Abstract
In this study, we investigated anti-inflammatory effects of esculin (ESC) on lipopolysaccharide (LPS)-induced acute lung injury (ALI). ALI was induced in mice by intratracheal instillation of LPS, and ESC (20 and 40 mg/kg) was given orally 1 h prior to LPS administration. After 6 h, bronchoalveolar lavage fluid (BALF) and lung tissue were collected. ESC pretreatment decreased LPS-induced evident lung histopathological changes, lung wet-to-dry weight ratio, and lung myeloperoxidase activity. In addition, pretreatment with ESC inhibited inflammatory cells and proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β, and interleukin-6 in BALF. Furthermore, we demonstrated that ESC inhibited the Toll-like receptor-2 (TLR2), Toll-like receptor-4 (TLR4), myeloid differentiation primary response gene-88 (MyD88), and nuclear factor-κB (NF-κB) p65 in LPS-induced ALI. The results indicated that the ESC had a protective effect on LPS-induced ALI in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute lung injury (ALI) or its more severe form, acute respiratory distress syndrome (ARDS), characterized by severe hypoxemia, pulmonary edema, and neutrophil accumulation in the lung, is a common clinical problem associated with significant morbidity and mortality in shock, sepsis, ischemia reperfusion, etc. [1, 2]. ALI is a major clinical problem that has a high mortality rate of 30 to 40 % despite significant advances in antimicrobial therapy and supportive care made in the past few decades. There are still few effective measures or specific medicines to treat it [3]. The inflammatory process played a key role in the development of ALI, and the main pathological change of ALI was acute leakage inflammatory response with leakage of protein into the alveolar space, inflammatory cells accumulation, interstitial edema, and disruption of epithelial integrity [4]. Therefore, it was significant that anti-inflammatory drugs were used to reduce lung injury at early stage of ALI. Intratracheal administration of LPS has gained wide acceptance as a clinically relevant model of severe lung injury [5]. Thus, we use this model to determine whether ESC could prevent ALI induced by LPS administration in mice.
Nuclear factor-kappaB (NF-κB), a nuclear transcription factor, is a regulator of inflammatory processes. Chen et al. have reported that NF-κB plays an important role in the pathogenesis of lung diseases [6, 7]. NF-κB is required for maximal transcription of numerous cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [8]. These cytokines are thought to be important in the generation of ALI. Therefore, it has been suggested that inhibitors of NF-κB function may be useful as anti-inflammatory agents [9].
Toll-like receptors (TLRs) are transmembrane receptors with an extracellular domain that interacts with a pathogen ligand and an intracellular domain that is involved in signaling [10].
Esculin is a coumarin derivative that has been demonstrated to have multiple biological functions including intestinal antioxidant activity [11] and anticancer activity including growth inhibition of human leukemia cells [12]. However, no available study has evaluated the effects of ESC treatment on LPS-induced acute lung injury in a mouse model. Therefore, we sought to investigate whether ESC could protect against nonspecific pulmonary inflammation in mice.
MATERIALS AND METHODS
Chemicals and Reagents
ESC and dexamethasone (Dex) were purchased from the National Institutes for Food and Drug Control (Beijing, China). LPS (Escherichia coli055:B5) was purchased from Sigma Co. Mouse dexamethasone was purchased from Xiansheng drug store (Nanjing, China). LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA). Superoxide dismutase (SOD), myeloperoxidase (MPO), and Wright–Giemsa staining (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were purchased from the Institute of Jiancheng Bioengineering (Nanjing, China). The enzyme-linked immunosorbent assay (ELISA) kits for determination of IL-6 and TNF-α were produced by Nanjing KeyGen Biotech. Co., Ltd. (Nanjing, China). All antibodies were purchased from Cell Signaling Technology Inc (Beverly, MA, USA).
Animals
BALB/c mice (male, 8–10 weeks old, 18–20 g each) were purchased from the Center of Experimental Animals of Changchun University of Chinese Medicine (Changchun, China). Mice were allowed to acclimatize to the laboratory for at least 7 days under climate-controlled conditions.
Experimental Protocol for Acute Lung Injury Model
The intratracheal instillation was done as previously described with slight modifications [13]. Female BALB/c mice were randomly divided into five groups with 10 mice in each group: (1) control group (saline), (2) LPS group, (3) LPS + dexamethasone (LPS + Dex, 2 mg/kg), (4) LPS + ESC (ESC, 20 mg/kg), and (5) LPS + ESC (ESC, 40 mg/kg). The animals were administered with saline or drugs intragastrically. After 30 min of treatment, mice were anesthetized with chloraldurate (3 %), and 20 μg of LPS in 50 μL phosphate buffered saline (PBS) was administered intratracheally to induce acute lung injury. Control group mice were given 50 μL PBS intratracheally.
Bronchoalveolar Lavage
Six hours after intratracheal instillation, the animal was killed and bronchoalveolar lavage (n = 10) was performed three times through a tracheal cannula with 0.5 mL (total volume 1.5 ml) of autoclaved PBS to obtain the BALF. The total leukocyte count was determined using a hemocytometer. BAL fluid (BALF) samples were centrifuged at 1500 rpm for 10 min at 4 °C, the supernatants were stored in −80 °C for analysis of cytokine concentrations, and the pellet was resuspended in 100 μl of saline, centrifuged onto slides and stained for 8 min with Wright–Giemsa staining (Nanjing Jiancheng Bioengineering Institute Nanjing, China). The slides were quantified for macrophages, neutrophils, and lymphocytes by counting a total of 200 cells/slide at ×40 magnification as the differential cell count.
Measurement of MPO in Lung
MPO activities in lung were determined, and all procedures were according to the manual. MPO activity was determined by using o-dianisidine as peroxidase substrates, and data were presented as unit per gram tissue.
Measurement of SOD and MDA in BALF
The levels of SOD and MDA were determined by standard methods. All procedures were according to the manual.
Cytokine Assay
Levels of TNF-α, IL-1β, and IL-6 in BALF were determined by ELISA kits according to the instructions recommended by the manufacturers. The optical density of each well was read at 450 nm.
Measurement of wet-to-Dry Ratio of the Lungs
The right lungs were removed at the end of the experiment. The trachea and esophagus were separated from the lungs by blunt dissection, and the wet weight of the latter was determined. Subsequently, the lungs were incubated at 60 °C for 3 to 4 days to remove all moisture, then the dry weight was measured and the ratio of wet-to-dry weight calculated.
Histopathology
In order to evaluate tissue inflammation, hematoxylin and eosin (HE) staining was performed on paraffin-embedded sections.
Western Blot Analysis
Lung tissues were harvested at 6 h after LPS administration and frozen in liquid nitrogen immediately until homogenization. Proteins were extracted with lysis buffer (RIPA with protease and phosphatase inhibitor) for 15 min on ice. Protein concentrations were determined by BCA protein assay kit. Equal amounts of protein were loaded per well on a 10 % sodium dodecyl sulfate polyacrylamide gel and transferred to PVDF membranes. The membranes were blocked with 5 % bovine serum albumin (BSA) (5 g BSA was dissolved in 100 ml TTBS) for 2 h at room temperature and incubated with primary antibody diluted 1:1000 in 5 % BSA overnight at 4 °C. After that, with the use of peroxidase-conjugated secondary antimouse and antirabbit antibodies (1:1000 dilution), the bound antibodies were detected by SuperSignal West Pico chemiluminescent substrate. GAPDH was detected as an internal control of protein loading.
Statistical Analysis
The data are expressed as mean values ± SD. Differences between groups were analyzed by one-way ANOVA or Student’s t tests, with p < 0.05 considered as significant.
RESULT
Effects of ESC on Inflammatory Cells in BALF
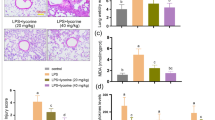
LPS increased the number of total cells and neutrophils as compared to the control group. Pretreatment with ESC (20 and 40 mg/kg) and Dex (2 mg/kg) significantly decreased the number of total cells and neutrophils, compared to those in the LPS group (Fig. 1).
Effects of ESC on SOD and MDA in BLAF
As shown in Fig. 2, LPS increased the levels of MDA and decreased the levels of SOD as compared to the control group. Pretreatment with ESC (20 and 40 mg/kg) and Dex (2 mg/kg) significantly decreased the levels of MDA and decreased the levels of SOD, compared to those in the LPS group.
Effects of ESC on MPO in Lung
As shown in Fig. 3, LPS increased MPO in lung as compared to the control group. Pretreatment with ESC (20 and 40 mg/kg) and Dex (2 mg/kg) significantly decreased MPO, compared to those in the LPS group.
Effects of ESC on Cytokine in BALF
To determine the effects of ESC on LPS-induced cytokine production, we measured the contents of TNF-α, IL-1β, and IL-6 in BALF by ELISA. As shown in (Fig. 4), TNF-α, IL-1β, and IL-6 levels were found to be significantly increased in the LPS group compared with the control group. ESC (20 and 40 mg/kg) and Dex (2 mg/kg) pretreatment efficiently decreased the levels of TNF-α, IL-1β, and IL-6.
Effects of ESC on Lung Wet-to-Dry Ratio
Six hours after LPS challenge, the lung wet-to-dry (W/D) ratios in LPS group were significantly higher than those in the control group. ESC groups (20 and 40 mg/kg) and the Dex (2 mg/kg) decreased lung wet-to-dry ratio (Fig. 5).
Effect of ESC on Pathological Changes of the Lung
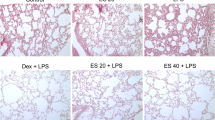
Histological evaluation of lungs (Fig. 6) by light microscopy demonstrated a large number of neutrophil sequestration and infiltration around the pulmonary vessel and airway, distributed in the alveolar and interstitial after intratracheal LPS. Treatment groups significantly reduced the degree of inflammatory cell infiltration. The results indicate that ESC can reduce the degree of pathological inflammation in lung tissues in acute lung injury.
Effect of ESC on TLR/NF-κB Activation
The expressions of inflammation-related proteins TLR2, TLR4, MyD88, IRAK1, and phosphor-NF-κB p65 were changed by LPS in lung. As shown in (Fig. 7) compared with the control group, the levels of TLR2, TLR4, MyD88, IRAK1, and phosphor-NF-κB p65 in LPS group were significantly increased. In ESC (20 mg/kg, 40 mg/kg), the levels of TLR2, TLR4, MyD88, IRAK1, and phosphor-NF-κB p65 were significantly decreased compared to the model group.
DISCUSSION
ALI and ARDS are the syndromes of acute respiratory failure that results from a disturbance of the alveolar-capillary barrier associated with several clinical disorders. ALI associated with inflammation is a severe disease that presents high morbidity and mortality rates, and there are no effective drugs in the clinic [14]. Therefore, prevention of ALI is an important therapeutic goal. LPS is a principal component of the outer membrane of Gram-negative bacteria and can enter the blood stream and elicit inflammatory responses that may lead to shock and ultimately to death [15]. Intraperitoneal administration of LPS is a widely used model of ALI in mice. The symptoms of LPS-induced ALI expressed by the mouse model have close resemblance to the observed pathology in humans [16]. Thus, we used this model to study the prevention of ESC on LPS-induced ALI in mice. To the best of our knowledge, the current study is the first to demonstrate the effects of ESC on LPS-induced ALI.
Lung W/D ratio was evaluated as an index of pulmonary edema, which is a typical symptom of inflammation not only in systemic inflammation but also in local inflammation [17]. In the present study, it was found that ESC could decrease the LPS-induced lung W/D ratio. These results suggested that ESC has a protective effect on LPS-induced ALI. The character of ALI is the acute inflammatory process in the airspaces and lung parenchyma injury involving the release of inflammatory mediators such as TNF-α, IL-1β, and IL-6. Several lines of evidence from several clinical studies indicated that proinflammatory cytokines, notably TNF-α, IL-1β, and IL-6, participate in the early development of inflammation; they have been shown to play a crucial role in ALI and ARDS [18]. Increased concentrations of TNF-α, IL-1β, and IL-6 in the BALF have been observed in patients with ALI and were related to poor outcome [19]. TNF-α, IL-1β, and IL-6 served as predictive markers for ALI severity. In the present study, LPS caused a significant increase in TNF-α, IL-1β, and IL-6 expressions in BALF compared with the control group; ESC was found to downregulate TNF-α, IL-1β, and IL-6 secretions at 6 h after LPS challenge. These results suggest that the protective effects of ESC on LPS-induced ALI were partly attributed to inhibition of TNF-α, IL-1β, and IL-6.
To date, it has been shown that TLR2 plays a key role in the microbial antigen activation of nuclear factor kappa B (NF-κB) [20]. Signaling through TLR2/myeloid differentiation primary-response protein 88 (MyD88) activates NF-κB and promotes the production of proinflammatory cytokines such as interleukin 1 (IL-1), IL-6, IL-8, IL-12, and monocyte chemotactic peptide 1 [21, 22]. We examined the effects of ESC on the activation TLR2/MyD88 pathway, and we found ESC inhibits the production of TLR2, TLR4, and MyD88. To further characterize the nature of the inhibitory effect of ESC on cytokine production, we examined the effects of ESC on the activation of the NF-κB signaling pathways. It is well known that NF-κB is key signaling pathways accounting for the expressions of proinflammatory cytokines induced by LPS. Therefore, we investigated the possibility that ESC inhibits the production of TNF-α, IL-1β, and IL-6 by interfering with the activation of NF-κB. The NF-κB pathway has been considered to play a pivotal role in the pathogenesis of ALI. The nuclear accumulation of NF-κB p65 was observed in alveolar macrophages from patients with acute lung injury caused by severe infection, in contrast to alveolar macrophages from control patients [23]. In the current study, we found that increased nuclear factor-κB (NF-κB) p65 in LPS-induced tissue were detected. However, pretreatment with ESC inhibited phosphorylation of IκBα, nuclear factor-κB (NF-κB) p65 in the lung.
In conclusion, the present study showed that ESC played a potent anti-inflammatory role in LPS-induced ALI in mice. Our results indicated that ESC attenuated LPS-induced inflammatory cell infiltration. Administration of ESC reduced inflammatory cytokines (TNF-α, IL-1β, and IL-6) in BALF, decreased W/D ratio, and improved the pulmonary functions. Furthermore, we demonstrated that ESC inhibited TLR/NF-κB pathway. Therefore, ESC may be considered as a potential agent for preventing ALI in the future. Also, further and comprehensive studies are needed before clinical application.
References
Rubenfeld, G.D. 2003. Epidemiology of acute lung injury. Critical Care Medicine 31: S276–S284.
Zhang, X.M., K.J. Song, H.Z. Xiong, H.Y. Li, X. Chu, and X.M. Deng. 2009. Protective effect of florfenicol on acute lung injury induced by lipopolysaccharide in mice. International Immunopharmacology 9: 1525–1529.
Matthay, M.A., G.A. Zimmerman, C. Esmon, J. Bhattacharya, B. Coller, C.M. Doerschuk, et al. 2003. Future research directions in acute lung injury summary of a national heart lung and blood institute working group. American Journal of Respiratory and Critical Care Medicine 167: 1027–1035.
Chen, Z., X. Zhang, X. Chu, X. Zhang, K. Song, Y. Jiang, L. Yu, and X. Deng. 2010. Preventive effects of Valnemulin on lipopolysaccharide-induced acute lung injury in mice. Inflammation 33: 306–314.
Rubenfeld, G.D., E. Caldwell, E. Peabody, J. Weaver, D.P. Martin, M. Neff, et al. 2005. Incidence and outcomes of acute lung injury. The New England Journal of Medicine 353: 1685–1693.
Pahl, H.L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18: 6853–6866.
Chen, Z., X. Zhang, X. Chu, X. Zhang, K. Song, Y. Jiang, L. Yu, and X. Deng. 2010. Preventive effects of valnemulin on lipopolysaccharideinduced acute lung injury in mice. Inflammation 33: 306–314.
Yang, R., L. Yang, X. Shen, W. Cheng, B. Zhao, K.H. Ali, Z. Qian, and H. Ji. 2012. Suppression of NF-κB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. European Journal of Pharmacology 674: 391–396.
Di, R., M.T. Huang, and C.T. Ho. 2011. Anti-inflammatory activities of mogrosides from Momordica grosvenoriin murine macrophages and a murine ear edema model. Journal of Agricultural and Food Chemistry 59: 7474–7481.
Drexler, S.K., and B.M. Foxwell. 2010. The role of Toll-like receptors in chronic inflammation. International Journal of Biochemistry & Cell Biology 42: 506–518.
Lee, B.C., S.Y. Lee, H.J. Lee, G.S. Sim, J.H. Kim, J.H. Kim, Y.H. Cho, D.H. Lee, H.B. Pyo, T.B. Choe, D.C. Moon, Y.P. Yun, and J.T. Hong. 2007. Anti-oxidative and photo-protective effects of coumarins isolated from Fraxinus chinensis. Archives of Pharmacal Research 30: 1293–1301.
Park, C., C.Y. Jin, G.Y. Kim, I.W. Choi, T.K. Kwon, B.T. Choi, S.J. Lee, W.H. Lee, and Y.H. Choi. 2008. Induction of apoptosis by esculetin in human leukemia U937 cells through activation of JNK and ERK. Toxicology and Applied Pharmacology 227: 219–228.
Kumazawa, Y., K. Kawaguchi, and H. Takimoto. 2006. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Current Pharmaceutical Design 12: 4271–4279.
Rubenfeld, G.D. 2003. Epidemiology of acute lung injury. Critical Care Medicine 31: 276–284.
Knapp, S., S. Florquin, D.T. Golenbock, and T. van der Poll. 2006. Pulmonary lipopolysaccharide (LPS)-binding protein inhibits the LPS-induced lung inflammation in vivo. Journal of Immunology 176: 3189–3195.
Chen, H., C. Bai, and X. Wang. 2010. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Review of Respiratory Medicine 4: 773–783.
Zhang, X.M., K.J. Song, H.Z. Xiong, H.Y. Li, X. Chu, and X.M. Deng. 2009. Protective effect of florfenicol on acute lung injury induced by lipopolysaccharide in mice. International Immunopharmacology 9: 1525–1529.
Bhatia, M., and S. Moochhala. 2004. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. The Journal of Pathology 202: 145–156.
Minamino, T., and I. Komuro. 2006. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. The Journal of Clinical Investigation 116: 2316–2319.
Zhang, G., and S. Ghosh. 2001. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. Journal of Clinical Investigation 107: 13–19.
Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunology 2: 675–680.
Horng, T., G.M. Barton, R.A. Flavell, and R. Medzhitov. 2002. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420: 329–333.
Moine, P., R. McIntyre, M.D. Schwartz, et al. 2000. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock 13: 85–91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tianzhu, Z., Shumin, W. Esculin Inhibits the Inflammation of LPS-Induced Acute Lung Injury in Mice Via Regulation of TLR/NF-κB Pathways. Inflammation 38, 1529–1536 (2015). https://doi.org/10.1007/s10753-015-0127-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0127-z