Abstract
Acute lung injury (ALI), a common component of systemic inflammatory disease, is a life-threatening condition without many effective treatments. Fisetin, a natural flavonoid from fruits and vegetables, was reported to have wide pharmacological properties such as anti-inflammatory, antioxidant, and anticancer activities. The aim of this study was to detect the effects of fisetin on lipopolysaccharide (LPS)-induced acute lung injury and investigate the potential mechanism. Fisetin was injected (1, 2, and 4 mg/kg, i.v.) 30 min before LPS administration (5 mg/kg, i.v.). Our results showed that fisetin effectively reduced the inflammatory cytokine release and total protein in bronchoalveolar lavage fluids (BALF), decreased the lung wet/dry ratios, and obviously improved the pulmonary histology in LPS-induced ALI. Furthermore, fisetin inhibited LPS-induced increases of neutrophils and macrophage infiltration and attenuated MPO activity in lung tissues. Additionally, fisetin could significantly inhibit the Toll-like receptor 4 (TLR4) expression and the activation of NF-κB in lung tissues. Our data indicates that fisetin has a protective effect against LPS-induced ALI via suppression of TLR4-mediated NF-κB signaling pathways, and fisetin may be a promising candidate for LPS-induced ALI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), which is the severest form of injury, occurs in the setting of acute severe illness complicated by systemic inflammation [1]. There are several pathogenic factors that participate in the induction of ALI, such as sepsis, pneumonia, aspiration of gastric contents, transfusions, major trauma, and acute pancreatitis [2]. Among these pathogenic factors, Gram-negative bacteria-induced sepsis is the most common pathological condition of ALI/ARDS [3]. The lipopolysaccharide (LPS) components of Gram-negative bacteria are thought to play a key role in initiating the inflammatory processes that result in ALI/ARDS [4]. LPS can bind to TLR4 and lead to the activation of NF-κB to induce the production of inflammatory cytokines [5–7]. Administration of LPS to experimental animals causes the pathological condition of ongoing sepsis and concomitant ARDS-like lung injury. Despite extensive effects in research and clinical medicine, the mortality rate of ALI/ARDS still remains high [2, 8]. Therefore, the development of novel and targeted drug therapies for ALI/ARDS is urgently needed to reduce lethality.
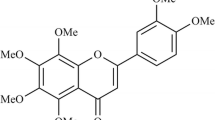
Fisetin (3,7,3′,4′-tetrahydroxy flavone, Fig. 1) is a member of flavonoids, and it is commonly found in plants such as smoke trees and various types of fruits and vegetables such as strawberries, grapes, apples, persimmons, onions, and cucumbers. Various in vitro and in vivo studies have shown that fisetin exerts wide pharmacological properties such as anticancer [9], antioxidant [10], and anti-inflammation [11–13]. Previous research indicated that fisetin may protect against the progression of inflammatory diseases by limiting interactions between mast cells and activated T cells [14]. Recently, it has been reported that fisetin exerts anti-inflammatory effect by inhibiting c-Jun N-terminal kinase (JNK) and NF-κB pathways in LPS-stimulated RAW264.7 macrophage cells [12]. Furthermore, it has been shown that fisetin can inhibit lipopolysaccharide-induced macrophage activation and dendritic cell maturation [15].
A number of studies have shown the anti-inflammatory potential of fisetin. However, the pharmacological actions of fisetin on acute lung injury have not been clarified. In this study, we investigated the protective effects of fisetin on LPS-induced acute lung injury and elucidated the potential mechanism underlying these protective effects.
MATERIALS AND METHODS
Chemicals and Reagents
Fisetin and LPS (E. coli 055:B5) were obtained from Sigma (St. Louis, MO, USA). TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kit were obtained from American R&D Corporation (R&D Systems Inc., Minneapolis, MN, USA). The myeloperoxidase (MPO) determination kit was provided by the Jiancheng Bioengineering Institute of Nanjing (Nanjing, Jiangsu, China). Anti-TLR4, anti-p-NF-κB p65, and anti-NF-κB p65 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-IκB-α antibodies were obtained from Cell Signaling Technology Inc. (Beverly, MA).
Animals
Adult male Sprague-Dawley rats, weighing approximately 250 to 300 g, were obtained from the Experimental Animal Center of Xuzhou Medical College. Rats were kept in a temperature- and humidity-controlled room with free access to pellet food and water on a 12-h light/dark cycle. All procedures were performed in accordance with the Declaration of Helsinki of the World Medical Association.
Experimental Design
All rats were randomly divided into six groups: control group, fisetin (4 mg/kg) group, LPS group (received LPS injection 5 mg/kg, i.v.), LPS + fisetin (1 mg/kg) group, LPS + fisetin (2 mg/kg) group, and LPS + fisetin (4 mg/kg) group. LPS (5 mg/kg) or vehicle (saline) was administered intravenously to induce acute lung injury [16]. Fisetin (1, 2, or 4 mg/kg) was intravenously injected 30 min before LPS injection. The doses of these drugs were on the basis of previous studies and our preliminary experiments [17, 18]. Six hours after LPS injection, rats were euthanized and samples were collected.
Determination of Bronchoalveolar Lavage Proteins and Cell Counts
Bronchoalveolar lavage (BAL) was performed with intratracheal injection of 5 mL ice-cold phosphate-buffered saline (PBS) through a tracheal cannula followed by gentle aspiration. Then, the recovered fluid was centrifuged at 1200×g for 10 min at 4 °C. Supernatants were used for the measurement of total protein and inflammatory cytokines concentrations. The cell pellet was resuspended in 100 μl PBS, and total cells recovered in BALF were counted. The cell differentiation was determined for 200 cells by examination of the HE-stained smears.
Lung Wet/Dry Weight Ratio
The lung wet/dry weight ratio was evaluated to indicate the pulmonary edema. The inferior lobe of right lung was excised, rinsed briefly in PBS, blotted, and then weighed to obtain the “wet” weight. Subsequently, the lungs were incubated at 60 °C for 72 h to remove all moisture, then the “dry” weight was measured and the wet/dry weight ratio was calculated.
ELISA Assay for TNF-α and IL-6
The concentrations of TNF-α and IL-6 in BALF were detected using a commercially available ELISA kit (R&D Systems, Minneapolis, MN) according to the protocol recommended by the manufacturer. The levels were calculated according to standard curves.
MPO Activity Assay
The accumulation of neutrophils in lung tissues was assessed by MPO activity. MPO activity in homogenates of lung tissues was determined using test kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) according to the manufacturer’s instructions.
Histological Evaluation
The lungs were harvested at 6 h after LPS administration and fixed with 10 % neutral phosphate-buffered formalin for 48 h at 4 °C. Then, the lung tissues were embedded in paraffin and cut into 5-μm sections. After hematoxylin and eosin (H&E) staining, pathological changes of lung tissues were observed under a light microscope.
Western Blot Analysis
Proteins were extracted from the lungs using Nuclear and Cytoplasmic Protein Extraction Kit. Protein concentrations were determined by BCA protein assay kit, and equal amounts of protein were fractionated on 12 % polyacrylamide SDS gel. Then, proteins were transferred to polyvinylidene difluoride membrane. The membrane was blocked by 5 % nonfat dry milk for 2 h at room temperature followed by primary antibody (1:1000) overnight at 4 °C. Subsequently, the membrane was treated with the secondary antibody (1:10,000) at room temperature for 2 h and developed using NBT/BCIP color substrate. The density of the bands on the membrane were scanned and analyzed with an image analyzer (Lab Works Software, UVP Upland, CA, USA).
Statistical Analysis
All values were expressed as means ± SEM. Statistical analysis of the results was carried out by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test in SPSS11.0 (Chicago, IL, USA), and P values <0.05 were considered to be statistically significant.
RESULTS
Effects of Fisetin on the Lung Wet/Dry Weight Ratio and the Concentration of Total Protein in BALF
Six hours after LPS administration, the lung wet/dry ratio and the concentration of total protein in BALF were analyzed in order to detect the lung injury of LPS-challenged rats. As shown in Figs. 2 and 3, the lung wet/dry ratio and the levels of total protein in BALF were significantly higher in LPS group than in control group. However, fisetin administration significantly decreased the wet/dry ratio and the concentration of total protein in BALF of LPS-challenged rats.
Effect of fisetin on the lung wet/dry ratio in LPS-induced ALI rats. Rats were given an intravenous injection of fisetin (1, 2, and 4 mg/kg) 30 min before LPS administration. The lung wet/dry weight ratio was determined at 6 h after LPS administration. Data are presented as means ± SEM (n = 6). ##P < 0.01 versus control group; *P < 0.05, versus LPS group; **P < 0.01, versus LPS group.
Effect of fisetin on the total protein concentration in BALF of LPS-induced ALI rats. Rats were given an intravenous injection of fisetin (1, 2, and 4 mg/kg) 30 min before LPS administration. BALF was collected at 6 h after LPS administration to analyze the total protein concentration. Data are presented as means ± SEM (n = 6). ##P < 0.01 versus control group; *P < 0.05, versus LPS group; **P < 0.01, versus LPS group.
Effects of Fisetin on LPS-Mediated Lung Histopathologic Changes
Under light microscopy, normal structure of lung showed integral alveoli structure and no edema (Fig. 4a). However, It is obvious that continuing lung destruction, as indicated by the HE assay, was observed at 6 h after injection of LPS, which manifesting as serious pulmonary edema, haemorrhagia in stroma, alveolar collapse and massly inflammatory cell infiltration (Fig. 4c). Administration with fesitin effectively alleviated the destruction of the lung structure (Fig. 4d–f).
Histologic assessment of the effect of fisetin on LPS-induced ALI. Rats were given an intravenous injection of fisetin (1, 2, and 4 mg/kg) 30 min before LPS administration. Lungs from each experimental group were processed for histological evaluation at 6 h after LPS administration (magnification ×200). a Control group, b fisetin (4 mg/kg) group, c LPS group, d LPS + fisetin (1 mg/kg) group, e LPS + fisetin (2 mg/kg) group, f LPS + fisetin (4 mg/kg) group.
Effects of Fisetin on the Inflammatory Cell Counts in BALF
In this study, total cells, neutrophils, and macrophages in BALF were analyzed to examine the effects of fisetin on LPS-induced pulmonary inflammation. As shown in Fig. 5, the accumulation of total cells, neutrophils, and macrophages in BALF significantly increased compared to the control group. However, this increase was apparently reduced by administration with fisetin.
Effects of fisetin on the numbers of total cells, neutrophils, and macrophages in BALF of LPS-induced ALI rats. Rats were given an intravenous injection of fisetin (1, 2, and 4 mg/kg) 30 min before LPS administration. BALF was collected at 6 h after LPS administration to measure the numbers of total cells (a), neutrophils (b), and macrophages (c). Data are presented as means ± SEM (n = 6). ##P < 0.01 versus control group; *P < 0.05, versus LPS group; **P < 0.01, versus LPS group.
Effects of Fisetin on MPO Activity in Lung Tissues
Neutrophils secrete MPO and lead to productions of MPO-derived oxidants and damages of lung tissues. The activity of MPO enzyme in tissues is an important index of neutrophil infiltration, which plays a key role in the progression of ALI. In this study, MPO activity in the homogenates of lung tissues was detected at 6 h after LPS injection. As shown in Fig. 6, after LPS stimulation, MPO activity increased sharply compared to the control group. However, fisetin administration apparently inhibited MPO activity in lung tissues.
Fisetin inhibited LPS-induced MPO activity in lung tissues. Rats were given an intravenous injection of fisetin (1, 2, and 4 mg/kg) 30 min before LPS administration. MPO activity was determined at 6 h after LPS administration. Data are presented as means ± SEM (n = 6). ##P < 0.01 versus control group; *P < 0.05, versus LPS group; **P < 0.01, versus LPS group.
Effects of Fisetin on the Concentrations of TNF-α and IL-6 in BALF
To further evaluate the anti-inflammatory effect of fisetin, the concentrations of TNF-α and IL-6 in BALF were analyzed by ELISA. As shown in Fig. 7, compared with control group, the concentrations of TNF-α and IL-6 in BALF significantly increased after LPS injection. However, fisetin administration effectively reduced the LPS-induced production of TNF-α and IL-6.
LPS-induced alterations of TNF-α and IL-6 in BALF and the suppression of fisetin. Rats were given an intravenous injection of fisetin (1, 2, and 4 mg/kg) 30 min before LPS administration. BALF was collected at 6 h after LPS administration to analyze the inflammatory cytokines TNF-α (a) and IL-6 (b). Data are presented as means ± SEM (n = 6). ##P < 0.01 versus control group; *P < 0.05, versus LPS group; **P < 0.01, versus LPS group.
Effects of Fisetin on TLR4 Expression and NF-κB Activation in Lung Tissues
Western blot analysis was used to determine the expression of TLR4 and the phosphorylation of NF-κB p65 subunits in lung tissues. As illustrated in Fig. 8a, b, rats challenged with LPS showed significant increase of the expression of TLR4 and the activation of NF-κB in lung tissues, as compared with control group. However, fisetin administration markedly inhibited the expression of TLR4 and the activation of NF-κB. These results indicat that fisetin may inhibit TLR4-mediated NF-κB signaling pathway in LPS-induced ALI.
Fisetin inhibited the expression of TLR4 and NF-κB activation in lung tissues. Rats were given an intravenous injection of fisetin (1, 2, and 4 mg/kg) 30 min before LPS administration. The expression of TLR4 (a), the phosphorylation of NF-κB p65 (b), and the expression of cytoplasmic IκB-α (c) were detected by Western blotting. Data are presented as means ± SEM (n = 6). ##P < 0.01 versus control group; *P < 0.05, versus LPS group; **P < 0.01, versus LPS group.
On the other hand, to investigate whether IκB-α is regulated by fisetin, we assessed the level of IκB-α expression in the cytosol extracts of lung tissues. As shown in Fig. 8c, rat challenged with LPS showed significant decrease of cytoplasmic IκB-α protein compared with control group. However, the loss of cytoplasmic IκB-α was markedly inhibited by administration with fisetin.
DISCUSSION
Fisetin, a natural compound available in many fruits and vegetables, such as strawberry, apple, persimmon, grape, onion, and cucumber [3], recently allured much attention as a developing anti-inflammation drug [12, 15, 18, 19]. In the current study, we investigated the anti-inflammatory effects of fisetin on LPS-induced ALI in rats and elucidated its potential anti-inflammatory mechanism. The results showed that fisetin had a protective effect on LPS-induced ALI. Furthermore, our data showed that fisetin inhibited the expression of TLR4 and the activation of NF-κB in lung tissues. These results suggest that the effects of fisetin against the LPS-induced ALI may be due to its ability to inhibit TLR4-mediated NF-κB signaling pathways and fisetin may be a beneficial candidate for acute lung injury treatment.
To evaluate the effects of fisetin on LPS-induced ALI, lung histological changes were detected. Microscopically, the lungs in LPS group showed capillary congestion, marked interstitial edema, a moderate amount of intra-alveolar fibrinous exudate, and focal alveolar hemorrhage. After fisetin administration, the integrity of alveoli and some respiratory bronchioles were mostly recovered. Besides, pulmonary edema is one of the major characteristics of ALI. Previous studies indicate that the protein-rich pulmonary interstitial edema is meaningful for the prognosis of ALI/ARDS [20]. In the present study, we found that fisetin administration significantly alleviated the interstitial edema compared with LPS group. This finding was also testified by the lung wet/dry weight ratio test in our study. We found that fisetin early treatment could obviously reduce the increment of lung wet/dry weight ratio induced by LPS.
The infiltration of inflammatory cells into lung tissues is a typical characteristic of LPS-induced ALI [21, 22]. MPO is an enzyme located mainly in the primary granules of neutrophils; thus, lung tissue MPO levels may suggest neutrophil infiltration into lung parenchyma or alveolar spaces [23]. Based on our findings, administration with fisetin significantly decreased MPO activity in lung tissues, which suggests that fisetin could inhibit the infiltration of neutrophils into lung tissues in LPS-induced ALI rats. Moreover, because protein extravasation is considered as an indicator of vascular leakage, we measured total protein contents in BALF and detected lower protein amounts in fisetin-pretreated rats. These date indicated that fisetin attenuated neutrophils influx and vascular leakage in LPS-induced ALI rats, which thereby alleviating the pulmonary inflammation.
Pro-inflammatory cytokines appear in the early phase of ALI inflammatory response. A largely unopposed early pro-inflammatory response is known to occur in ALI and leads to death [24]. Among various cytokines, TNF-α and IL-6 have been reported to play important roles in the pathogenesis of ALI. After LPS injection, TLR4-mediated NF-κB activation enhances the production of TNF-α and IL-6 and both of these cytokines are in turn known to activate NF-κB. This positive feedback could amplify the original inflammatory reaction and play an important role in the mechanism of LPS-induced ALI [2]. In our experiment, fisetin early administration obviously downregulated activated NF-κB, thus terminating new cytokine transcription to prevent cytokine cascade reaction and limiting the inflammatory response.
Previous study demonstrated that TLR4 is expressed in various types of lung cells, such as vascular endothelial and airway epithelial cells [25]. TLR4 mediates many forms of ALI and TLR4 pathway is actively involved in the pathogenesis of LPS-induced ALI [5, 26]. Being a main receptor for LPS, TLR4 may trigger the activation of NF-κB signaling pathway and result in the upregulation of inflammatory mediators [6]. NF-κB is an important transcription factor which plays an essential role in regulating immune responses, including the expression of many inflammatory cytokines’ genes. Previous study showed that NF-κB regulated inflammatory and immune responses to extracellular stimulus and played a critical role in the development of ALI [27]. To further illuminate the potential mechanisms of fisetin alleviating LPS-induced ALI, we investigated if the anti-inflammatory activity of fisetin was exerted via TLR4-mediated NF-κB pathway. The expression of TLR4 and the activation of NF-κB were investigated in rat lung tissues. Our study showed that fisetin could obviously inhibit the expression of TLR4 and the activation of NF-κB in LPS-stimulated rat lung tissues. These data indicated that fisetin administration could effectively inhibit TLR4-mediated NF-κB signaling pathway in LPS-induced ALI.
There were also some limitations in the present study. Firstly, the LPS-induced model of ALI cannot fully reproduce the complexity of clinical ALI/ARDS in human patients. Extrapolation of our results to the clinical situation may be limited by species differences. Therefore, it will be necessary to reproduce these findings in more clinically relevant models, such as in animal models of sepsis and pneumonia. Secondly, many anti-inflammatory drugs have been studied for ALI treatment. But, most compounds exhibit significant side effects. Although fisetin is commonly present in dietary fruits and vegetables and this compound presumably is a safer immunosuppressive agent, future studies are still required to further evaluate the side effects of fisetin for anti-inflammatory treatments. Moreover, it will be meaningful to explore whether fisetin has the similar protective effects on acute lung injury caused by other reasons. Therefore, future studies should focus on these factors. Further and more comprehensive studies are still needed before the clinical application of this drug to treat ALI.
In conclusion, our study suggested that fisetin may regulate the inflammatory process to produce beneficial actions in LPS-induced acute lung injury. The protective effect of fisetin in acute lung injury may be due to its ability to inhibit the expression of TLR4 and subsequently leads to the suppression of TLR4-mediated NF-κB pathway in lung tissues. Therefore, fisetin may be a potential therapeutic reagent for acute lung injury.
References
Villar, J., D. Sulemanji, and R.M. Kacmarek. 2014. The acute respiratory distress syndrome: incidence and mortality, has it changed? Current Opinion in Critical Care 20: 3–9.
Ware, L.B., and M.A. Matthay. 2000. The acute respiratory distress syndrome. The New England Journal of Medicine 342: 1334–49.
Arai, Y., S. Watanabe, M. Kimira, K. Shimoi, R. Mochizuki, and N. Kinae. 2000. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. The Journal of Nutrition 130: 2243–50.
Blackwell, T.S., and J.W. Christman. 1997. The role of nuclear factor-kappa B in cytokine gene regulation. American Journal of Respiratory Cell and Molecular Biology 17: 3–9.
Hu, R., H. Xu, H. Jiang, Y. Zhang, and Y. Sun. 2013. The role of TLR4 in the pathogenesis of indirect acute lung injury. Frontiers in Bioscience (Landmark Ed) 18: 1244–55.
Imai, Y., K. Kuba, G.G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. van Loo, et al. 2008. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–49.
Martin, T.R., and M.M. Wurfel. 2008. A TRIFfic perspective on acute lung injury. Cell 133: 208–10.
Spieth, P.M., and H. Zhang. 2014. Pharmacological therapies for acute respiratory distress syndrome. Current Opinion in Critical Care 20: 113–21.
Suh, Y., F. Afaq, N. Khan, J.J. Johnson, F.H. Khusro, and H. Mukhtar. 2010. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis 31: 1424–33.
Khan, N., D.N. Syed, N. Ahmad, and H. Mukhtar. 2013. Fisetin: a dietary antioxidant for health promotion. Antioxidants & Redox Signaling 19: 151–62.
Gelderblom, M., F. Leypoldt, J. Lewerenz, G. Birkenmayer, D. Orozco, P. Ludewig, et al. 2012. The flavonoid fisetin attenuates postischemic immune cell infiltration, activation and infarct size after transient cerebral middle artery occlusion in mice. Journal of Cerebral Blood Flow and Metabolism 32: 835–43.
Kim, S.C., S.H. Kang, S.J. Jeong, S.H. Kim, and H.S. Ko. 2012. Inhibition of c-Jun N-terminal kinase and nuclear factor kappa B pathways mediates fisetin-exerted anti-inflammatory activity in lipopolysccharide-treated RAW264.7 cells. Immunopharmacology and Immunotoxicology 34: 645–50.
Lyu, S.Y., and W.B. Park. 2005. Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Archives of Pharmacal Research 28: 573–81.
Nagai, K., Y. Takahashi, I. Mikami, T. Fukusima, H. Oike, and M. Kobori. 2009. The hydroxyflavone, fisetin, suppresses mast cell activation induced by interaction with activated T cell membranes. British Journal of Pharmacology 158: 907–19.
Liu, S.H., C.H. Lin, S.K. Hung, J.H. Chou, C.W. Chi, and S.L. Fu. 2010. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. Journal of Agricultural and Food Chemistry 58: 10831–9.
Shen, W., J. Gan, S. Xu, G. Jiang, and H. Wu. 2009. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Pharmacological Research 60: 296–302.
Ragelle, H., S. Crauste-Manciet, J. Seguin, D. Brossard, D. Scherman, P. Arnaud, et al. 2012. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. International Journal of Pharmaceutics 427: 452–9.
Goh, F.Y., N. Upton, S. Guan, C. Cheng, M.K. Shanmugam, G. Sethi, et al. 2012. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-kappaB. European Journal of Pharmacology 679: 109–16.
Yoo, H., S.K. Ku, M.S. Han, K.M. Kim, and J.S. Bae. 2014. Anti-septic effects of fisetin in vitro and in vivo. Inflammation 37: 1560–74.
Wilkins, P.A., and T. Seahorn. 2004. Acute respiratory distress syndrome. The Veterinary Clinics of North America. Equine Practice 20: 253–73.
Matthay, M.A., and R.L. Zemans. 2011. The acute respiratory distress syndrome: pathogenesis and treatment. Annual Review of Pathology 6: 147–63.
Lucas, R., A.D. Verin, S.M. Black, and J.D. Catravas. 2009. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochemical Pharmacology 77: 1763–72.
Reumaux, D., M. de Boer, A.B. Meijer, P. Duthilleul, and D. Roos. 2003. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. Journal of Leukocyte Biology 73: 841–9.
Antonov, A., C. Snead, B. Gorshkov, G.N. Antonova, A.D. Verin, and J.D. Catravas. 2008. Heat shock protein 90 inhibitors protect and restore pulmonary endothelial barrier function. American Journal of Respiratory Cell and Molecular Biology 39: 551–9.
Zarember, K.A., and P.J. Godowski. 2002. Tissue expression of human toll-like receptors and differential regulation of toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. Journal of Immunology 168: 554–61.
He, Z., X. Chen, S. Wang, and Z. Zou. 2014. Toll-like receptor 4 monoclonal antibody attenuates lipopolysaccharide-induced acute lung injury in mice. Experimental Therapeutics and Medecine 8: 871–876.
Hoesel, B., and J.A. Schmid. 2013. The complexity of NF-kappaB signaling in inflammation and cancer. Molecular Cancer 12: 86.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81200056).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10753-017-0607-4.
Rights and permissions
About this article
Cite this article
Feng, G., Jiang, Zy., Sun, B. et al. Fisetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury via TLR4-Mediated NF-κB Signaling Pathway in Rats. Inflammation 39, 148–157 (2016). https://doi.org/10.1007/s10753-015-0233-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0233-y