Abstract—
Dihydrotanshinone (DIH) is an extract of Salvia miltiorrhiza Bunge. It has been reported that DIH could regulate NF-κB signaling pathway. The aim of this study was to investigate whether DIH could protect mice from lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. In this study, sixty mice were randomly divided into five groups, one group as blank control group, the second group as LPS control group, and the last three groups were pre-injected with different doses of DIH and then inhaled LPS for experimental comparison. After 12 h of LPS treatment, the wet-dry ratio, histopathlogical changes, and myeloperoxidase (MPO) activity of lungs were measured. In addition, ELISA kits were used to measure the levels of TNF-α and IL-1β inflammatory cytokines in bronchoalveolar lavage fluids (BALF), and western blot analysis was used to measure the activity of NF-κB signaling pathway. The results demonstrated that DIH could effectively reduce pulmonary edema, MPO activity, and improve the lung histopathlogical changes. Furthermore, DIH suppressed the levels of inflammatory cytokines in BALF, such as TNF-α and IL-1β. In addition, DIH could also downregulate the activity of NF-κB signaling pathway. We also found that DIH dose-dependently increased the expression of LXRα. In addition, DIH could inhibit LPS-induced IL-8 production and NF-κB activation in A549 cells. And the inhibitory effects were reversed by LXRα inhibitor geranylgeranyl pyrophosphate (GGPP). Therefore, we speculate that DIH regulates LPS-induced ALI in mice by increasing LXRα expression, which subsequently inhibiting NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute lung injury is an acute inflammation of the lung caused by external or internal factors. Mild patients may have respiratory insufficiency, and severe patients may develop acute respiratory distress syndrome [1, 2]. It is a developmental process of lung injury development and spread, and also a process of immune cells in vivo initiating immune response to protect themselves to over-immune-induced inflammation [3]. Acute lung injury (ALI) is often accompanied by pulmonary cell infiltration, increased levels of inflammatory cytokines, and various proteins, leading to pulmonary edema and dyspnea [4, 5].
Lipopolysaccharide (LPS), a combination of lipids and polysaccharides in the cell wall of gram-negative bacteria, is one of the most common pathogenic factors of ALI [6]. When the body is infected with LPS, LPS interacts with TLR4 in target receptor cells to stimulate the body’s inflammatory response. LPS binds to the plasma protein LPS-binding protein (LBP) and CD14 forming high affinity complexes LPS-LPB-CD14. Then, it recruits MyD88 and the downstream TAK1 molecule to activate the NF-κB signaling pathway, thereby activating the expression of inflammatory cytokines and type I interferon [7, 8]. LXRα is a ligand-dependent transcription factor that plays an important role in lipid metabolism and inflammation [9, 10]. Previous studies showed that activation of LXRα could inhibit LPS-induced inflammatory response [11]. Furthermore, studies showed that LXRα agonist could inhibit LPS-induced NF-κB activation [12]. In addition, a large body of studies demonstrated that many herbal compounds could activate LXRα and can be used as LXRα agonists [13,14,15].
Dihydrotanshinone (DIH) is the rhizome extract of Salvia miltiorrhizaBge, which has antibacterial and anti-inflammatory effects [16]. The previous reports have demonstrated DIH exhibited an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization [17]. In addition, DIH could ameliorate DSS-induced experimental ulcerative colitis in mice [18] and attenuate atherosclerosis in apolipoprotein E-deficient mice [19]. Furthermore, the combination of DIH and Sanqi had synergistic effects in inhibiting inflammation mediators [20]. The aim of this study was to investigate whether DIH could reduce the damage of LPS-induced ALI and clarify the possible mechanism.
MATERIALS AND METHODS
Animals
The 7-week-old male BABL/c mice were purchased from the animal experiment center of Jilin University. The mice were raised in an environment with a temperature of 25 °C, humidity of 40–60% and good ventilation. The mice were free to eat and drink and maintained a 12-/12-h cycle of day and night.
Reagents
DIH standard products (purity > 98%) were purchased from China pharmaceutical and biological products inspection institute. LPS and myeloperoxidase (MPO) assay kits were obtained from Abclonal (Wuhan, China). The ELISA kits were supplied by Abcam (Cambridge, MA, USA). The other reagents without special labels were purchased from Jiancheng Bioengineering Institute (Nanjing, China). GGPP was purchased from Sigma-Aldrich (CA, USA).
LPS-Induced ALI Model
Sixty mice were randomly divided into 5 groups with 12 mice in each group: control group, LPS group, and DIH groups with three doses (10 mg/kg, 20 mg/kg, 30 mg/kg). The mice of DIH groups were injected with 10 mg/kg, 20 mg/kg, and 30 mg/kg of DIH, respectively, while the control group and LPS group were injected with the same volume of normal saline. After 1 h of DIH treatment, the mice were anesthetized; then, LPS was dropped from the nostrils of the LPS group [21] and DIH groups, while the control group was injected with the same volume of saline. The animal experiments were conducted in accordance with international guidelines on the ethical use of animals and approved by the Institutional Animal Care and Use Committee of Jilin University.
The Ratio of Wet to Dry Measurement
At 12 h after LPS treatment, part of the right lung tissue was removed, and the lung tissue was washed with PBS and weighed to get the wet weight. Subsequently, the tissue was placed in an incubator at 80 °C. After 48 h, the dry weight was weighed and the wet weight was divided by the dry weight to get the wet to dry ratio.
The Number of Inflammatory Cell Measurement
The mice were killed after 12 h of dripping into LPS. The trachea was separated and inserted into the trachea of the mice with tracheal cannula. The trachea was slowly washed with 3 ml PBS for three times and then recovered. The BALF was obtained and centrifuged in a 2000 RPM centrifuge for 10 min. The supernatant was left for follow-up test, and the cell precipitate was stained with Wright-Giemsa. The cell counter counts the number of macrophages, neutrophils, and total cells.
The Activity of MPO Measurement
In order to measure MPO activity, the right lung of mice was frozen, ground, dissolved with PBS, and supernatant was measured MPO activity by MPO assay kits according to the manufacturer’s instructions. The spectrophotometer was set at 450 nm to measure the OD.
The Changes of Lung Histopathological Changes
To observe the pathological changes of lung tissues, lung tissues were extracted and embedded in 4% formaldehyde for 48 h after LPS treatment for 12 h. Next, the lung tissue was rinsed clean and dehydrated, then embedded in paraffin and made into glass slides. The morphology of the cells was observed under microscope after H&E staining.
The Inflammatory Cytokines of BALF Measurement
The previously obtained bronchoalveolar lavage fluid was used to measure the concentrations of inflammatory cytokines. The levels of TNF-α and IL-1β were measured using ELISA assay kits according to the manufacturer’s instructions.
Western Blot Analysis
The NF-κB, LXRα, and ABCA1 expressions in lung tissue were determined by Western Blot. The protein concentration in the supernatant was analyzed using BCA assay kits. The samples were separated by 12% SDS polyacrylamide gel electrophoresis and then separated to the bottom. The samples were then transferred to the PVDF film. Subsequently, the membrane was placed in 5% skim milk for 2 h and incubated with the primary antibodies overnight at room temperature. After 3 times of TBST cleaning, the membranes were incubated with secondary antibody at room temperature for 1 h and then visualized by ECL.
In Vitro Experiment
A549 cells were cultured in DMEM supplemented with 10% FBS. The effects of DIH on A549 cell viability were detected by MTT assay. The cells were pre-treated with DIH 1 h before LPS treatment. IL-8 production and NF-κB activation were measured by ELISA and western blot analysis. In addition, to inhibit LXRα, its inhibitor GGPP (20 μM) was added and the cytokines and NF-κB activation were measured.
Statistical Analysis
All data results were analyzed using ANOVA; the expression of the results was means ± S.E.M. ANOVA and Student’s T were used as test methods for the difference analysis of any two groups of data. P < 0.05 stands for significant difference, while P < 0.01 stands for extremely significant difference.
RESULTS
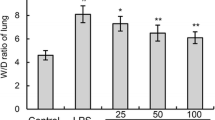
Effects of DIH on Pulmonary Edema
In order to investigate the effect of DIH on pulmonary edema, the wet to dry ratio of the lung was calculated. The lung wet to dry ratio in LPS group increased significantly after LPS treatment, while the lung wet to dry ratio in pretreatment DIH groups decreased to a certain extent. The results showed that DIH could alleviate LPS-induced pulmonary edema (Fig. 1).
Effects of DIH on Lung MPO Activity
The activity index of MPO is closely related to the status of immune cells. In the present study, we determined the activity status of MPO. The results showed that MPO activity was significantly increased after LPS stimulation. However, LPS-induced MPO activity was markedly decreased in mice pretreated with DIH (10 mg/kg, 20 mg/kg, 30 mg/kg) and the decreases were in a dose-dependent manner (Fig. 2).
Effects of DIH on Inflammatory Cell Number
The number of inflammatory cells correlates with the immune response. LPS stimulation directly increased the number of inflammatory cells, neutrophils, and macrophages, as shown in Fig. 3. However, the increased numbers of inflammatory cells, neutrophils, and macrophages were significantly inhibited by DIH (10 mg/kg, 20 mg/kg, 30 mg/kg). These results suggested that DIH could attenuate LPS-induced inflammatory cell infiltration in lung tissues.
Effects of DIH on Lung Histopathological Changes
LPS-induced histopathological changes in the lung tissues were detected in this study. Compared to the control group, LPS group showed severe histopathologic changes, such as cell wall thickened, cell edema, and inflammatory cell infiltration in the lung tissues. However, treatment with DIH (10 mg/kg, 20 mg/kg, 30 mg/kg) significantly ameliorated LPS-induced lung histopathological changes (Fig. 4).
Effects of DIH on histopathological changes in lung tissues in LPS-induced ALI mice. Representative histological changes of lung obtained from mice of different groups. A Control group, B LPS group, C LPS + DIH (10 mg/kg) group, D LPS + DIH (20 mg/kg) group, E LPS + DIH (30 mg/kg) group (hematoxylin and eosin staining, magnification 200 ×).
Effects of DIH on Inflammatory Cytokine Production
Inflammatory cytokines play a critical role in the development of lung injury. As can be seen in Fig. 5, the inflammatory cytokines of TNF-α and IL-1β production in LPS group were much higher than the control group. However, treatment with DIH (10 mg/kg, 20 mg/kg, 30 mg/kg) significantly inhibited LPS-induced inflammatory cytokine production. The results showed that DIH could inhibit LPS-induced inflammatory cytokine production.
Effects of DIH on Lung NF-κB Activity
The expression of inflammatory cytokines was regulated by NF-κB. To investigate the anti-inflammatory mechanism of DIH, NF-κB signaling pathway was measured in this study. According to the results of western blot, the expression of phosphorylation of NF-κB p65 and I-κBα in LPS group was upregulated compared with the control group. However, in DIH treated groups, the expression of phosphorylation of NF-κB p65 and I-κBα was attenuated by DIH in a dose-dependent manner (Fig. 6). The results suggested that DIH could downregulate the NF-κB activity induced by LPS.
Effects of DIH on Lung LXRα Expression
Previous studies showed that activation of LXRα could inhibit NF-κB activation [12]. To further clarify the anti-inflammatory mechanism of DIH, LXRα and its downstream signaling molecule ABCA1 expression was measured in this study. According to the results of western blot, LXRα and ABCA1 expression in LPS group was downregulated compared with control group. However, DIH (10 mg/kg, 20 mg/kg, 30 mg/kg) increased the expression of LXRα and ABCA1 in a dose-dependent manner (Fig. 7).
In vitro studies, our results showed that DIH could inhibit LPS-induced IL-8 production and NF-κB activation in A549 cells. And the inhibition of DIH on IL-8 production and NF-κB activation in A549 cells was blocked by GGPP (Fig. 8).
DISCUSSION
In present study, BABL/c mice were used to establish the ALI model. Our results demonstrated that DIH had protective effects against ALI and the mechanism may be through activating LXRα, which subsequently inhibited LPS-induced inflammatory response.
ALI is usually the concentration of inflammatory response in the lungs caused by pathogenic factors [2]. The accompanying symptoms include pulmonary interstitial edema, infiltration of neutrophils and macrophages, and histopathological changes. In severe cases, ALI progresses to acute respiratory distress syndrome (ARDS), which greatly increases the risk of death [22].
As a common pulmonary disease, ALI has a very high clinical fatality rate [23]. Thus, the researchers have been looking for effective drugs to treat ALI. In the present study, the experimental results showed that compared with the control group, the lung wet to dry ratio and MPO activity in LPS group were significantly increased. And the histopathological changes are more severe. ALI is characterized by the infiltration of inflammatory cells [24]. And these inflammatory cells could release the inflammatory cytokines and amply the inflammatory response [25]. In this study, there was an obvious increase in the number of inflammatory cells and inflammatory factors TNF-α and IL-1β in BALF. As expected, all the indicators in DIH groups were lower than those in the LPS group. These results indicated that DIH had protective effects against LPS-induced ALI.
As an important receptor, activation of TLR4 can directly stimulate the nuclear transcription expression of NF-κB after bacteria enter the body [26]. Previous studies have shown that LPS can activate the expression of NF-κB through TLR4 [27]. When ligands bind to cell surface receptors, adaptor proteins are recruited to the binding site to activate the IKK complex. IKK phosphorylates IκB and exposes NF-κB to nuclear transcription [28]. In previous studies, it has demonstrated that DIH could exhibit an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization [17]. The regulatory effect of DIH on NF-κB signaling pathway is not clear, and the effect of DIH on LPS-induced ALI is still unknown. Therefore, this paper explores the anti-inflammatory mechanism of DIH by establishing LPS-induced mouse ALI model. The results showed that DIH significantly attenuated NF-κB signaling pathway activity induced by LPS. LXRα has been known to have anti-inflammatory activity [29, 30]. Previous studies showed that activation of LXRα could inhibit LPS-induced TLR4 dimerization and NF-κB activation [13, 31]. Therefore, we investigated whether DIH exhibited anti-inflammatory effects through activating LXRα. And our results showed DIH could increase the expression of LXRα and downstream signaling molecule ABCA1 in a dose-dependent manner.
In this study, we found that DIH could activate LXRα, which leads to the inhibition of the nuclear transcription of NF-κB and inflammatory response. In the follow-up research, DIH will be used to participate in clinical experiments and we will subsequently examine the possibility of this as a specific treatment for ALI.
Availability of Data and Materials
The data used to support the findings of this study are available from the corresponding author upon request.
References
Parker, J.C. 2011. Acute lung injury and pulmonary vascular permeability: Use of transgenic models. Comprehensive Physiology 1: 835–882.
Matuschak, G.M., and A.J. Lechner. 2010. Acute lung injury and the acute respiratory distress syndrome: Pathophysiology and treatment. Missouri Medicine 107: 252–258.
Tomashefski, J.F. 2000. Pulmonary pathology of acute respiratory distress syndrome. Clinics in Chest Medicine 21: 435–466.
Chignard, M., and V. Balloy. 2000. Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. American Journal of Physiology-Lung Cellular and Molecular Physiology 279: L1083–L1090.
Blank, R., and L.M. Napolitano. 2011. Epidemiology of ARDS and ALI. Critical Care Clinics 27: 439–458.
Chen, H., C. Bai, and X. Wang. 2010. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Review of Respiratory Medicine 4: 773–783.
Lu, Y.C., W.C. Yeh, and P.S. Ohashi. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151.
Kawai, T., and S. Akira. 2007. Signaling to NF-kappaB by Toll-like receptors. Trends in Molecular Medicine 13: 460–469.
Endo-Umeda, K., and M. Makishima. 2019. Liver X receptors regulate cholesterol metabolism and immunity in hepatic nonparenchymal cells. International Journal of Molecular Sciences 20: 5045.
Liebergall, S.R., J. Angdisen, S.H. Chan, Y.J. Chang, T.F. Osborne, A.F. Koeppel, S.D. Turner, and I.G. Schulman. 2020. Inflammation triggers liver X receptor-dependent lipogenesis. Molecular and Cellular Biology 40: e00364-e419.
Miao, C.M., K. He, P.Z. Li, Z.J. Liu, X.W. Zhu, Z.B. Ou, X.Z. Ruan, J.P. Gong, and C.A. Liu. 2016. LXRalpha represses LPS-induced inflammatory responses by competing with IRF3 for GRIP1 in Kupffer cells. International Immunopharmacology 35: 272–279.
Fu, Y.H., Y. Tian, Z.K. Wei, H. Liu, X.J. Song, W.B. Liu, W.L. Zhang, W.L. Wang, Y.G. Cao, and N.S. Zhang. 2014. Liver X receptor agonist prevents LPS-induced mastitis in mice. International Immunopharmacology 22: 379–383.
Liu, B., Z.Q. He, J.J. Wang, Z.Y. Xin, J.X. Wang, F. Li, and Y.H. Fu. 2018. Taraxasterol inhibits LPS-induced inflammatory response in BV2 microglia cells by activating LXR alpha. Frontiers in Pharmacology 2018 (9): 278.
Fu, Y.H., Z.Y. Xin, B. Liu, J.X. Wang, J.J. Wang, X. Zhang, Y.N. Wang, and F. Li. 2018. Platycodin D inhibits inflammatory response in LPS-stimulated primary rat microglia cells through activating LXR alpha-ABCA1 signaling pathway. Frontiers in Immunology 8: 1929.
Su, K., G.X. Zhang, X. Zhang, and W. Jiang. 2019. Chikusetsusaponin V attenuates lipopolysaccharide-induced acute lung injury in mice by modulation of the NF-kappa B and LXR alpha. International Immunopharmacology 70: 174–179.
Lee, D.S., S.H. Lee, J.G. Noh, and S.D. Hong. 1999. Antibacterial activities of cryptotanshinone and dihydrotanshinone I from a medicinal herb Salvia miltiorrhiza Bunge. Bioscience Biotechnology and Biochemistry 63: 2236–2239.
Yuan, R.Y.K., L.T. Huang, L.J. Du, J.F. Feng, J. Li, Y.Y. Luo, Q.M. Xu, S.L. Yang, H.W. Gao, and Y.L. Feng. 2019. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacological Research 142: 102–114.
Guo, Y.L., X.X. Wu, Q. Wu, Y.F. Lu, J.S. Shi, and X.P. Chen. 2018. Dihydrotanshinone I, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice. Toxicology and Applied Pharmacology 344: 35–45.
Zhao, W.W., C.X. Li, H.W. Gao, Q. Wu, J.S. Shi, and X.P. Chen. 2016. Dihydrotanshinone I attenuates atherosclerosis in ApoE-deficient mice: Role of NOX4/NF-kappa B mediated lectin-like oxidized LDL receptor-1 (LOX-1) of the Endothelium. Frontiers in Pharmacology 7: 418.
Zhou, X., V. Razmovski-Naumovski, D. Chang, C.G. Li, A. Kam, M. Low, A. Bensoussan, and K. Chan. 2016. Synergistic effects of Danshen (Salvia Miltiorrhiza Radix et Rhizoma) and Sanqi (Notoginseng Radix et Rhizoma) combination in inhibiting inflammation mediators in RAW264.7 cells. Biomed Research International.
San, Z.H., Y.H. Fu, W. Li, E.S. Zhou, Y.M. Li, X.J. Song, T.C. Wang, Y. Tian, Z.K. Wei, M.J. Yao, et al. 2014. Protective effect of taraxasterol on acute lung injury induced by lipopolysaccharide in mice. International Immunopharmacology 19: 342–350.
Ruffini, E., A. Parola, E. Papalia, P.L. Filosso, M. Mancuso, A. Oliaro, G. Actis-Dato, and G. Maggi. 2001. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. European Journal of Cardio-Thoracic Surgery 20: 30–37.
Bruijn, M., L.B. van der Aa, R.R. van Rijn, A.P. Bos, and J.B.M. van Woensel. 2007. High incidence of acute lung injury in children with Down syndrome. Intensive Care Medicine 33: 2179–2182.
Abraham, E., A. Carmody, R. Shenkar, and J. Arcaroli. 2000. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology 279: L1137–L1145.
Yamasawa, H., Y. Ishii, and S. Kitamura. 1999. Cytokine-induced neutrophil chemoattractant in a rat model of lipopolysaccharide-induced acute lung injury. Inflammation 23: 263–274.
Wright, J.G., and J.W. Christman. 2003. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: Implications for therapy. American Journal of Respiratory Medicine 2: 211–219.
Maruyama, K., Y. Takada, N. Ray, Y. Kishimoto, J.M. Penninger, H. Yasuda, and K. Matsuo. 2006. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. Journal of Immunology 177: 3799–3805.
Rahman, A., and F. Fazal. 2011. Blocking NF-kappaB: An inflammatory issue. Proceedings of the American Thoracic Society 8: 497–503.
Zhu, R.T., Z.B. Ou, X.Z. Ruan, and J.P. Gong. 2012. Role of liver X receptors in cholesterol efflux and inflammatory signaling. Molecular Medicine Reports 5: 895–900.
Jin, S.H., J.H. Yang, B.Y. Shin, K. Seo, S.M. Shin, I.J. Cho, and S.H. Ki. 2013. Resveratrol inhibits LXR alpha-dependent hepatic lipogenesis through novel antioxidant Sestrin2 gene induction. Toxicology and Applied Pharmacology 271: 95–105.
Fu, Y.H., X.Y. Hu, Y.G. Cao, Z.C. Zhang, and N.S. Zhang. 2015. Saikosaponin a inhibits lipopolysaccharide-oxidative stress and inflammation in Human umbilical vein endothelial cells via preventing TLR4 translocation into lipid rafts. Free Radical Biology and Medicine 89: 777–785.
Author information
Authors and Affiliations
Contributions
Xueshibojie Liu designed the experiment; Xueshibojie Liu, Jinghui Yang, Jing Yue, Guangxin Zhang, and Kai Su did the experiment; Chengbi Xu analyzed the data; Xueshibojie Liu wrote the paper; Jing Yue, Kai Su, and Guangxin Zhang revised the paper.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All the experimental protocols in this study were approved by the Institutional Animal Care and Use Committee of Jilin University. All authors consent to participate this research.
Consent for Publication
All authors consent to publish this article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yue, J., Su, K., Zhang, G. et al. Dihydrotanshinone Attenuates LPS-Induced Acute Lung Injury in Mice by Upregulating LXRα. Inflammation 45, 212–221 (2022). https://doi.org/10.1007/s10753-021-01539-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01539-3