Abstract
Piperine, one of the active components of black pepper, has been reported to have antioxidant and anti-inflammatory activities. However, the effects of piperine on lipolysaccharide (LPS)-induced acute lung injury (ALI) have not been reported. Thus, the protective effects of piperine against LPS-induced ALI were investigated in this study. LPS-induced lung injury was assessed by histological study, myeloperoxidase (MPO) activity, and inflammatory cytokine production. Our results demonstrated that piperine attenuated LPS-induced MPO activity, lung edema, and inflammatory cytokines TNF-α, IL-6, and IL-1β production. Histological studies showed that piperine obviously attenuated LPS-induced lung injury. In addition, piperine significantly inhibited LPS-induced NF-κB activation. In conclusion, our results demonstrated that piperine had a protective effect on LPS-induced ALI. The anti-inflammatory mechanism of piperine is through inhibition of NF-κB activation. Piperine may be a potential therapeutic agent for ALI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), are the leading causes of mortality in critically ill patients [1]. It is characterized by severe pulmonary edema, neutrophil accumulation in the lung, and hypoxemia [2]. ALI is associated with the overproduction of inflammatory cytokines, such as TNF-α and IL-1β [3]. Mice model of lipopolysaccharide (LPS)-induced ALI has been extensively used to investigate the pathogenesis of human ALI [4, 5]. LPS induces neutrophil infiltrations and triggers inflammatory responses [6]. LPS also induces inflammatory mediator production, and these inflammatory mediators lead to severe lung injury [7]. Nowadays, the mortality of ALI remains high [8]. Thus, it is urgently needed to identify novel agents to improve the treatment of ALI/ARDS.
Piperine, one of the active components of black pepper, has been reported to have antioxidant and anti-inflammatory activities [9, 10]. Previous studies showed that piperine inhibited LPS-induced inflammatory mediators in RAW264.7 cells [11]. Piperine also inhibited LPS-induced maturation of bone marrow-derived dendritic cells [12]. Piperine has been reported to attenuate the severity of cerulean-induced acute pancreatitis [13]. Furthermore, piperine was found to inhibit cerebral ischemia-reperfusion-induced inflammation in middle cerebral artery occlusion rat model [14]. However, the effects of piperine on LPS-induced ALI remain unclear. In the present study, we investigated whether piperine had protective effects on LPS-induced ALI and clarified the underlying anti-inflammatory mechanism.
MATERIALS AND METHODS
Animals
Male BALB/c mice, weighing approximately 18 to 22 g, were obtained from the Animal Center of Jilin University (Changchun, China). All mice were housed under standard laboratory conditions. The mice were provided with water and food ad libitum. All animal experiments were performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Reagents
Piperine (purity >98 %) was purchased from the National Institutes for Food and Drug Control (Beijing, China). LPS (Escherichia coli 0111:B4) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). ELISA kits of TNF-α, IL-1β, and IL-6 were purchased from R&D Systems (Minneapolis, MN). The kit of MPO was purchased from Jiancheng Bioengineering Institute (Nanjing, China). Antibodies against p65, p-p65, IκBα, and p-IκBα were purchased from Cell Signaling Technology Inc. (Beverly, MA). Antibodies against β-actin and HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Autogen, Bioclear, UK). All other chemicals were of reagent grade.
Murine Model of LPS-Induced ALI
Ninety BALB/c mice were divided into six groups randomly: control group, LPS group, LPS + piperine (15, 30, and 60 mg/kg) groups. The control group and LPS group were given an equal volume of vehicle. LPS+ piperine (15, 30, and 60 mg/kg) groups were given intraperitoneally of piperine 1 h after LPS challenge. Twelve hours after LPS treatment, the mice were sacrificed and the bronchoalveolar lavage fluid (BALF) and lung tissues were collected. The choice of 12 h after LPS treatment was based on a previous study [15, 16].
Histological Study
To assess the lung histopathological changes, the lungs were harvested, fixed in 10 % formaldehyde, embedded in paraffin, and sliced into 5-μm sections. Then, the sections were stained with hematoxylin and eosin (H&E). The pathological changes of lung tissues were observed under a light microscope.
Measurement of Lung W/D Ratio
The lung wet/dry (W/D) weight ratio was used to assess the edema of the lung. Briefly, the right lungs were harvested and weighted to obtain the ‘wet’ weight. To obtain the ‘dry’ weight, the lungs were kept in an oven at 60 °C for 48 h. The lung W/D ratio was calculated by dividing the wet weight by the dry weight.
MPO Assay
The activity of lung MPO was measured by MPO kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Briefly, the lung tissue was collected 12 h after LPS treatment and 100 mg lung tissue was homogenized. Subsequently, 0.9 ml homogenate and 0.1 ml of reaction buffer was heated at 37 °C and determined in absorbance at 460 nm.
ELISA Assay
The levels of TNF-α, IL-6, and IL-1β in the BALF were examined by commercially available ELISA kits (R&D, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Western Blot Analysis
Twelve hours after LPS treatment, the lungs were collected, homogenized, and incubated with lysis buffer which includes a protease inhibitor cocktail (Sigma). After protein concentration was determined through BCA method, equal amounts (50 μg) of proteins were separated by 10 % SDS-PAGE. Then, the proteins were transferred onto a PVDF membrane. After blocking with 5 % nonfat dry milk, the membrane was probed with primary antibody at 4 °C overnight. After washing, the membrane was probed with horseradish peroxidase-conjugated second antibody for 2 h. The detection of labeling proteins were detected with enhanced-chemiluminescence Western blot detection kits.
Statistical Analysis
The values were expressed as the mean ± SD of three independent experiments. Statistically significant differences for multiple groups were assessed by ANOVA, followed by Dunnett’s test. Statistical significance was accepted p < 0.05 or p < 0.01.
RESULTS
Effects of Piperine on LPS-Mediated Lung Histopathologic Changes
Histopathological analysis was used to investigate the protective effects of piperine on LPS-induced ALI. As shown in Fig. 1, the lung section of the control group displayed normal structure. The lung section of LPS-treated group showed obvious histopathological changes, including interstitial edema, hemorrhage, thickening of the alveolar wall, and inflammatory cells infiltration (Fig. 1b). However, treatment of piperine attenuated the severity of lung injuries induced by LPS (Fig. 1c–e).
Effects of piperine on histopathological changes in lung tissues in LPS-induced ALI mice. Mice were given an intraperitoneal injection of piperine (15, 30, and 60 mg/kg) 1 h after administration of LPS. Representative histological changes of lung obtained from mice of different groups. a Control group, b LPS group, c LPS + piperine (15 mg/kg) group, d LPS + piperine (30 mg/kg) group, e LPS + piperine (60 mg/kg) group (hematoxylin and eosin staining, magnification ×200).
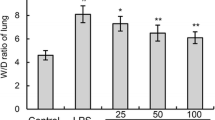
Piperine Inhibits LPS-Induced Lung W/D Ratio
The severity of lung edema was measured by lung W/D ratio. As shown in Fig. 2, there was a significant increase in the lung W/D ratio of LPS-treated group when compared with the control group. However, compared with LPS group, the levels of W/D ratio decreased in piperine-treated groups.
Effects of piperine on the lung W/D ratio of LPS-induced ALI mice. The lung wet/dry weight ratio was determined at 12 h after LPS challenge. The values presented are the means ± SEM (n = 12 in each group) of three independent experiments. Octothorpe, p < 0.01 vs. control group, **p < 0.01 vs. LPS group.
Effects of Piperine on MPO Activity
MPO activity was used to assess neutrophil accumulation in lung tissues. As shown in Fig. 3, MPO activity significantly increased in the LPS-treated group than in the control group. However, LPS-induced MPO activity was dose-dependently inhibited by treatment of piperine.
Effects of Piperine on TNF-α, IL-6, and IL-1β Production
Twelve hours after LPS treatment, the BALF were collected and the levels of inflammatory cytokines TNF-α, IL-6, and IL-1β were detected by ELISA. The results showed that the levels of TNF-α, IL-6, and IL-1β increased significantly after LPS treatment. However, treatment of piperine significantly inhibited LPS-induced inflammatory cytokines TNF-α, IL-6, and IL-1β production in a dose-dependent manner (Fig. 4).
Effects of piperine on TNF-α, IL-1ß, and IL-6 production in the BALF of LPS-induced ALI mice. BALF was collected at 12 h following LPS challenge to analyze the inflammatory cytokines TNF-α, IL-1ß, and IL-6. The values presented are mean ± SEM (n = 12 in each group) of three independent experiments. Octothorpe, p < 0.01 vs. control group; **p < 0.01 vs. LPS group.
Effects of Piperine on LPS-Induced NF-κB Activation
The effects of piperine on LPS-induced NF-κB activation were detected by Western blotting in this study. As shown in Fig. 5, LPS significantly induced NF-κB activation and IκBα degradation. However, piperine dose-dependently inhibited LPS-induced NF-κB activation and IκBα degradation.
DISCUSSION
Piperine, one of the active components of black pepper, has been reported to have anti-inflammatory activities. In the current study, we found that piperine had a protective effect on LPS-induced ALI. Treatment of piperine attenuated LPS-induced lung edema, infiltration of neutrophils in the lung, and inflammatory cytokine production. These results suggested that piperine might be a potential therapeutic agent for ALI.
Treatment of LPS caused severe lung injury in mice as evidenced by interstitial edema and infiltration of neutrophils [17, 18]. In this study, we investigated the effects of lung edema by measuring lung W/D ratio. Our results showed that piperine dose-dependently inhibited LPS-induced lung W/D ratio. MPO activity, a marker of neutrophil influx into tissue, was used to assess infiltration of neutrophils in this study [19]. Our results showed that treatment of piperine significantly inhibited LPS-induced MPO activity. Histopathological analysis showed that piperine significantly inhibited LPS-induced infiltration of neutrophils in the lung. Histopathological analysis also showed that piperine remarkably attenuated LPS-induced lung pathological changes. Taken together, these results of this study suggested that piperine exhibited protective effects against LPS-induced ALI in mice.
Inflammatory cytokines played an important role in the development of lung injury [3]. Among these cytokines, TNF-α, IL-1β, and IL-6 were known to be important inflammatory mediators that caused ALI [20, 21]. Meanwhile, previous studies showed that the production of inflammatory cytokines TNF-α, IL-1ß, and IL-6 increased in patients with ALI [22]. These inflammatory cytokines initiated and implied the inflammatory responses and led to lung injury [23]. In this study, we found that piperine significantly suppressed LPS-induced inflammatory cytokines TNF-α, IL-1β, and IL-6 production. NF-κB is a transcription factor that exists in an inactive form in the cytoplasm by its inhibitory proteins IκB [24, 25]. Once stimulation, NF-κB is released from the IκB and translocate from the cytoplasm to the nucleus to regulate TNF-α, IL-1β, and IL-6 gene transcription [26, 27]. To investigate the anti-inflammatory mechanism of piperine, the effects of piperine on LPS-induced NF-κB activation were detected. The results showed that piperine dose-dependently inhibited LPS-induced NF-κB activation.
In conclusion, piperine exerted its protective effects on LPS-induced ALI by inhibiting lung edema, neutrophil infiltration, and inflammatory cytokines production. These data suggested that piperine might be a therapeutic drug in the prevention and treatment of ALI. The protective effects of piperine on LPS-induced ALI were investigated in mice. Whether piperine has protective effects on humans remains unclear. However, further and more comprehensive studies are needed before piperine is used for the clinical practice.
References
Hughes, M., I.S. Grant, and F.N. MacKirdy. 2000. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. American Journal of Respiratory and Critical Care Medicine 162: 332–332.
Ware, L.B. 2006. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Seminars in Respiratory and Critical Care Medicine 27: 337–349.
Goodman, R.B., J. Pugin, J.S. Lee, and M.A. Matthay. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine & Growth Factor Reviews 14: 523–535.
Joh, E.H., W. Gu, and D.H. Kim. 2012. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-kappa B and MAPK pathways. Biochemical Pharmacology 84: 331–340.
Wu, J., Y.Y. Zhang, L. Guo, H. Li, and D.F. Chen. 2013. Bupleurum polysaccharides attenuates lipopolysaccharide-induced inflammation via modulating toll-like receptor 4 signaling. PLoS ONE 8: e78051.
Chandrasekar, B., J.B. Smith, and G.L. Freeman. 2001. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation 103: 2296–2302.
Bhatia, M., and S. Moochhala. 2004. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. Journal of Pathology 202: 145–156.
Ruffini, E., A. Parola, E. Papalia, P.L. Filosso, M. Mancuso, A. Oliaro, G. Actis-Dato, and G. Maggi. 2001. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. European Journal of Cardio-Thoracic Surgery 20: 30–37.
Vijayakumar, R.S., D. Surya, and N. Nalini. 2004. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Report 9: 105–110.
Rauscher, F.M., R.A. Sanders, and J.B. Watkins. 2000. Effects of piperine on antioxidant pathways in tissues from normal and streptozotocin-induced diabetic rats. Journal of Biochemical and Molecular Toxicology 14: 329–334.
Ying, X.Z., K.H. Yu, X.W. Chen, H. Chen, J.J. Hong, S.W. Cheng, and L. Peng. 2013. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cellular Immunology 285: 49–54.
Bae, G.S., J.J. Kim, K.C. Park, B.S. Koo, I.J. Jo, S.B. Choi, C.H. Lee, W.S. Jung, J.H. Cho, S.H. Hong, et al. 2012. Piperine inhibits lipopolysaccharide-induced maturation of bone-marrow-derived dendritic cells through inhibition of ERK and JNK activation. Phytotherapy Research 26: 1893–1897.
Bae, G.S., M.S. Kim, J. Jeong, H.Y. Lee, K.C. Park, B.S. Koo, B.J. Kim, T.H. Kim, S.H. Lee, S.Y. Hwang, et al. 2011. Piperine ameliorates the severity of cerulein-induced acute pancreatitis by inhibiting the activation of mitogen activated protein kinases. Biochemical and Biophysical Research Communications 410: 382–388.
Vaibhav, K., P. Shrivastava, H. Javed, A. Khan, M.E. Ahmed, R. Tabassum, M.M. Khan, G. Khuwaja, F. Islam, M.S. Siddiqui, et al. 2012. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-kappa B in middle cerebral artery occlusion rat model. Molecular and Cellular Biochemistry 367: 73–84.
Lv, H., C. Zhu, Y. Liao, Y. Gao, G. Lu, W. Zhong, Y. Zheng, W. Chen, and X. Ci. 2015. Tenuigenin ameliorates acute lung injury by inhibiting NF-kappaB and MAPK signalling pathways. Respiratory Physiology & Neurobiology 216: 43–51.
Fu, K., T. Piao, M. Wang, J. Zhang, J. Jiang, X. Wang, and H. Liu. 2014. Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology 23: 400–406.
Sato, K., M.B. Kadiiska, A.J. Ghio, J. Corbett, Y.C. Fann, S.M. Holland, R.G. Thurman, and R.P. Mason. 2002. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB Journal 16: 1713–1720.
Gong, J.H., J.P. Gong, J.Z. Li, K. He, P.Z. Li, and X.W. Jiang. 2013. Glycogen synthase kinase 3 inhibitor attenuates endotoxin-induced liver injury. Journal of Surgical Research 184: 1035–1044.
Matsuo, Y., H. Onodera, Y. Shiga, M. Nakamura, M. Ninomiya, T. Kihara, and K. Kogure. 1994. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke 25: 1469–1475.
Ran, X., S. Chao, Z. Jun-Gang, H. Yun, C. Kuan-Bing, and S. Wen-Jun. 2014. Protective effect of veratric acid on lipopolysaccharide-induced acute lung injury in mice. European Journal of Pharmacology 740: 227–232.
Tianzhu, Z., Y. Shihai, and D. Juan. 2014. The effects of morin on lipopolysaccharide-induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation 37: 1976–1983.
Halbertsma, F.J.J., M. Vaneker, G.J. Scheffer, and J.G. van der Hoeven. 2005. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Netherlands Journal of Medicine 63: 382–392.
Feng, J., Xiao, B., Chen, W., Ding, T., Chen, L., Yu, P., Xu, F., Zhang, H., Liu, Z., Liang, G. 2015. Synthesis and anti-inflammatory evaluation of novel C66 analogs for the treatment of LPS-induced acute lung injury. Chem Biol Drug Des.
Sun, Y., Y. Zhao, J. Yao, L. Zhao, Z. Wu, Y. Wang, D. Pan, H. Miao, Q. Guo, and N. Lu. 2015. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-kappaB and NLRP3 inflammasome activation. Biochemical Pharmacology 94: 142–154.
Zhao, Z., X. Tang, X. Zhao, M. Zhang, W. Zhang, S. Hou, W. Yuan, H. Zhang, L. Shi, H. Jia, et al. 2014. Tylvalosin exhibits anti-inflammatory property and attenuates acute lung injury in different models possibly through suppression of NF-kappaB activation. Biochemical Pharmacology 90: 73–87.
Wang, L.L., Y. Xu, Q. Yu, Q. Sun, Y. Xu, Q. Gu, and X. Xu. 2014. H-RN, a novel antiangiogenic peptide derived from hepatocyte growth factor inhibits inflammation in vitro and in vivo through PI3K/AKT/IKK/NF-kappa B signal pathway. Biochemical Pharmacology 89: 255–265.
Yang, P., Y. Han, L. Gui, J. Sun, Y.L. Chen, R. Song, J.Z. Guo, Y.N. Xie, D. Lu, and L. Sun. 2013. Gastrodin attenuation of the inflammatory response in H9c2 cardiomyocytes involves inhibition of NF-kappaB and MAPKs activation via the phosphatidylinositol 3-kinase signaling. Biochemical Pharmacology 85: 1124–1133.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Liu, J., Li, H. et al. Piperine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via Modulating NF-κB Signaling Pathways. Inflammation 39, 303–308 (2016). https://doi.org/10.1007/s10753-015-0250-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0250-x