Abstract
Objective
To investigate the participation of canonical Wnt and NF-κB signaling pathways in an experimental model of chronic arthritis induced by methylated bovine serum albumin (mBSA) in rat temporomandibular joint (TMJ).
Materials and methods
Wistar rats were sensitized by mBSA+Complete Freund Adjuvant (CFA)/Incomplete Freund Adjuvant (IFA) on the first 14 days (1 ×/week). Subsequently, they received 1, 2 or 3 mBSA or saline solution injections into the TMJ (1 ×/week). Hypernociceptive threshold was assessed during the whole experimental period. 24 h after the mBSA injections, the TMJs were removed for histopathological and immunohistochemical analyses for TNF-α, IL-1β, NF-κB, RANKL, Wnt-10b, β-catenin and DKK1.
Results
The nociceptive threshold was significantly reduced after mBSA injections. An inflammatory infiltrate and thickening of the synovial membrane were observed only after mBSA booster injections. Immunolabeling of TNF-α, IL-1β and Wnt-10b was increased in the synovial membrane in arthritic groups. The immunoexpression of nuclear β-catenin was significantly higher only in the group that received 2 booster TMJ injections. However, NF-κB, RANKL and DKK1 immunoexpression were increased only in animals with 3 mBSA intra-articular injections.
Conclusion
Our results suggest that canonical Wnt and NF-κB signaling pathways participate in the hypernociception and inflammatory response in TMJ synovial membrane during the development of rheumatoid arthritis in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an example of a chronic, progressive and systemic inflammatory disease characterized by synovial inflammation and hyperplasia, production of autoantibodies and destruction of cartilage and bone [1, 2]. RA affects approximately 1% of the adult population [3] and its etiology is still unknown [4]. It is estimated that 65% of patients with RA report symptoms of affections of the temporomandibular joint (TMJ), and 75% of those have bilateral involvement [5, 6]. Symptoms may include facial pain, edema, limited mandibular movements (due to the restriction of condylar translation), headache, trismus, crackling and muscle spasms [5].
During chronic inflammation, the synovial membrane of the TMJ is responsible for releasing pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α), to the synovial fluid, leading to synovial hyperplasia, connective tissue degeneration, fibrosis and perforation of the articular disc [7].
Experimental models of RA are useful to replicate many characteristic features of the human disease. The arthritis induced by methylated bovine serum albumin (mBSA) reproduces an immunomediated inflammation with the presence of pro-inflammatory cytokines, cellular hypertrophy, angiogenesis and cartilage and bone destruction [8,9,10]. An interesting goal of this experimental model is that it promotes a monoarthritis. Thus, the contralateral joint can be used as internal control [11].
Wnt pathway regulates cell homeostasis processes, such as cell differentiation, proliferation, migration and adhesion and participates in many pathological conditions [12,13,14], including RA [4, 15]. There are at least 3 types of Wnt pathways. Among them, the canonical pathway is the most studied and best understood so far. The biding of Wnt to its receptor inhibits the glycogen synthase kinase 3-β, releasing β-catenin from its destruction complex and increasing the presence of the cytoplasmic-free β-catenin. This β-catenin excess translocates into the nucleus and induces a cellular response throughout gene transduction [4]. The canonical Wnt pathway can promote synovial hyperplasia and inflammation, pannus formation and bone and cartilage erosion during the progression of this disease [15].
The activation of the nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-κB) pathway is the most critical signaling in inflammatory processes and it is closely involved in chronic inflammatory disorders, including RA [16]. NF-κB has the ability to control the expression of gene products affecting various cellular responses, including cell proliferation and apoptosis [17]. This signaling can stimulate neoangiogenesis and inflammation in the synovial membrane [18]. During the rheumatic disease, chondrocytes express many of NF-κB-mediated cytokines and chemokines, such as TNF-α, IL-1β, IL-8 and receptor activator of NF-kB (RANKL), increasing the synthesis of catabolic factors and further inflammation [18]. In human TMJ arthritis, the NF-κB activation in synovial fibroblasts is associated with COX-2 expression induced by TNF-α, contributing to nociceptive sensitization and inflammatory response [7].
There is a functional cross-regulation between canonical Wnt pathway and NF-κB signaling in the pathogenesis of various inflammatory diseases [19] but this correlation is not fully elucidated in TMJ RA. Therefore, considering the role of the host response in the destruction of the TMJ during RA, experimental studies are essential to understand the pathological mechanisms underlying the tissue breakdown, leading to the development of new therapeutic approaches. For this purpose, our group investigated the morphological changes of the synovial membrane during the development of TMJ arthritis, as well as the participation of canonical Wnt and NF-κB pathways in the progression of this chronic disease.
Materials and methods
Ethics statement
The experimental procedures were approved by the Institutional Animal Care and Use Committee of the Federal University of Ceará, Brazil (No. 35/15) and performed in accordance with their Animal Care Standards. All rats were housed in a room that was held at a constant ambient temperature (22–24 °C) with a 12-h light/dark cycle and easy access to food and water.
TMJ rheumatoid arthritis induction and experimental groups
The experiments were performed in 24 male Wistar rats (200–250 g). The animals were divided into the following groups (n = 6): control (C), with animals not submitted to RA induction; mBSA (1)/Saline (1), with animals that received 1 intra-articular injection of mBSA in the left TMJ and 0.9% saline solution in the right TMJ; mBSA (2)/Saline (2), with animals that received 2 intra-articular injections of mBSA in the left TMJ and saline solution in the right TMJ; mBSA (3)/Saline (3), with animals that received 3 intra-articular injections of mBSA in the left TMJ and saline solution in the right TMJ. Initially, the mBSA groups were sensitized with 500 μg of mBSA in 200 μL of an emulsion containing 100 μL of phosphate-buffered saline (PBS) and 100 μL of Complete Freund Adjuvant (CFA), which was subcutaneously administered. Booster injections of mBSA dissolved in Incomplete Freund Adjuvant (IFA) were administered 7 and 14 days after the first immunization [20]. Twenty-one days after subcutaneous injections, the animals were intraperitoneally anesthetized with ketamine 10% (70 mg/kg) and xylazine 2% (6 mg/kg) and the arthritis was induced in the left TMJ in immunized animals by an intra-articular injection of mBSA (10 μg/articulation) dissolved in 10 μL of PBS. Booster injections of mBSA were given on days 28 and 35 [20]. The animals were euthanized 24 h after each mBSA injection in the left TMJ. The control group did not receive any mBSA administration. The animals that received 1, 2 or 3 mBSA injections in the left TMJ, also received saline (0.9%; 10 µL; i.art) in the right TMJ [Saline (1), Saline (2) and Saline (3)]. (Supplementary File).
Evaluation of mechanical hyperalgesia
Mechanical hyperalgesia in TMJ was evaluated by measuring the intensity of force that needed to be applied to the TMJ region until a reflex response occurs (e.g., head withdrawal). For this purpose, a digital analgesymeter (electronic von Frey Digital, Insight Instruments, São Paulo, SP, Brazil) was used to measure the nociceptive threshold (in grams) when the transducer was applied to the surface of the TMJ area. The measurements were performed by a calibrated examiner unaware of the rendered treatments [21].
The animals were subjected to a conditioning session of head withdrawal threshold measurements in the experimental room for 5 consecutive days under controlled temperatures and low illumination. The mechanical hyperalgesia tests were performed on days 0, 1, 3, 6, 7, 11, 13, 14, 18 and 20 after the subcutaneous induction of mBSA and Freundʼs Adjuvant, and on days 21 (6 h after the first intra-articular injection of mBSA), 24, 28, 31 and 35.
Histopathological analysis
The rats were euthanized, and the TMJ and periarticular tissue were removed. The tissues were fixed in 10% neutral buffered formalin for 24 h, demineralized in 10% EDTA, embedded in paraffin and sectioned along the long axis of the TMJ. Sections (4 μm) with condyle, articular disc, synovial membrane, articular cartilage, and periarticular tissue were evaluated under light microscopy (Leica DM 2000, Wetzlar, Germany). The specimens were stained with hematoxylin–eosin (HE) and the sections were evaluated by a blinded and experienced pathologist. Scores were semi-quantitatively given for the evaluated parameters: inflammatory infiltrate and thickening of the synovial membrane. Sections were classified on a 0–3 scale for inflammatory infiltrate, where: 0 = no infiltrate; 1 = discrete infiltration in synovium; 2 = moderate synovial infiltrate; 3 = intense synovial infiltration. For thickening of the synovial membrane, a scale of 0–3 was used, where: 0 = no thickening; 1 = discrete thickening of the synovial membrane; 2 = moderate synovial thickening; 3 = intense thickening of the synovial membrane [22].
Immunohistochemical analysis for TNF-α, IL-1β, NF-κB, RANKL, Wnt-10b, β-catenin and DKK1
Immunohistochemistry for TNF-α, IL-1β, NF-κB, RANKL, Wnt-10b, β-catenin and DKK1 were performed on groups mBSA (2) and mBSA (3), using the streptavidin-biotin peroxidase method in formalin-fixed, paraffin-embedded tissue sections (4-μm thick) mounted on poly-l-lysine-coated microscope slides. The right TMJ of saline mBSA (2) and saline mBSA (3) were used as control. After deparaffinization, antigenic recuperation was performed with retrieval solution with citrate buffer (pH 6.0) for 20 min at 95 °C. Endogenous peroxidase was blocked with 3% H2O2 for 10 min to reduce non-specific binding. The sections were then incubated with anti-TNF-α 1:100 (Abcam; Cambridge, UK), anti-IL-1β 1:100 (Abcam; Cambridge, UK), anti-NF-κB P50 1:400 (Santa Cruz Biotechnology; California, USA), anti-RANKL (1:100 dilution; Santacruz Biotechnology; California, USA), anti-Wnt-10b (1:400 Abcam; Cambridge, UK), anti-β-Catenin (1:200 dilution; DAKO; California, USA) and anti-DKK1 (1:100 dilution; Santacruz Biotechnology; California, USA), diluted in DAKO antibody diluent for 1 h. The antibody binding sites were visualized by the incubation with diaminobenzidine–H2O2 (DAB, DAKO; California, USA) solution. A negative control lacking the primary antibody was performed in parallel with incubation. Slides were counterstained with hematoxylin, dehydrated in graded alcohol series, cleared in xylene and coverslipped. Positivity for TNF-α, IL-1β, NF-κB, RANKL, Wnt-10b and DKK1 was determined by brown staining at the level of the cytoplasm in synovial membrane. For β-catenin, positive staining was labeled separately for cytoplasm and nucleus. The sections were evaluated by an examiner unaware of the treatment. Cytoplasmatic and nuclear quantification of immunolabeled cells of the synovial membrane were performed in five randomly selected high-power (400 ×) fields under a microscope (Leica DM 2000, Wetzlar, Germany), and the percentage average value was used as the final immunoreactivity value [23].
Statistical analysis
The data were presented as mean ± SEM or as medians with variation range (maximum and minimum) when appropriate from 6 animals per group. One-way ANOVA or two-way ANOVA both followed by Tukey’s post hoc test were used. All tests were two-sided and considered to be statistically significant if probability level (p value) < 0.05. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Prism software, La Jolla, CA, USA) and SPSS 20.0 (SPSS Inc., Chicago, IL, USA) computer software program.
Results
Mechanical hyperalgesia analysis of mBSA-induced rheumatoid arthritis
The mechanical hyperalgesia evaluation in the left TMJ was performed on days 0, 1, 3, 6, 8, 11, 13, 15, 18 and 20 (sensitization phase). Significant differences were not observed during these days, suggesting that subcutaneous administration of mBSA did not modify the nociceptive threshold in the TMJ (supplementary file) (p = 0.3397; n = 6). On day 21, 6 h after the first mBSA intra-articular injection, a new measurement was registered and a significant reduction of the nociceptive threshold was observed (p < 0.0001; n = 6). On day 28, 6 h after the second mBSA injection, a significant reduction of the nociceptive threshold was observed, when compared to days 21 and 24 (p < 0.05; n = 6). In the third booster administration of mBSA in the left TMJ, on day 35, the nociceptive threshold remained low and it was statistically significant compared to days 20, 21 and 24 (Fig. 1a; p < 0.05; n = 6). Saline solution was administered in the right TMJ of rats that received 1, 2 or 3 injections of mBSA in the left TMJ and was it statistically different, when compared to its respective left side during all administrations of mBSA at TMJ (Fig. 1b, c, d; p < 0.05; n = 6). No statistical difference was observed when compared to the control group (p > 0.05; n = 6).
Evaluation of nociception in chronic RA during mBSA or saline administrations at TMJ. a The head withdrawal threshold of all groups. b The significant difference of the mBSA 1 and Saline 1 (right TMJ) groups after the first (day 21) injection of mBSA in the TMJ. c The significant difference of the mBSA 2 and Saline 2 (right TMJ) groups after the first (day 21) and second (day 28) injections of mBSA in the TMJ. d The significant difference of the mBSA 3 and Saline 3 (right TMJ) groups after the first (day 21), second (day 28) and third (day 35) injections of mBSA in the TMJ. Points represent the mean ± SEM of 6 animals per group. *Significant difference compared to the saline group (contralateral—right TMJ). #Significant difference compared to day 21 (p < 0.0001; two-way ANOVA followed by Tukey’s test)
Histopathological analysis of the TMJ
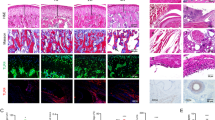
No alterations were observed in the articular tissues of the control group (Fig. 2a), as well as in the right TMJ of animals that received saline injections (Fig. 2b, c, d). On the other hand, the mBSA (1) group presented a discrete inflammatory infiltrate in the synovial membrane and thickening of the articular disc without the presence of joint cartilage destruction (Fig. 2e, e).
Photomicrographs of the TMJ of the experimental groups. a Normal TMJ. b TMJ of the saline (1) group (right TMJ). c TMJ of the saline (2) group (right TMJ). d TMJ of the saline (3) group (right TMJ). e TMJ of the mBSA (1) group. f TMJ of the mBSA (2) group. g TMJ of the mBSA (3) group. JC joint cartilage, AD articular disc, IDS infra-articular space. (*) inflammatory infiltrate. Hematoxylin & eosin (H&E). 100 × magnification
The mBSA (2) group, however, showed an intense mononuclear inflammatory infiltrate, thickening of the articular disc, but without joint destruction (Figs. 2f, 3f). In addition, mBSA (3) group presented an intense mononuclear infiltrate, thickening of the articular disc and synovial membrane, extensive joint destruction and pannus formation (Figs. 2g, 3g).
Photomicrographs of the TMJ synovial membrane. a Normal TMJ synovial membrane. b Synovial membrane of the saline (1) group. c Synovial membrane of the saline (2) group. d Synovial membrane of the saline (3) group. e Synovial membrane of the mBSA (1) group. f Synovial membrane of the mBSA (2) group. g Synovial membrane of the mBSA (3) group. Hematoxylin & eosin (H&E). 200 × magnification
After histopathological analysis, scores were applied to the experimental groups for inflammatory infiltrate and thickening of the synovial membrane. The mBSA (3) group was described by the pathologist and showed higher scores. The cell types observed in this group were predominantly macrophages, lymphocytes and plasma cells, characteristic features of chronic inflammation (Fig. 3g). Moreover, mBSA (2) group presented expressive scores for inflammatory infiltrate, but there was no statistical difference, compared to mBSA (3) group. Saline and mBSA (1) groups did not present any statistical difference, compared to the control group (Table 1; n = 6). Thereby, for immunohistochemical analysis, only groups mBSA (2) and mBSA (3) and their contralateral TMJs [Saline (2) and Saline (3)] were used.
Immunostaining assay for TNF-α, IL-1β, NF-κB, RANKL, Wnt-10b, β-catenin and DKK-1
Immunohistochemical analysis for TNF-α and IL-1β showed an increase in the immunoexpression of these cytokines, which was characterized by brown-colored cells in arthritic TMJ of rats injected with mBSA (Fig. 4c, d, g, h), compared to the contralateral TMJs (Fig. 4a, b, e, f). No immunolabeling was evidenced in any of the antibodies tested on the slides used as the negative control, where the primary antibody was replaced by the diluent antibody (Dako, St. Louis, USA).
Photomicrographs of immunopositive cells in synovial membrane for TNF-α, IL-1β, NF-κB and RANKL after mBSA booster injections in TMJ. Immunopositive cells in synovial membrane for TNF-α in Saline (2) (a), Saline (3) (b), mBSA (2) (c) and mBSA (3) (d) groups. Immunopositive cells in synovial membrane for IL-1β of Saline (2) (e), Saline (3) (f), mBSA (2) (g) and mBSA (3) (h) groups. Immunopositive cells in synovial membrane for NF-κB of Saline (2) (i), Saline (3) (j), mBSA (2) (k) and mBSA (3) (l) groups. Immunopositive cells in synovial membrane for RANKL of Saline (2) (m), Saline (3) (n), mBSA (2) (o) and mBSA (3) (p) groups
There was an increase of TNF-α immunolabeling in the synovial membrane in groups of animals that received mBSA booster injections. The mBSA (2) and mBSA (3) groups showed higher immunolabeling for TNF-α when compared to the Saline groups (p < 0.05; n = 5). There was no statistical difference between the arthritic groups (Fig. 5a; p > 0.05; n = 5).
Quantitative analysis of immunopositive cells in synovial membrane for TNF-α, IL-1β, NF-κB, RANKL, Wnt-10b, β-catenin (cytoplasmic and nuclear) and DKK-1, after mBSA booster injections in TMJ. The rats were treated with saline (right TMJ) or 2 and 3 mBSA injections (left TMJ). Count of immunopositive cells for TNF-α (a), IL-1β (b), NF-κB (c), RANKL (d), Wnt-10b (e), cytoplasmic β-catenin (f), nuclear β-catenin (g) and DKK-1 (h) in synovial membrane was performed. Results are expressed as the mean ± SEM of at least 5 animals per group. *p < 0.05 saline versus mBSA groups, #p < 0.05 mBSA (2) versus mBSA (3) group (one-way ANOVA and Tukey’s test)
The mBSA groups showed higher immunolabeling for IL-1β in the synovial membrane when compared to Saline groups (p < 0.0001; n = 5). Nonetheless, there was no significant difference between mBSA (2) and mBSA (3) groups (Fig. 5b; p > 0.05; n = 5).
Our results also showed an increased expression of NF-κB (Fig. 4l) and RANKL (Fig. 4p) in rats with 3 mBSA booster injections in TMJ when compared to the mBSA (2) (Fig. 4k, o) and saline groups (Fig. 4i, j, m, n) and these were statistically different (p < 0.0001; n = 5); Fig. 5c, d).
Photomicrographs of immunopositive cells in synovial membrane for Wnt-10b, β-catenin and DKK-1. Immunopositive cells in synovial membrane for Wnt-10b of Saline (2) (a), Saline (3) (b), mBSA (2) (c), mBSA (3) (d). Immunopositive cells in synovial membrane for β-catenin of Saline (2) (e), Saline (3) (f), mBSA (2) (g) and mBSA (3) (h). Immunopositive cells in synovial membrane for DKK-1 of Saline (2) (i), Saline (3) (j), mBSA (2) (k) and mBSA (3) (l) groups
Wnt-10b showed increased immunolabeling in TMJ of arthritic rats (Fig. 6c, d). In mBSA (2) and mBSA (3) groups, the Wnt-10b expression in synovial membrane was significantly higher when compared to Saline groups, and the mBSA (2) group showed a greater immunolabeling of this protein when compared to mBSA (3) (Fig. 5e; p < 0.05; n = 5).
There was no statistical difference in cytoplasmic β-catenin immunolabeling among the groups (Fig. 5g; p > 0.05; n = 5). However, there was a significant increase in immunoexpression of nuclear β-catenin only in the mBSA (2) group (Figs. 6g, h, 5h; p < 0.05; n = 5). In addition, the immunoexpression of DKK1, a Wnt antagonist, was significantly increased only in mBSA (3) group, when compared to other groups (Fig. 6l and 5h; p < 0.05; n = 5).
Discussion
In the present study, it was observed that the sensitization phase with subcutaneous administration of CFA or IFA and mBSA did not alter the head withdrawal threshold. This nociceptive threshold was reduced after 6 h of the first intra-articular mBSA injection in the left TMJ. In accordance with previous studies, 1 mBSA injection was able to reduce the nociceptive threshold in rat’s knee [24] and mice’s tibiofemoral joint [25]. Quinteiro et al. reported that 24 h after 1 mBSA injection in the TMJ of rats, the animals presented a painful behavior and an important inflammatory influx [8].
After 24 h of 1 mBSA injection in rats’ TMJ, a reduction of the nociceptive threshold and a discrete acute inflammatory infiltrate, marked by neutrophils, was observed. Therefore, morphologically, it could not simulate a chronic arthritic process. In such wise, booster injections of mBSA were used in the TMJ [20], to verify the development of RA. Those injections were able to reproduce the same findings of a human RA. Thereby, it was observed in the present study that only the mBSA (3) group showed an expressive chronic inflammatory infiltrate, with macrophages, lymphocytes and plasma cells, and pannus formation.
An interesting goal of this experimental model is that it promotes a monoarthritis and the contralateral joint can be used as internal control [11]. We confirm this fact in arthritic animals that received saline solution injection in the right TMJ and this joint did not show hyperalgesia to the mechanical stimuli or the presence of inflammatory infiltrate.
TNF-α and IL-1β are present in inflammation of the synovial membrane, and, considering their involvement of cytokines in the RA pathogenesis, novel therapeutic approaches have been used for RA management [26,27,28]. The administration of TNF-α inhibitors is a well-established therapeutic approach for RA in other joints [29]. Food and Drug Administration (FDA) approved the use of an IL-1 receptor antagonist (IL-1Ra) for RA treatment [30, 31]. An increase of IL-1β expression in the synovial membrane was observed in the pannus area of the animals that received 2 or 3 injections of mBSA. Mononuclear cells from the inflammatory pannus, spontaneously produce IL-1β [32] and this cytokine plays an important role in the recruitment of inflammatory cells to the joint [28].
TNF-α has a direct contribution to synovial inflammation and tissue degradation. High levels of TNF-α have been found in the RA joints. This can lead to an increase in adhesion molecules expression, chemokine production, angiogenesis and nociceptors activation [32]. Thus, TNF-α induces the osteoclastogenesis and contributes to the inhibition of osteoblastic differentiation [33]. In our study, TNF-α expression was significantly higher in the synovial membrane of the arthritic groups.
Classical pro-inflammatory cytokines, TNF-α and IL-1β, play an important role in the pathogenesis of chronic inflammatory diseases, and stimulate the canonical NF-kB pathway. Studies have shown that NF-kB activation promotes synovial hyperplasia, stimulating the proliferation and inhibiting the apoptosis of fibroblast-like synoviocytes [16, 17]. The NF-kB pathway results in the synthesis of pro-inflammatory mediators, causing synovitis accompanied by morphological alterations of the synovial membrane, such as infiltration of mononuclear cells and edema [18]. Although, both RA-induced groups showed increased expression of TNF-α and IL-1β, only the mBSA (3) group showed high expression of NF-κB in the synovial membrane. Lawrence demonstrated that the activation of NF-kB pathway is due to the increased expression of those cytokines, leading to the transcription of target genes of pro-inflammatory mediators [34]. These factors can increase inflammatory response, synovial hyperplasia and articular destruction, as found in this arthritic group.
Studies also have demonstrated that NF-kB pathway is activated by RANKL [35, 36]. It was shown that the RANKL expression is increased in the synovium of RA patients [37]. Although the authors relate the expression of RANKL more frequently to osteocytes and osteoblasts, it is also present in synovial cells, activated T cells, B cells, and natural killer cells [37, 38]. The abundance of monocytic cells in the synovial membrane, which respond to RANKL, enhances the formation of osteoclasts. The mBSA (3) group showed an increased immunoexpression of RANKL in the synovium, which can be related to NF-kB activation.
Wnt proteins have a central role in a variety of developmental processes and events, which include organogenesis, morphogenesis, cell differentiation and tissue remodeling [39], and are expressly activated in the synovial tissues of patients with RA [12, 40]. Wnt proteins participate in joint development (Wnt 4, 5 and 14) and chondrogenesis inhibition (Wnt 7a) [12]. Studies suggest that Wnt-10b increases angiogenesis and cell-growth regulation, but its behavior in TMJ arthritis was not yet elucidated. Studies have shown the presence of inflammatory cell aggregates in the synoviocytes layer and these cells have been involved in RA pathogenesis [41, 42]. Our data showed an increased immunolabeling for Wnt-10b in the synovial membrane, suggesting a specific activation of the Wnt pathway in the TMJ synovium of arthritic rats.
In RA patients, Wnt-10b and β-catenin were noticed in fibroblasts, endothelial and synovial lining cells [12]. This could lead to an increased production of cytokines, chemokines and matrix metalloproteinases (MMP), stimulating inflammation, synovial hyperplasia and cartilage erosion [12, 43, 44]. β-Catenin is the key mediator of the Wnt/β-catenin signaling pathway. In an inactivated stage, the β-catenin levels are low in the cytoplasm, while in an activated stage, it accumulates in the cytoplasm. The cytoplasmic β-catenin excess translocates into the nucleus, regulating target genes responsible for cell activation and proliferation [40]. Our results showed a lower expression of Wnt-10b and nuclear β-catenin in mBSA (3) group, when compared to mBSA (2). This fact corroborates with other studies that suggest that Wnt-10b expression may be increased in synovium in parallel with the decreased inflammatory infiltrate [45, 46].
The Dickkopf (DKK) family is an endogenous Wnt signaling inhibitor which blocks its signaling by biding to low-density lipoprotein receptor protein (LRP) homologues [47]. Studies have shown that DKK1 is secreted by the synovium in response to inflammation and its immunoexpression is inversely correlated with the radiographic disease severity in arthritic joints [4, 48]. Our results showed an increased labeling of DKK1 in the mBSA (3) group. This can be related to the decreased Wnt-10b and nuclear β-catenin expression in the mBSA (3) group, as a negative feedback for Wnt-10b synthesis. Studies have shown that canonical Wnt signaling leads to DKK1 production as a negative feedback through the activation of β-catenin/TCF transcription complex [49, 50]. Thus, DKK1 is involved in the remodeling and repairing processes of the articular bone in human systemic rheumatic diseases such as RA [4]. These results can indicate a possible mechanism of joint repair in the TMJ. The interaction among the mediators of the canonical Wnt signaling pathway must be further investigated.
Wnt signaling pathway can regulate some target genes of NF-κB and positive and negative cross-regulations have been observed [51, 52]. Another possible relation is that NF-κB activation could inhibit the nuclear translocation of β-catenin by interfering with the formation of the transcriptional complex β-catenin/TCF [52]. In the mBSA (3) group, a reduction of nuclear β-catenin occurs, and this leads us to question why cytoplasmic β-catenin does not translocate to the nucleus. This fact may be related to the increase of NF-κB immunoexpression in this group. Also, the components of the canonical Wnt signaling pathway can also modulate immune and inflammatory response by the interaction with NF-κB [52, 53]. The activation of both pathways in chondrocytes could synergistically upregulate the lymphoid enhancer-biding factor-1 (Lef1) expression by a complex formation that could lead to cartilage destruction [54], alterations only seen in mBSA (3) group.
Our results suggest the participation of the canonical Wnt and NF-κB signaling pathways during the development of TMJ arthritis in rats. The greater expression of DKK1 in the mBSA (3) group may be a result of negative feedback in the canonical Wnt signaling. A longer follow-up of the progression of TMJ RA experimental model and the investigation of canonical Wnt and NF-κB blockers would be helpful to better understand the participation of these signaling pathways in the chronification of this disease. Thereby, further investigation of these interactions must be studied.
References
Cunha CO, Pinto LMS, Mendonça LM, Saldanha ADD, Conti ACCF, Conti PCR. Bilateral asymptomatic fibrous-ankylosis of the temporomandibular joint associated with rheumatoid arthritis: a case report. Braz Dent J. 2012;23:77982. https://doi.org/10.1590/s0103-64402012000600025.
Sodhi A, Naik S, Pai A, Anuradha A. Rheumatoid arthritis affecting temporomandibular joint. Contemp Clin Dent. 2015;6:124–7. https://doi.org/10.4103/0976-237X.149308.
Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229. https://doi.org/10.1186/ar2669.
Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y, Zhang L, Lv XW, Jin Y, Li J. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25:2069–78. https://doi.org/10.1016/j.cellsig.2013.04.002.
Ruparelia PB, Shah DS, Ruparelia K, Sutaria SP, Pathak D. Bilateral TMJ involvement in rheumatoid arthritis. Case Rep Dent. 2014;2014:262430. https://doi.org/10.1155/2014/262430.
Ahmed N, Catrina AI, Alyamani AO, Mustafa H, Alstergren P. Deficient cytokine control modulates temporomandibular joint pain in rheumatoid arthritis. Eur J Oral Sci. 2015;123:235–41. https://doi.org/10.1111/eos.12193.
Ke J, Long X, Liu Y, Zhang YF, Li J, Fang W, Meng QG. Role of NF-κB, in TNF-α induced COX-2 expression in synovial fibroblasts from human TMJ. J Dent Res. 2007;86:363–7. https://doi.org/10.1177/154405910708600412.
Quinteiro MS, Napimoga MH, Macedo CG, Freitas FF, Abdalla HB, Bonfante R, Trindade-Clemente Napimoga J. 15-deoxy-Δ12,14-prostaglandin J2 reduces albumin-induced arthritis in temporomandibular joint of rats. Eur J Pharmacol. 2014;740:58–65. https://doi.org/10.1016/j.ejphar.2014.07.002.
Chandrupatla DMSH, Weijers K, Gent YYJ, Greeuw I, Lammertsma AA, Jansen G, Laken CJ, Molthoff CFM. Sustained macrophage infiltration upon multiple intra-articular injections: an improved rat model of rheumatoid arthritis for PET guided therapy evaluation. Biomed Res Int. 2015;2015:509295. https://doi.org/10.1155/2015/509295.
Bonfante R, Napimoga MH, Macedo CG, Abdalla HB, Pieroni V, Clemente Napimoga JT. The P2X7 receptor, cathepsin S and fractalkine in the trigeminal subnucleus caudalis signal persistent hypernociception in temporomandibular rat joints. Neuroscience. 2018;391:120–30. https://doi.org/10.1016/j.neuroscience.2018.09.005.
Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108–16. https://doi.org/10.1002/art.22321.
Imai K, Morikawa M, D’Armiento J, Matsumoto H, Komiya K, Okada Y. Differential expression of WNTs and FRPs in the synovium of rheumatoid arthritis and osteoarthritis. Biochem Biophys Res Commun. 2006;345:1615–20. https://doi.org/10.1016/j.bbrc.2006.05.075.
Gatica-Andrades M, Vagenas D, Kling J, Nguyen TTK, Benham H, Thomas R, Körner H, Venkatesh B, Cohen J, Blumenthal A. WNT ligands contribute to the immune response during septic shock and amplify endotoxemia-driven inflammation in mice. Blood Adv. 2017;1:1274–86. https://doi.org/10.1182/bloodadvances.2017006163.
Humphries AC, Mlodzik M. From instruction to output: Wnt/PCP signalling in development and cancer. Curr Opin Cell Biol. 2017;51:110–6. https://doi.org/10.1016/j.ceb.2017.12.005.
Sen M. Wnt signaling in rheumatoid arthritis. Rheumatology. 2005;44:708–13. https://doi.org/10.1093/rheumatology/keh553.
Liu YR, Yan X, Yu HX, Yao Y, Wang JQ, Li XF, Chen RN, Xu QQ, Ma TT, Huang C, Li J. NLRC5 promotes cell proliferation via regulating the NF-κB signaling pathway in rheumatoid arthritis. Mol Immunol. 2017;91:24–34. https://doi.org/10.1016/j.molimm.2017.08.024.
Xia ZB, Meng FR, Fang YX, Wu X, Zhang CW, Liu Y, Liu D, Li GQ, Feng FB, Qiu HY. Inhibition of NF-κB signaling pathway induces apoptosis and suppresses proliferation and angiogenesis of human fibroblast-like synovial cells in rheumatoid arthritis. Medicine. 2018;97:e10920. https://doi.org/10.1097/md.0000000000010920.
Rigoglou S, Papavassiliou AG. The NF-κB signaling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–4. https://doi.org/10.1016/j.biocel.2013.08.018.
Chandrakesan P, Jakkula LU, Ahmed I, Roy B, Anant S, Umar S. Differential effects of β-catenin and NF-κB interplay in the regulation of cell proliferation, inflammation and tumorigenesis in response to bacterial infection. PloS One. 2013;8:e79432. https://doi.org/10.1371/journal.pone.0079432.
Lora VRMM, Clemente-Napimoga JT, Abdalla HB, Macedo CG, Canales GT, Barbosa CMR. Botulinum toxin type A reduces inflammatory hypernociception induced by arthritis in the temporomandibular joint of rats. Toxicon. 2017;129:52–7. https://doi.org/10.1016/j.toxicon.2017.02.010.
Gondim DV, Costa JL, Rocha SS, Brito GA, Ribeiro RA, Vale ML. Antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint. Can J Physiol Pharmacol. 2012;90:395–405. https://doi.org/10.1139/y2012-003.
Chaves HV, do Val DR, Ribeiro KA, Lemos JC, Souza RB, Gomes FIF, da Cunha RMS, de Paulo Teixeira Pinto V, Filho GC, de Souza MHLP, Bezerra MM, de Castro Brito GA. Heme oxygenase-1/biliverdin/carbon monoxide pathway downregulates hypernociception in rats by a mechanism dependent on cGMP/ATP-sensitive K+ channels. Inflamm Res. 2018;67:407–22. https://doi.org/10.1007/s00011-018-1133-z.
Liu YD, Liao LF, Zhang HY, Lu L, Jiao K, Zhang M, Wang MQ. Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage. 2014;22:302–12. https://doi.org/10.1016/j.joca.2013.11.014.
Oliveira MC, Tavares LP, Vago JP, Batista NT, Queiroz-Junior CM, Vieira AT, Ferreira AVM. Tumor Necrosis Factor, but not neutrophils, alters the metabolic profile in acute experimental arthritis. Plos One. 2016;11:e0146403. https://doi.org/10.1371/journal.pone.0146403.
Farinon M, Lora PS, Francescato LN, Bassani VL, Henriques AT, Xavier RM, De Oliveira PG. Effect of Aqueous Extract of Giant Horsetail (Equisetum giganteum L.) in Antigen-Induced Arthritis. Open Rheumatol J. 2013;7:129–33. https://doi.org/10.2174/1874312901307010129.
Westacott CI, Witcher JT, Barnes IC, Thompson D, Swan AJ, Dieppe PA. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990;49:676–81. https://doi.org/10.1136/ard.49.9.676.
Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52:870–5. https://doi.org/10.1136/ard.52.12.870.
Towle CE, Hung HH, Bonassar LJ, Treadwell BV. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;5:293–300. https://doi.org/10.1016/s1063-4584(97)80008-8.
Fassio A, Adami G, Gatti D, Orsolini G, Giollo A, Idolazzi L, Benini C, Vantaggiato E, Rossini M, Viapiana O. Inhibition of tumor necrosis factor-alpha (TNF-alpha) in patients with early rheumatoid arthritis results in acute changes of bone modulators. Int Immunopharmacol. 2019;67:487–9. https://doi.org/10.1016/j.intimp.2018.12.050.
Raychaudhuri SP, Raychaudhuri SK. Biologics: target-specific treatment of systemic and cutaneous autoimmune diseases. Indian J Dermatol. 2009;54:100–9. https://doi.org/10.4103/0019-5154.53175.
Gertel S, Mahagna H, Karmon G, Watad A, Amital H. Tofacitinib attenuates arthritis manifestations and reduces the pathogenic CD4 T cells in adjuvant arthritis rat. Clin Immunol. 2017;184:77–81. https://doi.org/10.1016/j.clim.2017.04.015.
McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol. 2016;12:63–8. https://doi.org/10.1038/nrrheum.2015.171.
Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–8. https://doi.org/10.1038/319516a0.
Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. https://doi.org/10.1101/cshperspect.a001651.
Kim HJ, Park C, Kim GY, Park EK, Jeon YJ, Kim S, Hwang HJ, Choi YH. Sargassum serratifolium attenuates RANKL-induced osteoclast differentiation and oxidative stress through inhibition of NF-κB and activation of the Nrf2/HO-1 signaling pathway. Biosci Trends. 2018;12(3):257–65. https://doi.org/10.5582/bst.2018.01107.
Kim JM, Lee JH, Lee GS, Noh EM, Song HK, Gu DR, Kim SC, Lee SH, Kwon KB, Lee YR. Sophorae Flos extract inhibits RANKL-induced osteoclast differentiation by suppressing the NF-κB/NFATc1 pathway in mouse bone marrow cells. BMC Complement Altern Med. 2017;17(1):164. https://doi.org/10.1186/s12906-016-1550-x.
Lee EJ, So MW, Hong S, Kim YG, Yoo B, Lee CK. Interleukin-33 acts as a transcriptional repressor and extracellular cytokine in fibroblast-like synoviocytes in patients with rheumatoid arthritis. Cytokine. 2016;77:35–43. https://doi.org/10.1016/j.cyto.2015.10.005.
Boman A, Kokkonen H, Arlestig L, Berglin E, Rantapãã-Dahlqvist S. Receptor activator of nuclear factor kappa-B ligand (RANKL) but not sclerotin or gene polymorphisms is related to joint destruction in early rheumatoid arthritis. Clin Rheumatol. 2017;2017(36):1005–12. https://doi.org/10.1007/s10067-017-3570-4.
Rabelo FS, da Mota LM, Lima RA, Lima FA, Barra GB, de Carvalho JF, Amato AA. The Wnt signaling pathway and rheumatoid arthritis. Autoim Rev. 2019;9:207–10. https://doi.org/10.1016/j.autrev.2009.08.003.
Xiao CY, Pan YF, Guo XH, Wu YQ, Gu JR, Cai DZ. Expression of β-catenin in rheumatoid arthritis fibroblast-like synoviocytes. Scand J Rheumatol. 2011;2011(40):26–33. https://doi.org/10.3109/03009742.2010.486767.
Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. https://doi.org/10.1056/NEJM200103223441207.
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;2003(423):356–61. https://doi.org/10.1038/nature01661.
Van der Bosh MH, Blom AB, Sloetjes AW, Koenders MI, van de Loo FA, van den Berg WB, van Lent PL, van der Kraam PM. Induction of canonical Wnt signaling by synovial overexpression of selected Wnts leads to protease activity and early osteoarthritis-like cartilage damage. Am J Pathol. 2015;185(7):1970–80. https://doi.org/10.1016/j.ajpath.2015.03.013.
Van der Bosh MH, Blom AB, van de Loo FA, Koenders MI, Lafeber FP, van den Berg WB, van der Kraam PM, van Lent PL. Synovial Wnt signaling induces the expression. Of MMPs and is associated with disease progression in early symptomatic osteoarthritis patients. Arthritis Rheumatol. 2017;69(10):1978–83. https://doi.org/10.1002/art.40206.
Chu CQ, Field M, Feldmann M, Maini RA. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage- pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–32. https://doi.org/10.1002/art.1780340908.
Kraan MC, Patel DD, Haringman JJ, Smith MD, Weedon H, Ahern MJ, Breedveld FC, Tak PP. The development of clinical signs of rheumatoid synovial inflammation is associated with increased synthesis of the chemokine CXCL8 (interleukin-8). Arthritis Res. 2002;3(1):65–71. https://doi.org/10.1186/ar141.
Malysheva K, Rooji K, Lowik CWGM, Baten DL, Rose-John S, Stoika R, Korchynskyi O. Interleukin6/Wnt interactions in rheumatoid arthritis: interleukin 6 inhibits Wnt signaling in synovial fibroblasts and osteoblast. Croat Med J. 2016;57(2):89–98. https://doi.org/10.3325/cmj.2016.57.89.
Honsawek S, Tanavelee A, Yuktanandana P, Ngarmukos S, Saetan N, Tantavisut S. Dickkopf-1 (DKK-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskel Dis. 2010;11:257. https://doi.org/10.1186/1471-2474-11-257.
Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the b-catenin/TCF pathway. Oncogene. 2004;23:8520–6. https://doi.org/10.1038/sj.onc.1207892.
Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24(1):73–84. https://doi.org/10.1038/sj.emboj.7600460.
Ma B, Fey M, Hottiger MO. Wnt/β-catenin signaling inhibits CBP- mediated RelA acetylation and expression. Of proinflammatory NF-κB target genes. J Cell Sci. 2015;128:2430–6. https://doi.org/10.1242/jcs.168542.
Ma B, Hottiger MO. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front Immunol. 2016;7:378. https://doi.org/10.3389/fimmu.2016.00378.
Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-κB interactions in murine hepatocytes: a complex to die for. Hepatology. 2013;57:763–74. https://doi.org/10.1002/hep.26042.
Yun K, Choi YD, Nam JH, Park Z, Im SH. NF-kappaB regulates Lef1 gene expression in chondrocytes. Biochem Biophys Res Commun. 2007;357:589–95. https://doi.org/10.1016/j.bbrc.2007.03.170.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Howard Lopes Ribeiro Júnior, Flávia de Araújo Silva and Adalberto Nascimento de Lima Júnior. This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jason J. McDougall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sousa, L.M., dos Santos Alves, J.M., da Silva Martins, C. et al. Immunoexpression of canonical Wnt and NF-κB signaling pathways in the temporomandibular joint of arthritic rats. Inflamm. Res. 68, 889–900 (2019). https://doi.org/10.1007/s00011-019-01274-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01274-4