Abstract
An autoimmune disease, rheumatoid arthritis (RA) is characterized by the onset of inflammation and subsequent damage to the joints. Although several therapies are available for RA, none are effective, and many have undesirable side effects. The roots of Sinomenium acutum produce an alkaloid called Sinomenine (SIN), which has been used for centuries in Chinese medicine to treat arthritis due to its anti-inflammatory properties. This study aimed to explore the potential therapeutic benefits of SIN through oral administration following RA induction using Freund’s complete adjuvant (FCA) injections. The study monitored changes in the arthritic index, hind paw volume, inflammation and oxidative stress markers. Results demonstrated that SIN effectively inhibited the activity of NF-κB and IKKβ in knee joint tissues, which led to a decrease in tissue levels of TNF-α, IL-6, IL-1β, and iNOS in RA-induced rats. The production of anti-inflammatory cytokines such as IL-10, Arg-1, and Fizz1 also increased. In rat knee joints, SIN elevated the expression of TIMP-1 and TIMP-3 and decreased the expression of MMP-2 and MMP-9. Additionally, SIN modulated the RANK/RANKL/OPG pathway in RA-induced rat knee joint tissues, reducing RANKL expression and increasing OPG. SIN also effectively decreased MDA, NO, and elevated antioxidant enzymes (SOD, CAT, GPx, and GSH) in RA-induced rats via Nrf2/Keap 1 signaling pathway activation. In conclusion, this study suggests that SIN possesses potential therapeutic benefits for treating RA by modulating the RANK/RANKL/OPG pathway, which may impact osteoclast activity, oxidative stress, and inflammation in knee joint tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an inflammatory condition that affects the complete body, causing signs that include synovial hyperplasia, cartilage degradation, and joint fragmentation. These complications can lead to respiratory, cardiovascular, and psychological problems (Smolen et al. 2018). Clinical resistance and immune cell leakage from autoimmunity into the synovium cause RA synovitis (McInnes and Schett 2011). The prevalence of RA varies by region but is estimated to affect 1% of the global population, with an annual incidence rate of 3 cases per 10,000 individuals (Prasad et al. 2023). There were approximately 460 cases of RA per 100,000 persons observed globally between 1980 and 2019 (Vasdev et al. 2023). According to Pathade et al. (2022), forecast that the prevalence of RA in the eight major markets will rise from 4.6 million in 2019 to 5.1 million in 2029.
The exact pathophysiology of RA remains elusive (Zampeli et al. 2015). However, it is known that an interruption in the body’s immune system brings about a substantial leak of leukocytes (WBCs) right into the synovium, leading to synovial hyperplasia and inflammation (Tian et al. 2021). As inflammatory cells migrate in and new blood vessels form, a layer of synovial membrane called the pannus develops. Pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α play pivotal roles in development and progression of RA. These molecules have a pronounced effect on the degradation of bone and cartilage (Azizi et al. 2014). The synovial fluid of RA patients contains various constituents, including autoantibodies, endogenous immune cells, inflammatory cytokines, and matrix metalloproteinases (MMPs).
These molecules stimulate the activity of cells responsible for bone and cartilage formation, namely osteoblasts and chondrocytes, while inhibiting the function of bone-resorbing cells, known as osteoclasts, and fibroblast-like synoviocytes (FLS) (Guo et al. 2018). The RANK/RANKL/OPG system is a crucial trio responsible for regulating bone metabolism and immunity (Shaker and Elbaz 2020). RANKL, a molecule present on activated T cells and osteoblasts, binds to its receptor RANK on osteoclast precursors, inducing their differentiation and activation. OPG acts as a decoy receptor for RANKL, preventing its interaction with RANK and thus controlling osteoclast activity (Ono et al. 2020). An imbalance in the RANK/RANKL/OPG signaling pathway can lead to bone diseases and has significant implications for inflammatory diseases like RA (Papadaki et al. 2019). MMPs, IL-1β, IL-6, TNF-α, TGF-β, and chemokines are all chemicals that are produced by FLS during the progression of RA, when exposure to these compounds results in joint swelling, inflammation, cartilage degradation, and bone fragility (Kondo et al. 2021).
To manage RA, clinicians often prescribe a combination of medications, including glucocorticoids, nonsteroidal anti-inflammatory medicines, disease-modifying anti-rheumatic therapies. However, these treatments can produce a range of adverse effects, some of which can be severe, emphasizing the need for safer and more effective RA therapies (Bullock et al. 2018). Phytochemicals have been revealed to have valuable results on human wellness regarding condition avoidance and treatment. Sinomenine (SIN) is a pharmacologically active alkaloid that is found in the Chinese plant Sinomenium acutum (Zhao et al. 2012). SIN has been linked to a variety of therapeutic benefits. These include reducing consisting of anti-inflammatory (Wang and Li 2011), suppressing the immune system (Chen et al. 2017), treating central nervous system disorders (Hong et al. 2022), managing cardio-cerebrovascular disease (Zhang et al. 2021), and providing antioxidant properties (Fan et al. 2022).
Recent research suggests that SIN can decrease lymphocyte and synovial fibroblast advancement, macrophage intrusion, and inflammatory cytokine manufacturing, all of which are linked to RA pathogenesis (Gao et al. 2021). The exact mechanism responsible for SIN's protective effect in RA is still unknown. Our study aims to explore the therapeutic potential of SIN in rats with RA. Building on past research that highlighted the anti-inflammatory properties of SIN, we will delve deeper into its role in regulating the RANK/RANKL/OPG signaling pathway, which is linked to osteoclast activation. Moreover, we investigate mitigation of oxidative stress with SIN via the Nrf2/Keap1 signaling pathway. This study provides a comprehensive analysis of the antioxidant and immunomodulatory properties of SIN, in addition to its anti-inflammatory effects. Using measures like inflammatory markers, oxidative stress indicators, paw volume, and arthritic indices, we intend to provide a detailed understanding of SIN's effectiveness. Our work will also address the gaps in current knowledge by exploring SIN’s role in preventing lipid peroxidation (LPO) and its interaction with antioxidant enzymes. By highlighting the multiple curative benefits of SIN in RA, our research paves the way for subsequent studies and positions SIN as a promising option for clinical applications.
Materials and methods
Materials
Sinomenine (SIN) was purchased from Solarbio Life Sciences, located in Beijing, China, while Methotrexate (MTX) was obtained from Sigma Aldrich in St. Louis, MO, USA. The RF, CRP, TNF-, IL-6, IL-1, iNOS, IL-10, Arg-1, Fizz1, RANKL, and OPG kits were purchased from Cusabio Biotech Co., Ltd. located in Wuhan, China. MDA and NO, as well as GSH, SOD, CAT, and GPx kits were purchased from Nanjing Jiancheng in China. The IHC kits were purchased from Vectastain ABC Kit, Vector Laboratories in Burlingame, CA, USA. Moreover, the cDNA kit was purchased from Nanjing Vazyme Biotech Co, located in Nanjing, China.
Animals
Male Sprague–Dawley (SD) rats, each weighing an average of 180 ± 20 g were purchased in this study by Pizhou Oriental Breeding Co., Ltd. (Jiangsu, China). They were kept in controlled conditions, with each cage containing three rats. The conditions were maintained at a temperature of 22 ± 2°C, under a 12-h light/dark cycle, and at a humidity level ranging from 40 to 60%. The rats had access to standard pelleted water and food (Beijing Keao Xieli Feed Co., Ltd. Beijing, China) ad libitum. Before experiment, the rats were acclimatized for at least 1 week.
Rheumatoid arthritis induction
All rat groups, excluding the control group, received an injection of 0.1-mL FCA subcutaneously (s.c) into the in the left hind metatarsal footpad. On the 7th day, 0.05 mL of FCA was subcutaneously injected into the bases of the rats’ tails to promote inflammation (Sun et al. 2023). Fourteen days after the first FCA injections, a random assignment was made for the rats into five groups, each consisting of six rats (as depicted in Fig. 1).
-
Group 1: Normal rats receiving saline solution orally (normal control)
-
Group 2: Rats with rheumatoid arthritis-induced rats received saline solution orally (RA)
-
Group 3: Rats with rheumatoid arthritis induced received 25 mg/kg/day of SIN orally (RA + SIN 25)
-
Group 4: Rheumatoid arthritis induced received 50 mg/kg/day of SIN orally (RA + SIN 50)
-
Group 5: The study involved rats with rheumatoid arthritis, and they were administered methotrexate (MTX) at a dose of 3 mg/kg bw, delivered via orally twice a week (Liu et al. 2017).
Schematic representation of the experimental design. FCA: Freund’s complete adjuvant, SIN: sinomenine, MTX: methotrexate, TNF-α: tumor necrosis factor-alpha, IL-6: interleukin 6, IL-1β: interleukin 1β, iNOS: inducible nitric oxide synthase, IL-10: interleukin 10, Arg-1: arginase 1, FIZZ1: found in inflammatory zone 1, H&E: hematoxylin and eosin, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, IKK-β: inhibitor of nuclear factor kappa-B kinase subunit beta, MMP-2: matrix metallopeptidase 2, MMP-9: matrix metallopeptidase 9, TIMP-1: TIMP metallopeptidase inhibitor 1, TIMP-3 TIMP metallopeptidase inhibitor 3, RANK: receptor activator of nuclear factor kappa beta, RANKL: receptor activator for nuclear factor κ B ligand, OPG: osteoprotegerin, LPO: lipid peroxidation, SOD: superoxide dismutase, CAT: Catalase, GPx: glutathione peroxidase, GSH: reduced glutathione, Nrf2: nuclear factor erythroid 2–related factor 2, Keap1: Kelch-like ECH-associated protein 1, HO-1: Heme-oxygenase 1, NQO1: NAD(P)H quinone oxidoreductase 1
SIN and MTX were dissolved in sterile saline. They were then administered orally to rats, based on their body weight, at a dosage of 1 ml/100 g. The control groups were given an equivalent volume of normal saline, with the positive control group receiving the same quantity. Starting from the 14th day after the initial FCA injection, the low-dose SIN group was administered 25 mg/kg daily, while the high-dose SIN group received 50 mg/kg daily. Both treatments continued up to 35 days (Li et al. 2023).
On day 36, the rats were euthanized by cervical dislocation after receiving an anesthetic dose of 60 mg/kg (i.p) pentobarbital salt. After obtaining blood from a cardiac puncture, the serum was isolated. The remaining blood was then stored at a temperature of −80°C for further assessment. Spleen, thymus, and knee joints were removed for molecular evaluation and stored at −80° C or 4% paraformaldehyde.
Arthritic Assessment
Arthritis Index
To assess the severity of arthritis in rats, we utilized a paw volume measuring device to measure paw edema. In addition, we used the arthritis index requirements to review the severity of inflammation and swelling. These requirements entailed designating a quality of 0 for no inflammation or swelling, grade 1 for redness as well as swelling in the little toe joints, Grade 2 for redness as well as swelling in all joints and also toes, grade 3 for swelling below the ankle joint, and a score of 4 for generalized joint inflammation and edema, including the ankle joints (Cheng et al. 2016).
Hind paw volume assessment
A YLS-7B plethysmograph, located at the Facility Station of Shandong Academy of Medical Science in Shandong, China, was employed to assess the volume of the hind paws on various time points, specifically on days 0, 7, 14, 21, 28, and 35, by measuring how much water was displaced after submerging the back paw and able to calculate the paw volume (ml).
Immune organs index
The spleen and thymus of the rats used in the study were surgically removed after the trial. Thymus and spleen indices, which evaluate the weight of these organs among groups and examine how SIN affects the immune system, were calculated as body organ weight (mg)/body weight (g) × 100.
ELISA
We used ELISA kits developed by Cusabio Biotech Co., Ltd. (Wuhan, China). The homogenate was prepared from knee joints, and we were able to analyze indicators of inflammation and oxidative stress in blood and homogenate samples with the use of these kits. ELISA kits were used to measure RF, CRP, and several cytokines and proteins such as TNF-α, IL-6, IL-1β, iNOS, IL-10, Arg-1, Fizz1, RANKL, and OPG. The serum or tissue homogenate was transferred to micro-ELISA strip plates coated with specific antibodies and HRP-conjugated reagents in the ELISA method. After 15 min, the reaction was stopped utilizing a stop solution, and the measured absorbance at 450 nm with a spectrophotometer.
Oxidative stress and antioxidant markers
In the homogenate of knee joints, both oxidative stress markers (MDA and NO) and antioxidant markers (GSH) were measured in nmol/mg protein. Antioxidant enzymes SOD, CAT, and GPx levels were measured using kits from Nanjing Jiancheng, China, and expressed as U/mg protein.
Hematoxylin-eosin staining
After the animals were sacrificed the knee joints were removed and preserved in 4% paraformaldehyde. After being immersed in a 10% EDTA solution at 4°C for 30 days to remove calcium, the joints were embedded in paraffin. The tissue was sectioned into 5 μm slices using a microtome. The sections were dewaxed and 3 times changes in xylene and anhydrous ethanol before staining with hematoxylin and eosin solution (Baso Biotechnology Co., Ltd, Zhuhai, Guangdong, China). Photos were taken at 40× using phase-contrast microscopy (Nikon H550S and DS-Ri2 cameras, Japan). The nucleus and cytoplasm were stained blue and red respectively.
Immunohistochemical analysis
IHC was performed in accordance with earlier research (Wahab et al. 2022). Briefly, after deparaffinization and rehydration, antigen retrieval was accomplished on 5 μm paraffinized rat ankle joint sections. After cooling, the sections were placed in a 3% H2O2 incubator at room temperature for 10 min. Tissue samples were blocked with 5% for 1 h. The next step the samples were incubated overnight at a temperature of 4°C with primary antibodies, which were diluted at a ratio of 1:500. The primary antibodies such as NF-κB (ab32360), MMP-2 (ab86607), and RANKl (ab239607) purchased from Abcam, Cambridge, MA, USA. The samples were incubated with HRP conjugated secondary antibodies for 1 h at room temperature (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA). After washing the sections three times with PBS, diaminobenzidine (DAB) or red chromogen was applied, followed by hematoxylin counterstaining. The photos were captured at a magnification of 40× using phase-contrast microscopy (Nikon H550S and DS-Ri2 cameras, Japan).
qRT-PCR
qPCR was carried out according to previous studies (Khalil et al. 2021). Briefly, the total RNA extracted with the TRIzol reagent from knee joints. After checking the UV absorption ratio at 260/280 to assess the purity of the extracted RNA, the remaining samples convert into cDNA (Nanjing Vazyme Biotech Co, Nanjing, China) by following the manufacturer’s instructions. SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA) was utilized for the RT-PCR. The 2-ΔΔC technique was used to analyze three duplicates of each sample and determine relative gene expression levels. Primers used for amplification of the respective genes in this study, which included:
IKKβ | F 5’-GCCTCTTCTCATTCCTGCTTG-3’ |
R 5’-CTGATGAGAGGGAGGCCATT-3’ | |
iNOS | F 5’-GAGAAGTCCAGCCGCACC-3’ |
R 5’-CAATCCACAACTCGCTCCAAGA-3’ | |
Arg-1 | F 5’-AGCACTGAGGAAAGCTGGTC-3’ |
R 5’-CAGACCGTGGGTTCTTCACA-3’ | |
Fizz1 | F 5′-GACTGCTACTGGGTGTGCTT-3’ |
R 5’-GCTGGGTTCTCCACCTCTTC-3’ | |
Nrf2 | F 5’- CATTTGTAGATGACCATGAGTCGC-3’ |
R 5’- ATCAGGGGTGGTGAAGACTG-3’ | |
Keap 1 | F 5’- CTTCGGGGAGGAGGAGTTCT-3’ |
R 5’- CGTTCAGATCATCGCGGCTG-3’ | |
Nqo1 | F 5’- GACATCACAGGGGAGCCG-3’ |
R 5’- CTCAGGCGGCCTTCCTTATAC-3’ | |
Ho-1 | F 5’-GTGCACATCCGTGCAGAGAA-3’ |
R 5’-GTGCACATCCGTGCAGAGAA-3’ | |
MMP-2 | F 5’-AAAGGAGGGCTGCATTGTGAA-3’ |
R 5’- CTGGGGAAGGACGTGAAGAGG-3’ | |
MMP-9 | F 5’-AGGTGCCTCGGATGGTTATCG-3’ |
R 5’- TGCTTGCCCAGGAAGACGAA-3’ | |
TIMP-1 | F 5’-GGCGAACCGGAAACCTGT-3’ |
R 5’- GCGCCCTTTGCATCTCTGG-3’ | |
TIMP3 | F5’-CTTCTGCAACTCCGACATCGT-3’ |
R 5’- GGGGCATCTTACTGAATCCTC-3’ | |
β-actin | F 5’-CACGATGGAGGGGCCGGACTCATC-3’ |
R 5’-TAAAGACCTCTATGCCAACACAGT-3’ |
Statistical analysis
The data was analyzed using GraphPad Prism 9.0. The results of the experiment were presented as the mean ± SD. The data was analyzed using one-way analysis of variance (ANOVA) followed by post hoc Tukey test for multiple comparisons. A P value of <0.05 was considered statistically significant.
Results
Body weight changes
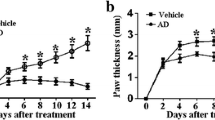
The RA group of rats had severe inflammation symptoms in their hind paws, including arthritic swelling, instability, inflammation, pus discharge, and difficulty walking on hard surfaces (Fig. 2A). At the start of the research study, there was no substantial (p > 0.05) distinction in body weight amongst the 5 groups prior to induce RA. Nonetheless, after induction RA, rats in the RA group revealed a considerable (p<0.05) weight reduction. Treatment with SIN as well as MTX in the RA rats caused reduced swelling and also raised (p<0.05) body weight gain (Fig. 2B).
A Photos of the hind paws of experimental rats. Effect of SIN on B body weight, C arthritis score, D paw volume in RA-induced rats. Data values are presented as the mean ± SD, and each group consists of six samples. *p < 0.05 were observed when compared to the control group, while #p< 0.05 were observed when compared to the RA group
Arthritis score
The arthritis score in the RA groups was dramatically greater (p<0.01) than the control group. However, the RA group that received SIN and MTX exhibited a notable (p<0.01) reduction in paw edema. To evaluate the severity of joint inflammation in RA rats, the study employed the arthritis score. Initially, there was no significant difference in arthritis scores between the groups of rats treated with RA and SIN (p>0.05). The initial measurements indicated successful replication of the RA rat model, with all rats except normal control rats having an arthritis score near the upper limit of the scale. Throughout the experiment, the arthritis scores of the RA group remained unchanged, while the groups treated with SIN and MTX showed a significant decrease (p<0.01) (Fig. 2C).
Changes in rat paw volume
Figure 1D shows that the RA group had a statistically larger paw volume compared to the NC group (p<0.05). However, the RA-induced rats treated with SIN showed a significant reduction in paw edema compared to the RA group.
Thymus and spleen index
The thymus and spleen are vital immunological body organs in animals, and their indices might show immune feature markers. The RA rats had significantly higher thymus and spleen indices than the NC rats. In contrast, RA groups treated with the SIN group had significantly lower thymus as well as spleen indices than those of the RA group, recommending that SIN can control the body’s immune response (Fig. 3C and D).
The impact of SIN on various factors in rats induced with RA including A RF (rheumatoid factor), B CRP (C-reactive protein), C spleen index, and D thymus index. Data values are presented as the mean ± SD, and each group consists of six samples. *p < 0.05 were observed when compared to the control group, while #p< 0.05 were observed when compared to the RA group
Effects of SIN on diagnostic indicators of RA
Diagnostic indicators of RA, such as RF and CRP were analyzed to confirm the RA model. As can be seen in Fig. 3A and B, the RF and CRP levels in the rat RA model were statistically (p<0.01) higher than in the control group. Significant (p<0.01) decreases in RF and CRP levels were seen in the SIN group compared to the RA group.
Effects of SIN on the histopathology of RA rats
H & E staining of normal rat knee joints revealed a clear structure, neat organization, and the absence of hyperplasia, inflammatory cell infiltration, or damage to the articular surface, as seen in Fig. 4. This included the presence of healthy cartilages and subchondral bones. Conversely, the synovial cells of the RA group were active and hyperplastic, with numerous inflammatory cells attacking the joint area. In addition, the articular surface was irregular, cartilage and subchondral narrow, cartilage, and subchondral bones were destroyed, and a pannus formed, all of which are hallmarks of RA’s pathology. RA-induced rats treated with SIN group showed considerably less synovial hyperplasia, inflammatory cell infiltration, and pannus development.
Levels of inflammatory and pro-inflammatory markers
Figure 5 depicts the impact of SIN on the inflammatory and pro-inflammatory markers of RA rats. The NF-κB protein assists make different molecules that trigger inflammation, like cytokines. Our research showed that rats with RA had higher expression levels of NF-κB p65 (Fig. 5A) and Iκκβ (Fig. 5B). However, these levels were drastically decreased in SIN-treated RA animals.
ELISA assay for knee joints homogenate in experimental rats. A NF-κβ, C TNF-α, D IL-6, E IL-1β, G IL-10 and relative mRNA expression of B Ikkβ; F iNOS; H Arg-1; I Fizz1 in RA-induced rats. Data values are presented as mean ± SD for a sample size of 6 in each group. *p < 0.05 were observed when compared to the control group, while #p< 0.05 were observed when compared to the RA group
TNF-α (Fig. 5C), IL-6 (Fig. 5D), IL-1β (Fig. 5E), and iNOS (Fig. 5F) were also found in increased amounts in RA-affected animals compared to controls. Anti-inflammatory markers IL-10, Arg-1, and Fizz1 were all lower in the RA group (see Fig. 5G, H, and I). TNF-α, IL-6, IL-1β, and iNOS levels were all significantly reduced in RA-induced rats treated with SIN as compared to the RA group. Increased IL-10, Arg-1, and Fizz1 were also seen in the SIN-treated group. As was shown above, SIN has the potential to lessen joint inflammation by promoting the production of anti-inflammatory cytokines while simultaneously lowering the production of pro-inflammatory ones.
Immunohistochemistry data clearly demonstrated strong red staining for the NF-κB p65 protein. Rheumatoid arthritis (RA) rats had a significantly greater distribution of the NF-κB p65 protein compared to healthy rats. On the other hand, SIN- or MTX-treated RA-induced animals showed a significant decrease in NF-κB p65 protein expression levels (Fig. 6).
Antioxidant and oxidative stress indicators in rats with RA
Figure 7 indicate the mRNA level of Nrf2, Keap-1 HO-1, and NQO1 levels in knee joints. In rats with RA, there was a decrease in Nrf2 (Fig. 7A), HO-1 (Fig. 7C), and NQO1 (Fig. 7D) while Keap-1 (Fig. 7B) mRNA levels increased in contrast to normal control rats. When the RA-induced rats were treated with SIN, there was a notable increase in Nrf2, HO-1, and NQO1 mRNA levels, and a simultaneous decrease in Keap-1 levels when compared to RA rats.
Figure 8A and B reveal that oxidative stress indicators, lipid peroxidation product such as MDA and also NO levels were significantly greater in the knee joints homogenate of rats with RA compared to the group of healthy control rats. Lower levels of GSH and SOD, CAT, GPx, was shown in rats with induced RA compared to normal control rats in Fig. 8C–F. In addition, there was a significant decrease in MDA, NO and an increase in SOD, CAT, GPx, and GSH in the RA group that had been treated with SIN, indicating increased antioxidant capacity.
Effects of SIN on RANK/RANKL/OPG singling pathway
The higher levels of RANKL (Fig. 9A) and lower level of OPG (Fig. 9B) with a higher level of RANKL/OPG ratio (Fig. 9C) in the knee joints homogenate of RA-induced rats when compared to normal rats. However, treatment with SIN in RA rats resulted in a lower level of RANKL and RANK/OPG ratio with higher level of OPG in knee joints homogenate as compared to RA rats.
Effect of SIN on levels of A RANKL; B OPG; C RANKL/OPG ratio and relative mRNA expression levels of D MMP-2; E MMP-9; F TIMP-1; G TIMP-3 in RA-induced rats. Data values are presented as mean ± SD for a sample size of 6 in each group. *p < 0.05 were observed when compared to the control group, while #p< 0.05 were observed when compared to the RA group
Effects of SIN on MMP and TIMP proteins
In knee joints homogenate, MMP-2 and MMP-9 enzymes remodel extracellular matrix (ECM) components and TIMP-1 and TIMP-3 are endogenous inhibitors. In RA-induced rats, MMP-2 (Fig. 9D) and MMP-9 (Fig. 9E) mRNA levels were considerably elevated, and TIMP-1 and TIMP-3 levels were significantly (p<0.01) lower in these rats compared to expression levels in normal control rats, as shown in Fig. 9F and G. The mRNA expression of MMP-2 and MMP-9 was reduced, while the expression of TIMP-1 and TIMP-3 was increased in RA rats treated with SIN.
Immunohistochemistry revealed brown staining for RANKL and red staining for MMP-2 protein expressions. Proteins RANKL and MMP-2 were more widely distributed in the RA group than in the control group. In contrast, the RA group treated with SINs showed the lower protein distribution of RANKL and MMP-2 compared to RA rats (Fig. 10).
Discussion
Rheumatoid arthritis (RA) is a chronic inflammatory condition that causes joint inflammation and tissue damage. Although there is no cure for RA, various medications can help reduce inflammation, alleviate pain and slow joint degeneration. The study investigated the therapeutic potential of sinomenine (SIN) from Sinomenium acutum root in a rat model of RA induced by Freund’s complete adjuvant (FCA). The study findings demonstrate that SIN has anti-inflammatory and immunomodulatory effects. It can also reduce oxidative stress and bone resorption, indicating its potential usefulness in the treatment of RA.
The current study found that RA-induced arthritis caused significant weight loss in rats, consistent with previous studies on weight loss in arthritic animals (Alabarse et al. 2018, Nasuti et al. 2019). Slower weight gain in edematous rats might be attributed to the inflammatory response and increased metabolic rate associated with arthritis, which could result in muscle wastage and increased energy consumption (Farrow et al. 2021). The significant increase in body weight after SIN and MTX treatment suggests that these medications may help alleviate the deleterious effects of arthritis on weight loss in rats. This finding is constant with previous results that SIN as well as MTX are helpful to prevent body weight loss in arthritic animals (Liu et al. 2018).
RA triggers inflammation and tissue damage in the joints and surrounding tissues, leading to swelling, inflammation, discomfort, as well as mobility restrictions. The arthritis score determines the extent of joint inflammation, with higher scores showing more severe inflammation. SIN therapy for 35 days reduced paw edema and arthritic score in RA rats, effectively reducing RA-related joint inflammation. Our findings support prior studies that indicated SIN to have therapeutic potential in the treatment of RA, decreasing paw edema and arthritic score (Jiang et al. 2023). Furthermore, inflammation-induced joint swelling in RA rats may increase foot volume, producing pain and difficulty walking. By reducing joint inflammation, SIN may help decrease joint swelling and, as a result, foot volume. Our data suggest that SIN may decrease foot volume in RA rats by reducing joint edema and inflammation. This finding follows a previous research that found SIN to have anti-inflammatory properties in several inflammatory diseases (Lan et al. 2016).
RA creates inflammation and damage to healthy joints, which might increase the size and weight of the thymus and spleen, recommending enhanced immune action in arthritic rats (Abdel Jaleel et al. 2021). In our present research, the thymus and spleen indices were dramatically more significant in the RA lower in the SIN group than in the RA group, suggesting that SIN might reduce inflammation and immune system activity (Jun et al. 1992). This finding follows previous study recommending that SIN has an immunomodulatory impact on RA. SIN has been shown to affect the immune system by changing cytokine production as well as controlling numerous immune cells.
Consequently, the reduction in thymus and spleen indices observed in the SIN group suggests that SIN has immunomodulatory properties that may lower immune system hyperactivation in RA rats. Elevated levels of RF and CRP antibodies in the blood are expected in RA patients. They are used to diagnose and follow the development of the condition (Pope and Choy 2021). The dysregulated immune feedback, identified by constant inflammation and manufacturing of numerous cytokines and chemokines that add to joint destruction, is a substantial aspect of the pathophysiology of RA. SIN has been found to reduce RF and CRP, all of which are RA disease activity indicators (Liu et al. 2018). The capacity of SIN to directly affect the task of immune cells such as T and B cells and to limit the production of pro-inflammatory cytokines are among the most likely descriptions for this decrease (Yap et al. 2018).
RA causes synovitis, inflammation, and joint deterioration by assaulting the synovial lining. When the immune system attacks healthy tissues, hyperplastic, and active synovial cells increase inflammatory cell assaults on the joint. This immune reaction destroys cartilage and subchondral bone, resulting in pannus, which may limit joint mobility and cause uneven articular surfaces. In our study, SIN therapy improved the histology of RA rats. This research study confirms previous outcomes that SIN has anti-inflammatory and anti-arthritic effects (Qian et al. 2018).
In RA, the immune system improperly assaults the body’s tissues, leading to joint damage and continual inflammation. The Iκκβ complex is a crucial part of the NF-κB pathway, which regulates immune response and inflammation. The Iκκβ complex is involved in breaking down Iκβ proteins, which triggers the activation of the NF-κB pathway. This activation, in turn, kickstarts the transcription of genes associated with inflammation. Rheumatoid arthritis (RA) patients have been demonstrated to have changes in levels of many cytokines, including TNF-α, IL-6, iNOS, IL-10, Arg-1, and Fizz1 (Dogan et al. 2022). TNF-α and IL-6 play a necessary function initially and also RA development by producing pro-inflammatory cytokines (Wei et al. 2015).
RA and immunological regulation are both profoundly affected by the pro-inflammatory cytokine IL-1β. Anti-inflammatory cytokines, on the other hand, have a role in immune response modulation. These include IL-10, Arg-1, and Fizz1 (Katsikis et al. 1994, Elbaz et al. 2020). An imbalance between pro-inflammatory and anti-inflammatory cytokines is responsible for the joint inflammation and degeneration seen in patients with RA. In the present research, SIN therapy for RA rats reduced inflammation, improved immunological features, and minimized joint damage (Yong et al. 2003). The inflammatory markers TNF-α, IL-6, and iNOS, and interleukin-1 were all reduced by SIN treatment, whereas IL-10, Arg-1, and Fizz1 levels were increased (Yao et al. 2017; Lai et al. 2002).
In RA, reactive oxygen species (ROS) levels increase due to continual inflammation, activated immune cells, and an accumulation of damaged cells and tissue (López-Armada et al. 2022). TNF-α, IL-6, and IL-1β are pro-inflammatory cytokines that increase ROS production while reducing the expression of antioxidant enzymes, both of which contribute to oxidative stress. Several detoxification and antioxidant genes are regulated by the transcription factor Nrf2. In experimental RA models, Nrf2 activation has been shown to decrease joint inflammation and cartilage degradation. Antioxidant and anti-inflammatory advantages of activating Nrf2 to increase production of HO-1 and NQO1 have also been shown in RA (Chadha et al. 2020). HO-1 and NQO1 antioxidant genes have been proven to protect against oxidative stress and inflammation in RA.
Protecting cells from ROS is the job of antioxidant enzymes like SOD, CAT, and GPx. Patients with RA have lower activity of these enzymes, which leads to ROS buildup and oxidative damage (Zeng et al. 2021). In order to reduce oxidative stress and inflammation, enzyme supplements or their precursor compounds may be helpful for RA sufferers. MDA is an indicator of oxidative stress and a byproduct of lipid peroxidation, and its levels are typically elevated in RA patients. Patients with RA may benefit from taking enzyme supplements or their precursors in order to reduce MDA levels, oxidative stress, and inflammation. The endogenous antioxidant GSH might lower MDA levels by scavenging ROS and repairing damaged cell membranes. Research has demonstrated that GSH supplementation may assist RA patients reduce oxidative stress and inflammation. According to previous studies, SIN is a bioactive molecule having anti-inflammatory and antioxidant properties (Bi et al. 2021, Xiao-Qing and Ke-Wu 2021). SIN has been reported to reduce oxidative stress and increase antioxidant enzymes in experimental RA models, which may contribute to explain its therapeutic advantages. SIN may exhibit antioxidant effects by stimulating the Nrf2-NF-κB pathway. When Nrf2 is activated by SIN, antioxidant genes including HO-1 and NQO1 may be increased (Zhang et al. 2019). SIN has been demonstrated to reduce MDA levels, a marker of oxidative stress, and boost the activity of antioxidant enzymes including SOD, CAT, and GPx in RA experimental models (Liao et al. 2021).
MMPs such as MMP-2 and MMP-9 are enzymes necessary to break down extracellular matrix (ECM) components in synovial tissues, triggering tissue damage and joint injury in inflammatory diseases such as RA. TIMP-1 and TIMP-3 are two TIMPs, which are endogenous inhibitors controlling MMP activity (Chang et al. 2008). There is an imbalance between MMPs and TIMPs in RA, and an excess of MMPs results in greater tissue damage and degradation of ECM (Yamamoto et al. 2021). The dysregulation of MMPs and TIMPs is thought to have a role in RA’s chronic inflammation and joint degradation. The present study suggests that SIN treatment may reduce collagen breakdown and inflammation in RA by lowering MMP and TIMP-1 expression, these findings are consistent with previous studies (Liu et al. 2018).
RANK, RANKL, and OPG are essential signaling molecules that control bone remodeling and osteoclast production (Geusens 2012). The deregulation of these molecules has been associated with bone and joint degeneration in RA patients (Papadaki et al. 2019). In our study, SIN has been observed to reduce bone loss in RA patients by diminishing osteoclast formation and activity and enhancing bone regeneration. A potential mechanism through which SIN might influence bone deterioration is via the modulation of RANK, RANKL, and OPG signaling. Our current research indicates that SIN may inhibit osteoclast formation and activity by decreasing RANK and RANKL expression, while simultaneously increasing OPG expression. Additionally, SIN might support bone formation by influencing osteoblast activity (Li et al. 2013). However, while SIN possesses anti-inflammatory and immunomodulatory qualities and can impact bone development and destruction, it remains unclear whether its effects on the RANKL/OPG pathway are direct or potentially a secondary outcome of its anti-inflammatory properties. Hence, while it might have some advantages, claiming superiority over other RA treatments like biologic DMARDs and bisphosphonates requires further validation.
Conclusion
SIN was tested in a rat model of RA using FCA. SIN decreases oxidative stress, collagen deterioration, and bone resorption by acting as an anti-inflammatory, immunomodulatory, and chondroprotective agent. In RA-induced rats, therapy with SIN reduced joint inflammation and immune function and degeneration. Pro-inflammatory cytokines were reduced whereas anti-inflammatory cytokines were increased after SIN treatment. One possible mechanism by which SIN reduces oxidative damage, inflammation, and collagen degradation is via activation of the Nrf2/HO-1 and NF-κβ/Iκκβ pathways.
Data availability
All the data generated or analyzed during this study are included in this published article.
References
Abdel Jaleel GA, Azab SS, el-Bakly WM, Hassan A (2021) ’Methyl palmitate attenuates adjuvant induced arthritis in rats by decrease of CD68 synovial macrophages. Biomed Pharmacother 137:111347
Alabarse PVG, Lora PS, Silva JMS, Santo RCE, Freitas EC, de Oliveira MS, Almeida AS, Immig M, Teixeira VON, Filippin LI, Xavier RM (2018) Collagen-induced arthritis as an animal model of rheumatoid cachexia. J Cachexia Sarcopenia Muscle 9:603–612
Azizi G, Boghozian R, Mirshafiey A (2014) The potential role of angiogenic factors in rheumatoid arthritis. Int J Rheum Dis 17:369–383
Bi F, Zhang Y, Liu W, Xie K (2021) Sinomenine activation of Nrf2 signaling prevents inflammation and cerebral injury in a mouse model of ischemic stroke. Exp Ther Med 21:1–9
Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, Ansari RA, Ahmed J (2018) Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract 27:501–507
Chadha S, Behl T, Kumar A, Khullar G, Arora S (2020) Role of Nrf2 in rheumatoid arthritis. Curr Res Transl Med 68:171–181
Chang Y-H, Lin IL, Tsay GJ, Yang S-C, Yang T-P, Ho K-T, Hsu T-C, Shiau M-Y (2008) Elevated circulatory MMP-2 and MMP-9 levels and activities in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Biochem 41:955–959
Chen Z, Tao ZZ, Zhou XH, Wu TT, Ye LF (2017) Immunosuppressive effect of sinomenine in an allergic rhinitis mouse model. Exp Ther Med 13:2405–2410
Cheng BCY, Yu H, Guo H, Su T, Fu X-Q, Li T, Cao H-H, Tse AK-W, Wu Z-Z, Kwan H-Y, Yu Z-L (2016) A herbal formula comprising Rosae Multiflorae Fructus and Lonicerae Japonicae Flos, attenuates collagen-induced arthritis and inhibits TLR4 signalling in rats. Sci Rep 6:20042
Dogan S, Kimyon G, Ozkan H, Kacmaz F, Camdeviren B, Karaaslan I (2022) TNF-alpha, IL-6, IL-10 and fatty acids in rheumatoid arthritis patients receiving cDMARD and bDMARD therapy. Clin Rheumatol 41:2341–2349
Elbaz EM, Amin HAA, Kamel AS, Ibrahim SM, Helmy HS (2020) Immunomodulatory effect of diallyl sulfide on experimentally-induced benign prostate hyperplasia via the suppression of CD4+T/IL-17 and TGF-β1/ERK pathways. Inflammopharmacology 28:1407–1420
Fan H, Tu T, Zhang X, Yang Q, Liu G, Zhang T, Bao Y, Lu Y, Dong Z, Dong J (2022) Sinomenine attenuates alcohol-induced acute liver injury via inhibiting oxidative stress, inflammation and apoptosis in mice. Food Chem Toxicol 159:112759
Farrow M, Biglands J, Tanner S, Hensor EMA, Buch MH, Emery P, Tan AL (2021) Muscle deterioration due to rheumatoid arthritis: assessment by quantitative MRI and strength testing. Rheumatology 60:1216–1225
Gao W-J, Liu J-X, Xie Y, Luo P, Liu Z-Q, Liu L, Zhou H (2021) Suppression of macrophage migration by down-regulating Src/FAK/P130Cas activation contributed to the anti-inflammatory activity of sinomenine. Pharmacol Res 167:105513
Geusens P (2012) The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis 4:225–233
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6:15
Hong H, Lu X, Lu Q, Huang C, Cui Z (2022) Potential therapeutic effects and pharmacological evidence of sinomenine in central nervous system disorders. Front Pharmacol 13:1015035
Jiang H, Lu Q, Xu J, Huo G, Cai Y, Geng S, Xu H, Zhang J, Li H, Yuan K, Huang G (2023) Sinomenine ameliorates adjuvant-induced arthritis by inhibiting the autophagy/NETosis/inflammation axis. Sci Rep 13:3933
Jun W, Pei-Gen X, Shi-Ying L, Ping G, Rui-Hai W (1992) The inhibitory effect of Sinomenine on immunological function in mice. Phytother Res 6:117–120
Katsikis PD, Chu CQ, Brennan FM, Maini RN, Feldmann M (1994) Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med 179:1517–1527
Khalil ASM, Giribabu N, Yelumalai S, Shahzad H, Kilari EK, Salleh N (2021) Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: restoration of spermatogenesis, steroidogenesis in diabetic rats. Life Sci 278:119605
Kondo N, Kuroda T, Kobayashi D (2021) Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int J Mol Sci 22:10922
Lai W-D, Wang S, You W-T, Chen S-J, Wen J-J, Yuan C-R, Zheng M-J, Jin Y, Yu J, Wen C-P (2002) Sinomenine regulates immune cell subsets: potential neuro-immune intervene for precise treatment of chronic pain. Front Cell Dev Biol 10:1041006. https://doi.org/10.3389/fcell.2022.1041006
Lan Z, Wei M, Chen L, Xie G, Liu X, Zhang X (2016) Role of sinomenine on complete Freund’s adjuvant-induced arthritis in rats. IUBMB Life 68:429–435
Li X, He L, Hu Y, Duan H, Li X, Tan S, Zou M, Gu C, Zeng X, Yu L, Xu J, Liu S (2013) Sinomenine suppresses osteoclast formation and Mycobacterium tuberculosis H37Ra-induced bone loss by modulating RANKL signaling pathways. PLoS ONE 8:e74274
Li RZ, Guan XX, Wang XR, Bao W-Q, Lian L-R, Choi SW, Zhang FY, Yan P-Y, Leung ELH, Pan H-D, Liu L (2023) Sinomenine hydrochloride bidirectionally inhibits progression of tumor and autoimmune diseases by regulating AMPK pathway. Phytomedicine 114:154751
Liao K, Su X, Lei K, Liu Z, Lu L, Wu Q, Pan H, Huang Q, Zhao Y, Wang M, Cai J, Liu L, Li T (2021) Sinomenine protects bone from destruction to ameliorate arthritis via activating p62Thr269/Ser272-Keap1-Nrf2 feedback loop. Biomed Pharmacother 135:111195
Liu X-Y, Xu L, Wang Y, Li J-X, Zhang Y, Zhang C, Wang S-S, Zhang X-M (2017) Protective effects of total flavonoids of Astragalus against adjuvant-induced arthritis in rats by regulating OPG/RANKL/NF-κB pathway. Int Immunopharmacol 44:105–114
Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long H, Wang Y (2018) Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front Immunol 9:2228
López-Armada MJ, Fernández-Rodríguez JA, Blanco FJ (2022) Mitochondrial dysfunction and oxidative stress in rheumatoid arthritis. Antioxidants (Basel) 11(6):1151. https://doi.org/10.3390/antiox11061151
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219
Nasuti C, Fedeli D, Bordoni L, Piangerelli M, Servili M, Selvaggini R, Gabbianelli R (2019) Anti-inflammatory, anti-arthritic and anti-nociceptive activities of Nigella sativa oil in a rat model of arthritis. Antioxidants (Basel) 8(9):342. https://doi.org/10.3390/antiox8090342
Ono T, Hayashi M, Sasaki F, Nakashima T (2020) RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen 40:2
Papadaki M, Rinotas V, Violitzi F, Thireou T, Panayotou G, Samiotaki M, Douni E (2019) New insights for RANKL as a proinflammatory modulator in modeled inflammatory arthritis. Front Immunol 10:97. https://doi.org/10.3389/fimmu.2019.00097
Pathade V, Nene S, Ratnam S, Khatri DK, Raghuvanshi RS, Singh SB, Srivastava S (2022) Emerging insights of peptide-based nanotherapeutics for effective management of rheumatoid arthritis. Life Sci 312:121257. https://doi.org/10.1016/j.lfs.2022.121257
Pope JE, Choy EH (2021) C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum 51:219–229
Prasad P, Verma S, Surbhi, Ganguly NK, Chaturvedi V, Mittal SA (2023) Rheumatoid arthritis: advances in treatment strategies. Mol Cell Biochem 478:69–88
Qian X, Zhao Z, Shang W, Xu Z, Zhang B, Cai H (2018) Serum proteomic analysis of the anti-arthritic effects of sinomenine on rats with collagen-induced arthritis. Mol Med Rep 18:49–58
Shaker OG, Elbaz EM (2020) Possible prognostic potential of RANKL and OPG in metastatic breast cancer egyptian females. Asian Pac J Cancer Prev 21:355–361
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K (2018) Rheumatoid arthritis. Nat Rev Dis Prim 4:18001
Sun Y, Liu J, Xin L, Wen J, Zhou Q, Chen X, Ding X, Zhang X (2023) Xinfeng capsule inhibits inflammation and oxidative stress in rheumatoid arthritis by up-regulating LINC00638 and activating Nrf2/HO-1 pathway. J Ethnopharmacol 301:115839
Tian Z, Zhang H, Shang C (2021) Farrerol ameliorate adjuvant-induced ankle injury via alteration of PPAR-γ signal pathway. J Food Biochem 45:e13585
Vasdev N, Pawar B, Gupta T, Mhatre M, Tekade RK (2023) A bird’s eye view of various cell-based biomimetic nanomedicines for the treatment of arthritis. Pharmaceutics 15:1150
Wahab NAA, Giribabu N, Kilari EK, Salleh N (2022) Abietic acid ameliorates nephropathy progression via mitigating renal oxidative stress, inflammation, fibrosis and apoptosis in high fat diet and low dose streptozotocin-induced diabetic rats. Phytomedicine 107:154464
Wang Q, Li XK (2011) Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol 11:373–376
Wei ST, Sun YH, Zong SH, Xiang YB (2015) Serum levels of IL-6 and TNF-α may correlate with activity and severity of rheumatoid arthritis. Med Sci Monit 21:4030–4038
Xiao-Qing Z, Ke-Wu Z (2021) Advances in anti-inflammatory and immunoregulatory mechanisms of sinomenine. Tradit Med Res 6:6
Yamamoto K, Wilkinson D, Bou-Gharios G (2021) Targeting dysregulation of metalloproteinase activity in osteoarthritis. Calcif Tissue Int 109:277–290
Yao R-B, Zhao Z-M, Zhao L-J, Cai H (2017) Sinomenine inhibits the inflammatory responses of human fibroblast-like synoviocytes via the TLR4/MyD88/NF-κB signaling pathway in rheumatoid arthritis. Die Pharmazie-An Int J Pharm Sci 72:355–360
Yap HY, Tee SZ, Wong MM, Chow SK, Peh SC, Teow SY (2018) Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells 7(10):161. https://doi.org/10.3390/cells7100161
Yong W, Yongfei F, Xin Z, Bing Z (2003) Effect of different concentration sinomenine on cytokines mRNA expression of synoviocytes in rats. Chin J Inf Tradit Chin Med 10:25–27
Zampeli E, Vlachoyiannopoulos PG, Tzioufas AG (2015) Treatment of rheumatoid arthritis: unraveling the conundrum. J Autoimmun 65:1–18
Zeng L, Yu G, Yang K, Li J, Hao W, Chen H (2021) The efficacy of antioxidative stress therapy on oxidative stress levels in rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. Oxid Med Cell Longev 2021:3302886
Zhang L, Zhang W, Zheng B, Tian N (2019) Sinomenine attenuates traumatic spinal cord injury by suppressing oxidative stress and inflammation via Nrf2 pathway. Neurochem Res 44:763–775
Zhang MW, Wang XH, Shi J, Yu JG (2021) Sinomenine in cardio-cerebrovascular diseases: potential therapeutic effects and pharmacological evidences. Front Cardiovasc Med 8:749113
Zhao X-X, Peng C, Zhang H, Qin L-P (2012) Sinomenium acutum: a review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm Biol 50:1053–1061
Funding
Shaanxi Provincial Key Research and Development Program (No. 2021SF-259); Xi’an Science and Technology Bureau Innovation Ability Strong Foundation Program Project (No. 21YXYJ0003).
Author information
Authors and Affiliations
Contributions
JL and RL conceived the idea and designed the study. JC and QC performed the experiments. JL helped in statistical analysis. JL and RL wrote the manuscript. All authors have read and approved the final version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by The First Ward of Rheumatology and Immunology, Xi'an No.5 Hospital, Xi'an, China in compliance with the institutional committee's rules for animal care and authorized procedures (Ethics No. 2022-1667).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Cao, J., Chen, Q. et al. Investigating the therapeutic potential of sinomenine in rheumatoid arthritis: anti-inflammatory, antioxidant, and immunomodulatory mechanisms. Naunyn-Schmiedeberg's Arch Pharmacol 397, 3945–3958 (2024). https://doi.org/10.1007/s00210-023-02810-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02810-0