.201700447.
Xiong, G., W. Ji, F. Wang, F. Zhang, P. Xue, M. Cheng, Y. Sun, X. Wang, and T. Zhang. 2019. Quercetin inhibits inflammatory response induced by LPS from porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-κB signaling pathway. BioMed Research International e2019: 1–10. https://doi.org/10.1155/2019/6282635.
Chen, S., H. Jiang, X. Wu, and J. Fang. 2016. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediators of Inflammation e2016: 1–16. https://doi.org/10.1155/2016/9340637.
Ren, K.W., Y.H. Li, G. Wu, J.Z. Ren, H.B. Lu, Z.M. Li, and X.W. Han. 2017. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. International Journal of Oncology 50: 1299–1311. https://doi.org/10.3892/ijo.2017.3886.
Haleagrahara, N., K. Hodgson, S. Miranda-Hernandez, S. Hughes, A.B. Kulur, and N. Ketheesan. 2018. Flavonoid quercetin–methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology 26: 1219–1232. https://doi.org/10.1007/s10787-018-0480-2.
Gokhale, J.P., H.S. Mahajan, and S.J. Surana. 2019. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomedicine & Pharmacotherapy 112: 108622. https://doi.org/10.1016/j.biopha.2019.108622.
Pan, F., L. Zhu, H. Lv, and C. Pei. 2016. Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. International Journal of Molecular Medicine 38: 1507–1514. https://doi.org/10.3892/ijmm.2016.2755.
Haleagrahara, N., S. Miranda-Hernandez, M.A. Alim, L. Hayes, G. Bird, and N. Ketheesan. 2017. Therapeutic effect of quercetin in collagen-induced arthritis. Biomedicine & Pharmacotherapy 90: 38–46. https://doi.org/10.1016/j.biopha.2017.03.026.
Javadi, F., A. Ahmadzadeh, S. Eghtesadi, N. Aryaeian, M. Zabihiyeganeh, A. Rahimi Foroushani, and S. Jazayeri. 2017. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. Journal of the American College of Nutrition 36: 9–15. https://doi.org/10.1080/07315724.2016.1140093.
de Sousa, L.M., J.M. dos Santos Alves, C. da Silva Martins, K.M.A. Pereira, P. Goes, and D.V. Gondim. 2019. Immunoexpression of canonical Wnt and NF-κB signaling pathways in the temporomandibular joint of arthritic rats. Inflammation Research 68: 889–900. https://doi.org/10.1007/s00011-019-01274-4.
Azevedo, M.I., A.F. Pereira, R.B. Nogueira, F.E. Rolim, G.A. Brito, D.V.T. Wong, R.C.P. Lima-Júnior, R. de Albuquerque Ribeiro, and M.L. Vale. 2013. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Molecular Pain 9: 1744–8069. https://doi.org/10.1186/1744-8069-9-53.
Gondim, D.V., J.L. Costa, S.S.G.A.D.C. Rocha, R.D. Brito, A. Ribeiro, and M.L. Vale. 2012. Antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint. Canadian Journal of Physiology and Pharmacology 90: 395–405. https://doi.org/10.1139/y2012-003.
Denadai-Souza, A., L. de Lucca Camargo, M.T. Ribela, J.E. Keeble, S.K. Costa, and M.N. Muscará. 2009. Participation of peripheral tachykinin NK1 receptors in the carrageenan-induced inflammation of the rat temporomandibular joint. European Journal of Pain 13: 812–819. https://doi.org/10.1016/j.ejpain.2008.09.012.
Chaves, H.V., R.D.A. Ribeiro, A.M.B. de Souza, A.S. Gomes, M.L. Vale, M.M. Bezerra, and G.A.D.C. Brito. 2011. Experimental model of zymosan-induced arthritis in the rat temporomandibular joint: role of nitric oxide and neutrophils. Journal of Biomedicine and Biotechnology e2011: 707985. https://doi.org/10.1155/2011/707985.
Brizeno, L.A.C., A.M.S. Assreuy, A.P.N. Alves, F.B. Sousa, P.G.D.B. Silva, S.C.O. Sousa, et al. 2016. Delayed healing of oral mucosa in a diabetic rat model: Implication of TNF-α, IL-1β and FGF-2. Life Sciences 155: 36–47. https://doi.org/10.1016/j.lfs.2016.04.033.
Guimarães, M.V., I.M. Melo, V.M.A. Araújo, D.V.T. Wong, C.S.R. Fonteles, L.K.A.M. Leal, et al. 2016. Dry Extract of Matricaria recutita L.(Chamomile) Prevents Ligature-Induced Alveolar Bone Resorption in Rats via Inhibition of Tumor Necrosis Factor-α and Interleukin-1β. Journal of Periodontology 87: 706–715. https://doi.org/10.1902/jop.2016.150411.
Teixeira, A.H., J.M. Freire, L.H. de Sousa, A.T. Parente, N.A. de Sousa, A. Arriaga, et al. 2017. Stemodia maritima L. extract decreases inflammation, oxidative stress, and alveolar bone loss in an experimental periodontitis rat model. Frontiers in Physiology 8: 988. https://doi.org/10.3389/fphys.2017.00988.
Quinteiro, M.S., M.H. Napimoga, K.P. Mesquita, and J.T. Clemente-Napimoga. 2012. The indirect antinociceptive mechanism of 15 d-PGJ 2 on rheumatoid arthritis-induced TMJ inflammatory pain in rats. European Journal of Pain 16: 1106–1115. https://doi.org/10.1002/j.1532-2149.2012.00114.x.
Bas, D.B., J. Su, G. Wigerblad, and C.I. Svensson. 2016. Pain in rheumatoid arthritis: models and mechanisms. Pain Management 6: 265–284. https://doi.org/10.2217/pmt.16.4.
Sessle, B.J. 2011. Peripheral and central mechanisms of orofacial inflammatory pain. International Review of Neurobiology 97: 179–206. https://doi.org/10.1016/B978-0-12-385198-7.00007-2.
Napimoga, M.H., G.R. Souza, T.M. Cunha, L.F. Ferrari, J.T. Clemente-Napimoga, C.A. Parada, W.A. Verri Jr., F.Q. Cunha, and S.H. Ferreira. 2008. 15d-prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. Journal of Pharmacology and Experimental Therapeutics 324: 313–321. https://doi.org/10.1124/jpet.107.126045.
Sakuraba, K., K. Fujimura, Y. Nakashima, K. Okazaki, J.I. Fukushi, M. Ohishi, A. Oyamada, Y. Esaki, H. Miyahara, Y. Iwamoto, Y. Yoshikai, and H. Yamada. 2015. Brief Report: Successful In Vitro Culture of Rheumatoid Arthritis Synovial Tissue Explants at the Air–Liquid Interface. Arthritis & Rheumatology 67: 887–892. https://doi.org/10.1002/art.39019.
Penatti, A., F. Facciotti, R. de Matteis, P. Larghi, M. Paroni, A. Murgo, O. de Lucia, M. Pagani, L. Pierannunzii, M. Truzzi, A. Ioan-Facsinay, S. Abrignani, J. Geginat, and P.L. Meroni. 2017. Differences in serum and synovial CD4+ T cells and cytokine profiles to stratify patients with inflammatory osteoarthritis and rheumatoid arthritis. Arthritis Research & Therapy 19: 1–9. https://doi.org/10.1186/s13075-017-1305-1.
McInnes, I.B., C.D. Buckley, and J.D. Isaacs. 2016. Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nature Reviews Rheumatology 12: 63–68. https://doi.org/10.1038/nrrheum.2015.171.
Roeleveld, D.M., and M.I. Koenders. 2015. The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 74: 101–107. https://doi.org/10.1016/j.cyto.2014.10.006.
Robert, M., and P. Miossec. 2019. IL-17 in rheumatoid arthritis and precision medicine: from synovitis expression to circulating bioactive levels. Frontiers in Medicine 5: 364. https://doi.org/10.3389/fmed.2018.00364.
Hot, A., and P. Miossec. 2011. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Annals of the Rheumatic Diseases 70: 727–732. https://doi.org/10.1136/ard.2010.143768.
Tsukamoto, M., N. Seta, K. Yoshimoto, K. Suzuki, K. Yamaoka, and T. Takeuchi. 2017. CD14 bright CD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Research & Therapy 19: 28. https://doi.org/10.1186/s13075-016-1216-6.
Ummarino, D. 2017. Defective IL-10-producing B reg cells. Nature Reviews Rheumatology 13: 132–132. https://doi.org/10.1038/nrrheum.2017.10.
Katsikis, P.D., C.Q. Chu, F.M. Brennan, R.N. Maini, and M. Feldmann. 1994. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. The Journal of Experimental Medicine 179: 1517–1527. https://doi.org/10.1084/jem.179.5.1517.
Marinou, I., J. Healy, D. Mewar, D.J. Moore, M.C. Dickson, M.H. Binks, D.S. Montgomery, K. Walters, and A.G. Wilson. 2007. Association of interleukin-6 and interleukin-10 genotypes with radiographic damage in rheumatoid arthritis is dependent on autoantibody status. Arthritis & Rheumatology 56: 2549–2556. https://doi.org/10.1002/art.22814.
Kawaguchi, K., M. Kaneko, R. Miyake, H. Takimoto, and Y. Kumazawa. 2019. Potent Inhibitory Effects of Quercetin on Inflammatory Responses of Collagen-Induced Arthritis in Mice. Endocrine, Metabolic & Immune Disorders-Drug Targets 19: 308–315. https://doi.org/10.2174/1871530319666190206225034.
Noack, M., and P. Miossec. 2019. Effects of Methotrexate Alone or Combined With Arthritis-Related Biotherapies in an in vitro Co-culture Model With Immune Cells and Synoviocytes. Frontiers in Immunology 10: 2992. https://doi.org/10.3389/fimmu.2019.02992.
Milenković, M., N. Arsenović-Ranin, Z. Stojić-Vukanić, B. Bufan, D. Vučićević, and I. Jančić. 2010. Quercetin ameliorates experimental autoimmune myocarditis in rats. Journal of Pharmacy & Pharmaceutical Sciences 13: 311–319. https://doi.org/10.18433/j3vs3s.
Inoue, K., and H. Yuasa. 2014. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metabolism and Pharmacokinetics 29: 12–19. https://doi.org/10.2133/dmpk.dmpk-13-rv-119.
Syed, K.M., and R.S. Pinals. 1996. Leukocytosis in rheumatoid arthritis. Journal of Clinical Rheumatology 2: 197–202. https://doi.org/10.1097/00124743-199608000-00007.
Das, S., and P. Padhan. 2017. An overview of the extraarticular involvement in rheumatoid arthritis and its management. Journal of Pharmacology & Pharmacotherapeutics 8: 81. https://doi.org/10.4103/jpp.JPP_194_16.
Radovanović-Dinić, B., S. Tešić-Rajković, V. Zivkovic, and S. Grgov. 2018. Clinical connection between rheumatoid arthritis and liver damage. Rheumatology International 38: 715–724. https://doi.org/10.1007/s00296-018-4021-5.
Conway, R., and J.J. Carey. 2017. Risk of liver disease in methotrexate treated patients. World Journal of Hepatology 9: 1092–1100. https://doi.org/10.4254/wjh.v9.i26.1092.
Cojocaru, M., I.M. Cojocaru, I. Silosi, and C.D. Vrabie. 2013. Liver involvement in patients with systemic autoimmune diseases. Maedica 8: 394.
Miltonprabu, S., M. Tomczyk, K. Skalicka-Woźniak, L. Rastrelli, M. Daglia, S.F. Nabavi, S.M. Alavian, and S.M. Nabavi. 2017. Hepatoprotective effect of quercetin: From chemistry to medicine. Food and Chemical Toxicology 108: 365–374. https://doi.org/10.1016/j.fct.2016.08.034.
Huang, Z.Q., P. Chen, W.W. Su, Y.G. Wang, H. Wu, W. Peng, and P.B. Li. 2018. Antioxidant activity and hepatoprotective potential of quercetin 7-rhamnoside in vitro and in vivo. Molecules 23: 1188. https://doi.org/10.3390/molecules23051188.
Acknowledgements
The authors gratefully acknowledge the Nucleus of Studies in Microscopy and Imaging Processing (NEMPI) and the Laboratory of Inflammation and Cancer (LAFICA) of Federal University of Ceará.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
A.C.F.C., L.M.S. and J.M.S.A. performed animal and laboratory experiments. A.C.F.C., L.M.S., K.M.A.P., A.P.N.N.A., P.G and D.V.G contributed to data analysis and interpretation. A.C.F.C. wrote a draft of the manuscript. D.V.G and M.L.V. contributed to the concept and design of the study. D.V.G coordinated the experiments and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experimental procedures were approved by the Ethical Committee on Animal Use (CEUA) of the Federal University of Ceará, Brazil with number protocol 148/17, according with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications no 8023, revised 1978) and the ARRIVE guidelines UK.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costa, A.C.d.F., de Sousa, L.M., dos Santos Alves, J.M. et al. Anti-inflammatory and Hepatoprotective Effects of Quercetin in an Experimental Model of Rheumatoid Arthritis. Inflammation 44, 2033–2043 (2021). https://doi.org/10.1007/s10753-021-01479-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01479-y