Abstract
Acute lung injury (ALI) is a kind of lung serious disease which leads to the damage of alveolar epithelial cells and capillary endothelial. Lipopolysaccharide (LPS) is one of the common factors inducing ALI. The previous study has reported that the anti-inflammatory effect of peiminine, but little is known about its effect on the ALI induced by LPS. The aim of this study is to investigate the therapeutic effect of peiminine on LPS-induced acute lung injury and potential mechanisms. Mice were given LPS through nasal cavity to establish ALI model, and then the peiminine (1, 3, or 5 mg/kg) was injected into the mice as the experimental group. In the present study, we would measure the W/D ratio, activity of MPO, the histopathological changes, and the levels of cytokines. The results showed that peiminine could reduce the W/D ratio and the MPO activity significantly. Furthermore, the histopathological changes and the expression of TNF-α, IL-1β, and IL-6 were inhibited after the peiminine treatment. In vitro, peiminine significantly inhibited LPS-induced IL-8 production in A549 lung epithelial cells. Meanwhile, the activity of NF-κB signaling pathway was suppressed obviously by peiminine with the western blot analysis. Also, peiminine significantly attenuated LPS-induced AKT and PI3K phosphorylation. In addition, peiminine was found to disrupt lipid rafts formation by attenuating the cholesterol content. In conclusion, peiminine could attenuate LPS-induced ALI in mice and it may become a new approach to treat ALI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Inflammation is a process of the defensive reaction between the immune system and the external stimulus [1]. When the body is stimulated, immune cells are attracted to the damaged area to participate in the immune response [2]. During the immune phase, the neutrophils and macrophages will secrete cytokines against the impaired factor, and lots of cytokines also recruit more immune cells [3, 4]. Thus, the anti-inflammatory cytokines play a crucial role, which include tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 [5]. Acute lung injury (ALI) is a kind of inflammatory disease in which alveolar cells are damaged [6]. ALI is often accompanied by pulmonary edema, hypoxemia, and respiratory distress, and its severe phase can develop into acute respiratory distress syndrome (ARDS) [7]. ARDS has a high mortality rate and many pathogenic factors [8].
Lipopolysaccharides (LPS) are a common pathogenic factor for ALI and it is the main component of the cell wall of Gram-negative bacteria [9]. The previous study has demonstrated that LPS could stimulate the neutrophils and macrophages to regulate the expression of cytokines to induce the inflammatory reaction [10]. In the process, NF-κB signaling pathway was activated to participate in the immune response [11].
Peiminine is a natural product isolated from Fritillaria plants [12]. There have been some reports about anti-inflammatory properties of peiminine, especially its protection against the LPS-induced mastitis [13] and dopaminergic neurons cell death [14]. Furthermore, peiminine had protective effects against DNCB-induced dermatitis through attenuating inflammatory cytokine production [15]. It is unclear about whether peiminine could protect ALI induced by LPS; thus, in this study, we investigated the regulating effect of peiminine on the acute lung injury in mice and clarify the mechanism.
MATERIALS AND METHODS
Materials
Peiminine standard products (purity > 98%) were obtained from China Institute of Pharmaceutical Biological Products. MPO assay kits were manufactured by Jiancheng Biological Engineering Institute (Jiancheng, Nanjing, China). Alexa Fluor 488-conjugated Cholera toxin subunit B (CTxB) was purchased from Invitrogen (CA, USA). Lipopolysaccharide (from Escherichia coli (055:B5)) and dimethyl sulfoxide (DMSO) were purchased from Sigma (USA). The ELISA assay kits of TNF-α, IL-1β, and IL-6 were manufactured by Biolegend (USA). The other agents were supplied by the Beijing Chemical Industry.
Animals
Sixty adult male BALB/c mice weighing between 14 and 20 g were obtained from animal medical laboratory of Nantong University. All the mice were fed freely in a standard laboratory environment. The house temperature was controlled at 23 ± 1 °C and the humidity was between 50 and 60%. The experimental protocol was conducted in accordance with the Laboratory Animal Management Regulations.
Murine Model of LPS-Induced ALI
Mice were divided into 5 groups (12 mice per group): control group, LPS group, and three peiminine groups. After fasting for 8 h, the LPS group and peiminine groups inhaled LPS (500 μL/kg) through the nostrils, and the control group mice were given 50 μL PBS respectively. For 1 h after the LPS treatment, the peiminine groups were intraperitoneally injected with peiminine (1, 3, 5 mg/kg). The doses of peiminine used in this study were based on previous study [16]. Accordingly, the control group and LPS group were treated with the same volume of PBS.
Lung Wet-to-Dry Weight Ratio
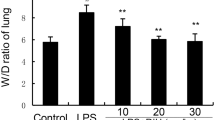
Mice were sacrificed after LPS treatment for 7 h. A part of lungs were washed by the PBS and drained the surface with filter paper; thus, the tissues were weighed to obtain the wet weight. Then the lung tissues were weighed again after they were placed in an 80 °C incubator for 48 h to get the dry weight. The wet-to-dry ratio was an important index to measure pulmonary edema.
Collection of BALF and Cell Counting
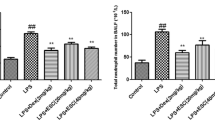
The detached tracheas were applied with tracheal cannula. The lungs were rinsed with 1.3 mL PBS through the tracheal cannula in three times to obtain bronchoalveolar lavage fluid (BALF). The BALF was placed in a centrifuge tube at 3000 RPM for 10 min. The supernatant fluid was collected for the subsequent experiments and the cells were stained with Wright-Giemsa. The cell counters were used to count the number of cells, macrophages, and neutrophils.
MPO Activity
Neutrophils and macrophages infiltrate under the LPS stimulation, while MPO activity reflects the severity of infiltration. MPO activity was measured with the MPO kits which were used accordance with the manufacturer’s instructions. The microplate was set at 470 nm to measure the number of OD to obtain the MPO activity level.
Histopathologic Evaluation of the Lung Tissue
Lung tissues of mice were immersed in 4% formaldehyde for 48 h; then, the tissue masses were dehydrated successively in different concentrations of ethanol. Afterwards, the tissues were sectioned and stained with hematoxylin and eosin. Histopathologic changes, such as the thickness of the alveolar wall and the degree of cell infiltration, can be observed from images under the microscope. The histological changes in the lungs were scored as previously described [17]. Each histological characteristic was scored 0 to 5.
ELISA
ELISA kits were used to measure the content of TNF-α, IL-1β, and IL-6 in BALF and the level of IL-8 in the supernatants. The operation process was strictly in accordance with the manufacturing instructions. In general, the antigens are added to the primary antibody and then overlaid with the secondary antibody to form a superimposed pattern. Then after TMB colored, we measured the OD at 450 nm with microplate.
Western Blotting
A549 cells were washed with PBS and homogenized 1 h after LPS treatment. The supernatants were left after centrifugation and the contents of protein were assayed by the Protein Extraction Kit (Thermo, USA). Samples were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then, transferred onto the polyvinylidene fluoride (PVDF) membranes. The membranes were sealed in 5% skim milk for 2 h and incubated with primary antibody overnight at 4 °C subsequently. Then the membranes were washed with TBST buffer three times and incubated with second antibody for 1 h at room temperature. Membranes were then detected by an ECL detection kit after washed with TBST buffer.
In vitro Study
A549 cells were cultured in DMEM medium (Hyclone, USA) containing 10% FBS (Hyclone, USA) at 37 °C in a 5% CO2 atmosphere. The effects of peiminine on cell viability were measured by MTT assay. The cells were treated with peiminine 1 h before LPS treatment. Twenty-four hours later, the production of IL-8 was measured by ELISA. One hour after LPS treatment, the expression of NF-κB, PI3K, and AKT were measured by western blot analysis.
Lipid Rafts Staining
A549 cells were cultured and fixed in 4% formaldehyde. Then, the cells were stained with 5 μg/mL Alexa Fluor 488-conjugated CTxB for 30 min. Finally, the cells were stained with Hochest and observed using a scanning confocal microscope (Olympus FluoView FV1000). Cholesterol content in lipid raft was assayed by gas–liquid chromatography as described previously.
Statistical Analyses
Measurement data conforming to normal distribution and homogeneity of variance were expressed as means ± SEM. One-way ANOVA and two-tailed Student’s t test was used for comparison among groups. p < 0.05 indicated that the difference was statistically significant.
RESULTS
Effects of Peiminine on Lung W/D Ratio in ALI Mice
The wet/dry lung ratio of mice can directly reflect the severity of pulmonary edema. As shown in Fig. 1, the W/D ratio significantly increased in the LPS group. Conversely, the lung W/D ratio obviously reduced after the peiminine treatment. The results showed that peiminine could inhibit the lung W/D ratio in LPS-induced ALI in mice.
Effects of Peiminine on the Number of Inflammatory Cells of BALF in ALI Mice
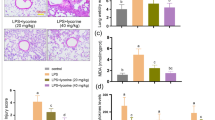
The BALF was collected and stained by Wright-Giemsa to count the number of inflammatory cells. As show in Fig. 2, the number of macrophages and neutrophils in LPS group was higher than that in the control group, while the numbers of peiminine groups were reduced clearly compared with the LPS group. The result indicated that peiminine could suppress the production of inflammatory cells, neutrophils, and macrophages.
Effects of Peiminine on MPO Activity in ALI Mice
The MPO measurements were made to measure LPS infection. The MPO activity significantly increased after LPS treatment, while the MPO activity was reduced after peiminine treatment. In addition, the amount of injection of peiminine was inversely proportional to the activity of MPO. The result demonstrated peiminine could downregulate the MPO activity in ALI which was induced by LPS.
Effects of Peiminine on Histological Changes in ALI Mice
Histological changes could reflect the cell damage caused by LPS directly. We could observe the normal situation in the control group and cell infiltration and cell wall thickening in the LPS group from the microscope. In peiminine groups, the histological changes were suppressed, and the higher dose of peiminine, the less histological changes (Fig. 3).
Effects of Peiminine on Cytokines Production in ALI Mice and A549 Cells
Inflammatory cytokines were crucial factors in measuring inflammation. We used the ELISA kits to assay the levels of TNF-α, IL-1β, and IL-6. As shown in Fig. 4, the levels of inflammatory cytokines increased markedly after LPS inhalation alone. As we expected, the levels reduced after peiminine treatment, and as the dose of peiminine increases, the reduction became more pronounced. In conclusion, the peiminine could limit the production of inflammatory cytokines in LPS-induced ALI mice (Fig. 5). In vitro, we found peiminine at the concentrations of 10, 20, and 40 μg/mL did not affect the viability of A549 cells (Fig. 6). Meanwhile, peiminine (10, 20, 40 μg/mL) significantly inhibited LPS-induced IL-8 production in A549 cells (Fig. 6).
Effects of peiminine on histopathological changes in lung tissues in LPS-induced ALI mice. Representative histological changes of lung obtained from mice of different groups. a Control group. b LPS group. c LPS + peiminine (1 mg/kg) group. d LPS + peiminine (3 mg/kg) group. e LPS + peiminine (5 mg/kg) group (hematoxylin and eosin staining, magnification × 200).
Effects of Peiminine on NF-κB Activation in A549 Cells
Because of the importance of NF-κB signaling pathway in the process of inflammation, we used the western blotting to obtain the NF-κB activity changes. As shown in Fig. 7, LPS promoted the activation of NF-κB signaling pathways. On the contrary, peiminine inhibited this activation trend.
Effects of Peiminine on AKT and PI3K Phosphorylation in A549 Cells
AKT and PI3K phosphorylation could lead to the activation of NF-κB. Thus, we used the western blotting to obtain the AKT and PI3K changes. As shown in Fig. 8, LPS promoted the phosphorylation of PI3K and AKT. On the contrary, peiminine inhibited this activation trend. These results demonstrated that peiminine had the effect to attenuate LPS-induced ALI in mice through PI3K/AKT/NF-κB signaling pathways.
Effects of Peiminine on the Formation of Lipid Rafts in A549 Cells by Depleting Cholesterol
Previous studies demonstrated that lipid rafts played a critical role in the PI3K/AKT signaling pathway and disrupting of lipid rafts could block PI3K/AKT signaling pathway. We found the formation of lipid rafts was disrupted (Fig. 9a) by peiminine through attenuating the content of cholesterol in lipid rafts (Fig. 9b).
Effects of peiminine on the formation of lipid rafts (a) and cholesterol content (B). A, control group; B, LPS group; C, LPS + peiminine (10 μg/mL) group; D, LPS + peiminine (20 μg/mL) group; E, LPS + peiminine (40 μg/mL) group. The values presented are the means ± SEM of three independent experiments. #p < 0.01 vs. control group; *p < 0.05 and **p < 0.01 vs. LPS group.
DISCUSSION
ALI is a clinically common pulmonary disease with high mortality, which is often accompanied by pulmonary edema, dyspnea, and other symptoms [18]. For a long time, researchers have been trying to find effective drugs for ALI. In the past studies, LPS-induced ALI model is often used, because LPS is a common pathogenic factor [19]. LPS is the main component of the cell wall of Gram-negative bacteria. When it stimulates the body, it will produce a series of inflammatory reactions. Especially, when LPS affects the lungs, it will induce lungs to develop into ALI and then into ARDS in severe cases [20].
The previous study had demonstrated that peiminine had the effect to protect against LPS-induced mastitis and dopaminergic neurons by inhibiting the NF-κB signaling pathway. In this study, we investigate the protective effect of peiminine in ALI in mice, and we also used the LPS-induced ALI model to explore the pharmacodynamic and pathological responses of peiminine.
In the present study, we need to obtain some crucial factors, including W/D ratio, histological changes, MPO activity, the number of inflammatory cells, the inflammatory cytokines production, and the NF-κB signaling pathway. The W/D ratio is the most intuitive manifestation of pulmonary edema and histopathological changes are observed the change of inflammatory cells through a microscope. The results showed peiminine could reduce the W/D ratio and attenuate histopathological changes compared with LPS treatment alone. When lung tissues were stimulated by LPS, inflammatory cells including neutrophils and macrophages were recruited, and the number of cells increased significantly [21]. Neutrophil infiltration was often accompanied by an increase in MPO activity, so MPO activity was also one of the indicators to measure LPS infection [22]. In this study, peiminine distinctly inhibited LPS-induced aggregation of inflammatory cells and enhanced MPO activity. It is acknowledged that LPS stimulate the body to produce inflammatory cytokines involved in the immune response and excessive inflammatory factors will produce excessive inflammatory reactions [23]. The cytokines including TNF-α, IL-1β, and IL-6 significantly increased after LPS inhalation, while peiminine could inhibit the increase in this study. Furthermore, NF-κB signaling pathway is also one of the crucial goals we need to study [24]. Under normal circumstances, IκB and NF-κB are bound to each other in the cell. When LPS invades, IκB is phosphorylated to release the inhibition of NF-κB, and then NF-κB dissociates into the nucleus, so LPS will promote the activation of the NF-κB signaling pathway [25]. In this study we found that the NF-κB signaling pathway was inhibited by peiminine in vitro. AKT and PI3K, the upstream molecules of NF-κB signaling pathway, its phosphorylation could lead to the activation of NF-κB [26]. In this study, we found peiminine attenuated phosphorylation of PI3K and AKT, which indicated peiminine suppressed LPS-induced ALI through attenuating PI3K/AKT/NF-κB signaling pathway. Lipid rafts played a critical role in the PI3K/AKT signaling pathway and studies showed disrupting of lipid rafts could block PI3K/AKT signaling pathway. In this study we found peiminine could disrupt the formation of lipid rafts by deleting cholesterol. This suggested peiminine blocked PI3K/AKT/NF-κB signaling pathway through disrupting lipid rafts.
In conclusion, the results demonstrated that peiminine could reduce the damage of inflammatory response to the body and the possibility of pulmonary edema. In particular, peiminine could attenuate ALI induced by LPS through inhibiting PI3K/AKT/NF-κB signaling pathway via disrupting lipid rafts. This may provide a new scheme for the clinical treatment of ALI.
References
Pinsky, M.R. 2004. Dysregulation of the immune response in severe sepsis. The American Journal of the Medical Sciences 328: 220–229.
Smith, J.A. 1994. Neutrophils, host defense, and inflammation: a double-edged sword. Journal of Leukocyte Biology 56: 672–686.
Fujiwara, N., and K. Kobayashi. 2005. Macrophages in inflammation. Current Drug Targets. Inflammation and Allergy 4: 281–286.
Nadra, I., J.C. Mason, P. Philippidis, O. Florey, C.D. Smythe, G.M. McCarthy, R.C. Landis, and D.O. Haskard. 2005. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circulation Research 96: 1248–1256.
Zhang, J.M., and J. An. 2007. Cytokines, inflammation, and pain. International Anesthesiology Clinics 45: 27–37.
Grommes, J., and O. Soehnlein. 2011. Contribution of neutrophils to acute lung injury. Molecular Medicine 17: 293–307.
Matthay, M.A., and G.A. Zimmerman. 2005. Acute lung injury and the acute respiratory distress syndrome - four decades of inquiry into pathogenesis and rational management. American Journal of Respiratory Cell and Molecular Biology 33: 319–327.
Ware, L.B. 2006. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Seminars in Respiratory and Critical Care Medicine 27: 337–349.
Johnson, E.R., and M.A. Matthay. 2010. Acute lung injury: epidemiology, pathogenesis, and treatment. Journal of Aerosol Medicine and Pulmonary Drug Delivery 23: 243–252.
Cassatella, M.A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunology Today 16: 21–26.
Lu, Y.C., W.C. Yeh, and P.S. Ohashi. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151.
Pan, F., K. Hou, F. Gao, B. Hu, Q. Chen, and W. Wu. 2014. Peimisine and peiminine production by endophytic fungus Fusarium sp isolated from Fritillaria Unibracteata var. wabensis. Phytomedicine 21: 1104–1109.
Gong, Q., Y.W. Li, H. Ma, W.J. Guo, X.C. Kan, D.W. Xu, J.X. Liu, and S.P. Fu. 2018. Peiminine protects against lipopolysaccharide-induced mastitis by inhibiting the AKT/NF-kappa B, ERK1/2 and p38 signaling pathways. International Journal of Molecular Sciences 19.
Chen, G.X., J.X. Liu, L.Q. Jiang, X. Ran, D.W. He, Y.H. Li, B.X. Huang, W. Wang, D.F. Liu, and S.P. Fu. 2018. Peiminine protects dopaminergic neurons from inflammation-induced cell death by inhibiting the ERK1/2 and NF-kappa B signalling pathways. International Journal of Molecular Sciences 19.
Lim, J.M., B. Lee, J.H. Min, E.Y. Kim, J.H. Kim, S. Hong, J.J. Kim, Y. Sohn, and H.S. Jung. 2018. Effect of peiminine on DNCB-induced atopic dermatitis by inhibiting inflammatory cytokine expression&IT in vivo&IT and &ITin vitro&IT. International Immunopharmacology 56: 135–142.
Gong, Q., Y. Li, H. Ma, W. Guo, X. Kan, D. Xu, J. Liu, and S. Fu. 2018. Peiminine protects against lipopolysaccharide-induced mastitis by inhibiting the AKT/NF-kappaB, ERK1/2 and p38 signaling pathways. International Journal of Molecular Sciences 19.
Liu, M.-H., A.-H. Lin, H.-F. Lee, H.-K. Ko, T.-S. Lee, and Y.R. Kou. 2014. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediators of Inflammation 2014.
DeClue, A.E., and L.A. Cohn. 2007. Acute respiratory distress syndrome in dogs and cats: a review of clinical findings and pathophysiology. Journal of Veterinary Emergency and Critical Care 17: 340–347.
Matute-Bello, G., C.W. Frevert, and T.R. Martin. 2008. Animal models of acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 295: L379–L399.
Mei, S.H.J., S.D. McCarter, Y.P. Deng, C.H. Parker, W.C. Liles, and D.J. Stewart. 2007. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Medicine 4: 1525–1537.
Abraham, E. 2003. Neutrophils and acute lung injury. Critical Care Medicine 31: S195–S199.
Lee, W.L., and G.P. Downey. 2001. Neutrophil activation and acute lung injury. Current Opinion in Critical Care 7: 1–7.
Goodman, R.B., J. Pugin, J.S. Lee, and M.A. Matthay. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine & Growth Factor Reviews 14: 523–535.
Everhart, M.B., H. Wei, T.P. Sherrill, M. Arutiunov, V.V. Polosukhin, J.R. Burke, R.T. Sadikot, J.W. Christman, F.E. Yull, and T.S. Blackwell. 2006. Duration and intensity of NF-kappa B activity determine the severity of endotoxin-induced acute lung injury. Journal of Immunology 176: 4995–5005.
Covert, M.W., T.H. Leung, J.E. Gaston, and D. Baltimore. 2005. Achieving stability of lipopolysaccharide-induced NF-kappa B activation. Science 309: 1854–1857.
Qi, S.M., Y.Q. Xin, Y.T. Guo, Y. Diao, X.J. Kou, L. Luo, and Z.M. Yin. 2012. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-kappa B signaling pathways. International Immunopharmacology 12: 278–287.
Funding
This study was supported by the National Natural Science Foundation of China (NO. 81700078) and Natural Science Foundation ofJiangsu Province (NO. BK20171172).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, B., Cao, L., Wang, K. et al. Peiminine Attenuates Acute Lung Injury Induced by LPS Through Inhibiting Lipid Rafts Formation. Inflammation 43, 1110–1119 (2020). https://doi.org/10.1007/s10753-020-01198-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01198-w