Abstract
The lung is relatively sensitive to irradiation. It is shown that acetylsalicylic acid (ASA) might reduce oxidative injury and that it has a place in protection from cancer. The aim of this study is to evaluate the potential radioprotective effects of ASA. Whole-body irradiation (6 Gy, single dose) was applied to the rats. Glutathione (GSH), malondialdehyde (MDA), myeloperoxidase (MPO), and nitric oxide (NO) levels in the lung tissue were measured. Control (C), Radiation (R), Radiation + ASA (R + ASA; received irradiation and 25 mg/kg of ASA intraperitoneally (i.p.)), and Radiation + Amifostine (R + WR-2721; received irradiation and 200 mg/kg of WR-2721 i.p.) groups were used. The MPO levels decreased statistically significantly in the group administered ASA. Histopathologically, a radioprotective effect of ASA was more evident in the R + ASA group. ASA is an agent which has not been used as a radioprotector in the clinic yet, and it is worth supporting with more advanced studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The lung is relatively sensitive to irradiation [1]. The histopathological changes of radiation-induced pulmonary toxicity can be divided into early and late stages on the basis of the time course and intensity of the radiation injury. Pulmonary fibrosis can also develop after exposure to radiotherapy, and it is characterized by the progressive and irreversible destruction of lung architecture produced by scar formation that finally leads to organ malfunction and causes morbidity [1, 2]. Following radiation exposure, lung damage occurs as a consequence of early epithelial and endothelial injury [3]. The role of inflammation in this process and inflammatory responses in blood vessels are some of the main uncertain points [3, 4].
Oxidative stress, which represents an imbalance between reactive oxygen species (ROS) and cellular mechanisms for detoxifying the reactive intermediates, perpetuates profibrotic inflammatory responses [5]. Radiation can cause various lesions by direct interaction with DNA or indirectly through damage induced by free radicals mainly resulting from the radiolysis of water molecules—the most abundant molecules in the cell [5]. The use of antioxidants has aroused increasing interest since it has been noticed that the protection of normal tissues may provide an increase in tumor control by providing an increase in the radiation dose [6]. Protection by numerous different agents (e.g., WR-2721, captopril, and 5-aminosalicylic acid (5-ASA) agonist) from functional and histopathological damage when administered both before and after irradiation has been shown [1, 6–8].
Acetylsalicylic acid (ASA) is a salicylate drug. ASA was the first discovered member of the non-steroidal antiinflammatory drug (NSAID) group. However, even though they all have similar effects and the mechanism of action for most of them is the inhibition of the enzyme cyclooxygenase (COX), not all of them are salicylates [9]. Moreover, ASA has an antiplatelet effect by preventing thromboxane production [9]. Likewise, it is thought that ASA and its derivates are antioxidants which scavenge free radicals—the potentially damaging by-products of the metabolism [7, 10]. Extensive research has been made on the role of ASA in reducing the incidence of many types of cancers. The drug might have an effect of reducing the risk of various types of cancers such as colon cancer [11] and lung cancer [12]. Briefly, it is argued that ASA can exert its therapeutic potential as a free radical scavenger and as an antiischemic substance [7, 9, 10].
This study aimed to investigate the potential radioprotective effects of ASA by using an animal model and to compare its effect with that of WR-2721 (amifostine), as a representative of clinically used radioprotector, in the prevention of oxidative damage caused by gamma irradiation on the lung tissue.
MATERIAL AND METHOD
All experiments and protocols described in the present study were performed in accordance with the guidelines of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes and also approved by the Medical Faculty Experimentation Ethics Committee of Gaziantep University. The rats were housed under standardized conditions (12-h light/dark cycle, 22 ± 2 °C, and relative humidity 50–70 %) and provided free access to standard rat nutrients and purified drinking water ad libitum.
Experimental Design
Prior to experimental manipulation, the rats were acclimatized to our laboratory conditions for a week. After the stabilization period, the rats were divided randomly into four equal-sized groups (six rats per group), namely, the Control (C), irradiation (R), Radiation + ASA (R + ASA), and Radiation + Amifostine (R + WR-2721). C rats received neither any radioprotector nor any irradiation; however, the rats were treated with 2.2 ml of saline intraperitoneally (i.p.). All groups of rats in the study (R, R + ASA, and R + WR-2721) received whole-body gamma irradiation as a single dose of 6 Gy (50 % lethal total body irradiation dose for rats). Besides the irradiation, R rats received 2.2 ml of saline by intraperitoneal injection (i.p.), while the R + ASA and R + WR-2721 rats received both irradiation and 25 mg/kg of ASA i.p. (acetysalicylic acid, Bayer İlaç, Gebze, Kocaeli, Turkey) or 200 mg/kg of WR-2721 i.p. (containing 500 mg of amifostine, Ethyol flacon, Er-Kim İlaç, İstanbul, Turkey), respectively. Saline, ASA, and WR-2721 injections in the study groups were performed 15 min before irradiation. The drug doses were chosen according to the previous studies since they were observed to have beneficial effects on the prevention of radiation-induced oxidative damage following irradiation after treatment with ASA or WR-2721 by using a similar protocol [13, 14]. A cobalt-60 teletherapy unit (Shandong Xinhua SCC-8000 F Zibo, China) was used for all irradiation applications. The dose rate was 1.80 Gy/min at a distance of 80 cm.
The study was terminated 72 h after irradiation. The lung tissues of rats were resected for the biochemical analysis.
Measurement of GSH
The glutathione (GSH) concentrations of the tissues were measured using the method described by Beutler et al. [15]. Fifty-milligram specimens of rat tissues were homogenized in 5 % NaCl. Three milliliters of the precipitating solution (1.67 g of metaphosphoric acid, 0.2 g of EDTA, and 30 g of NaCl in 100 ml of distilled water) was mixed with 2 ml of homogenate. The mixtures were centrifuged at 3000 rpm for 5 min. Two milliliters of filtrate was taken and added to another tube, and then 8 ml of the phosphate solution (0.3 M disodium phosphate) and 1 ml of Ellmanʼs reagent (DTNB) were added. A blank was prepared with 8 ml of phosphate solution, 2 ml of diluted precipitating solution (3 parts to 2 parts distilled water), and 1 ml of DTNB reagent. A standard solution of the glutathione was prepared (40 mg/100 ml). The optical density was measured at 412 nm in the spectrophotometer. GSH values were expressed in mmol/mg of total protein. The amount of total protein was measured with Lowry’s method [15, 16].
Measurement of MDA
A 50-mg tissue specimen was homogenized in 0.15 mol/l of KCI to determine the malondialdehyde (MDA). After the homogenate had been centrifuged at 3000 rpm, the MDA levels in 50 μl of tissue homogenate were determined by the thiobarbituric acid (TBA) reaction according to Yagi. The principle of the method is based on measuring absorbance of the pink color produced by the interaction of TBA with MDA at 530 nm. The values were expressed in nmol/ml [17].
Determination of NO
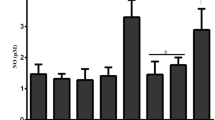
The lung tissues were homogenized in the lysis buffer and centrifuged at 14,000×g for 15 min at 4 °C. The cytosolic extracts were removed gently. Nitric oxide (NO) levels were determined in supernatants. The levels of NO in tissues were analyzed by a colorometric assay kit (nitric oxide colorimetric kit, Roche Diagnostics, GmbH Mannheim, Germany). Nitrate was reduced to nitrite by reduced nicotinamide adenine dinucleotide phosphate (NADPH) in the presence of the enzyme nitrate reductase. The resultant nitrite reacted with sulphanilamide and N-(1-naphtyl)-ethylene-diamine dihydrochloride to give a red–violet diazo dye. The diazo dye was measured on the basis of its absorbance in the visible range at 550 nm. The level of NO was marked in mg/g of tissue.
Measurement of MPO
A 300-mg tissue specimen was homogenized in 0.02 M EDTA (pH 4.7) by a homogenizer (SilentCrusher M Heidolph Instruments GmbH & Co. KG, Germany). Homogenates were centrifuged at 20,000×g for 15 min at +4 °C. The pellet was re-homogenized in 1.5 ml of 0.5 % hexa-decyl-tirimethyl-ammonium bromide (HETAB) prepared in 0.05 M KPO4 (pH 6) buffer and then re-centrifuged at 20,000×g for 15 min at +4 °C. The determination of supernatant’s myeloperoxidase (MPO) activity is based on the fact that it reduces o-dianizidine. Reduced o-dianizidine was measured at 410 nm by a spectrophotometer. Tissue MPO values were expressed in U/g of total protein [18].
Lung Tissue Preparation and Histological Examination
Histopathological analysis: The tissue specimens collected from each lung were fixed in 10 % formalin solution and processed to paraffin wax. Five-micrometer-thick sections were deparaffinized and stained with hematoxylene–eosin for the light microscopic examination. The slides were examined blindly by an experienced pathologist. Microscopic findings including pulmonary collapse, pulmonary edema, congestion, hemorrhage, lymphoid aggregation, and subtypes of inflammatory cells (eosinophil/neutrophil) were detected. The severity of these parameters was graded as follows: normal (0), minimal (1), moderate (2), and severe (3). The semiquantitative scores reflected the population examined as follows: Collapse was scored as grade 1 in one area, as grade 2 in two to three areas, and as grade 3 in four and more areas. Congestion and hemorrhage were scored as follows: <11 % (1), 11–30 % (2), and 30–100 % (3). Lymphoid aggregates were scored as follows: grade 1 in two focal areas, grade 2 in two to four focal areas, and grade 3 in five and more focal areas.
Statistical Analysis
The descriptive values of data were represented in means ± standard deviation (SD) of mean. After checking for normal distribution with Shapiro–Wilk’s test, the data were analyzed by one-way ANOVA, followed by Tukey HSD or Kruskal–Wallis test for multiple comparisons as applicable. A value of p less than 0.05 was considered significant. A statistical analysis was performed by using SPSS 11.5.1 software (Lead Technologies, Inc., Chicago, IL, USA) and Statistica 6.1 software (StatSoft Inc., Tulsa, OK, USA) as applicable.
RESULTS
GSH, MDA, MPO, and NO Levels in the Lung Tissues of Rats
We found a significant difference between the MDA and MPO levels of the R and C rats (p < 0.05), while R + ASA and R + WR-2721 rats were not significantly different from the C rats (p > 0.05, Table 1). The MDA levels significantly increased in the R rats, indicating that the irradiation might stimulate the lipid peroxidation in the lung tissue (p < 0.05). The effect of ASA was more noticeable than the effect observed for WR-2721, although insignificant (p > 0.05).
The tissue MPO levels of R rats were found to increase significantly when compared to those of the C rats (p < 0.05), while the R + ASA and R + WR-2721 rats were not statistically significantly different (p > 0.05). However, the tissue MPO levels of R + ASA rats significantly decreased in comparison to those of the R rats after irradiation (p < 0.01). On the other hand, the tissue MPO activities of R + WR-2721 rats were not significantly reduced when compared to those of the R rats (p > 0.05). In addition, ASA administration statistically significantly decreased the MPO levels, which is statistically different from the administration of WR-2721 (p < 0.05). ASA’s alleviating capability on the stimulated MPO, an essential enzyme for the normal neutrophil function, is more pronounced.
Histopathological Evaluations
The histopathological findings including pulmonary collapse, edema, congestion, hemorrhage, lymphoid aggregation, and subtypes of inflammatory cells are summarized in Table 2. The mean values of the congestion parameter in the lung specimens of irradiated rats markedly decreased after the administration of ASA and WR-2721, when compared to those of the R group (p < 0.05). The R group displayed microscopic vascular damage which culminated in wide hemorrhagic areas on the lung. This resulted in alveolar collapse (Fig. 1a, b). Nevertheless, hemorrhagic areas, congestion, and collapse were slightly obscure in the R + ASA group that was partially protected from the deleterious effect of radiation; however, there were statistically significant differences in congestion parameters only (Fig. 1c). On the lung specimens of R + WR-2721 group were hemorrhagic areas and vascular congestion (Fig. 1d). It was observed that when compared with the R group, the R + WR-2721 group had a significant increase in lymphoid aggregates. The edema in the R + WR-2721 group was significantly inapparent (p < 0.05).
The histological changes in irradiated rats compared with control rats. The specimens of R group microscopically showed pulmonary damage, resulting in wide hemorrhagic areas on the lung (a, H&E × 110) and marked alveolar collapse (b, H&E × 44). The lung was partially protected against the effects caused by radiation in the R + ASA group: Hemorrhagic areas and collapse were slight and obscure (c, H&E × 44), but alveolar congestion was slightly apparent. On the lung specimens of R + WR-2721 group, there were hemorrhagic areas and vascular congestion (d, H&E × 110).
DISCUSSION
In the present experimental study, it was especially intended to find out whether ASA supplementation would have any significant radioprotective effect on the lung tissue. Whole-body irradiation in rats led to tissue damage in the lung. This was shown by increased lipid peroxidation (MDA, a marker of lipid peroxidation) and inflammation (MPO, a marker of inflammation). ASA application significantly decreased the MPO level after irradiation and tended to reduce the levels of MDA. The present study indicated the noteworthy antiinflammatory and radioprotective effects of ASA on the lung tissue.
The development of radiation pneumonitis and subsequent fibrosis can result from the exposure to radiation for the treatment of thoracic tumors. Fibrogenesis is often defined as an uncontrolled wound healing response which includes a clotting/coagulation phase, an inflammatory phase, a fibroblast migration/proliferation phase, and a final remodeling phase where normal tissue architecture is restored [1, 5]. Oxidative damage to DNA, proteins, lipids, and other biomolecules appears as a result of radiation exposure, as has been observed partially in the present study. The increased MDA levels in the lung tissue after irradiation could be a consequence of increased lipid peroxidation. Inflammatory and profibrotic mediators are released and ROS are generated in the tissues after exposure to radiation [1]. We found that irradiation significantly increased one of the indices of the inflammation (MPO) level in our study. Early epithelial and endothelial injury gives rise to vascular congestion and thrombosis and causes the exudation of fluid into alveoli and leakage of plasma protein into the alveolar space [3, 4]. Similar histopathological findings have been observed in the present study. The stress response, stimulated by radiation, involves a repair process that encompasses cytokines and growth factors (e.g., vascular prostaglandin synthesis) as well as the regeneration of capillaries. The damage of type II pneumocyte (surfactant-producing cells) results in surfactant deficiency and alveolar collapse. There is loss of integrity of pulmonary capillaries [3, 4]. Although the relevance of very early events is not clear, lung damage is believed to be induced by a strong inflammatory response at least at the early stage [3, 5].
Some agents given either before and after irradiation or immediately after irradiation such as pentoxifylline, corticosteroid, angiotensin-converting enzyme (ACE) inhibitors, 5-ASA, and WR-2721 have been reported to protect the normal tissue against both the inflammatory response and fibrosis [7, 10, 19]. It is believed that the agents act by decreasing the effects of irradiation on the pulmonary endothelial cell dysfunction [7, 10, 19, 20]. It has been reported that WR-2721, a well-known protector, can protect rats when given before hemithoracic irradiation against both pneumonitis and fibrosis and reduces plasma TGF-b levels [21]. However, there are many aspects of WR-2721 which limit its use clinically [3, 4]. In the present study, we could not observe any remarkable effect of WR-2721 on the irradiated lung tissue. Histopathologically, a slight radioprotective effect was observed with ASA or WR-2721 (Table 2 and Fig. 1).
Recently, the data proving the antioxidant properties of ASA have been increasing rapidly [22, 23]. It is demonstrated that ASA, a member of the NSAID group, can inhibit the release of prostaglandins and might prevent tissue/cell damage in various diseases with the help of its antioxidant properties [22, 24, 25]. To investigate the reaction of ASA to ROS, electron spin resonance (ESR) was employed in an experimental study. According to the results, ASA was an efficient *OH radical scavenger and faster than several well-established antioxidants, including ascorbate, glutathione, and cysteine [22].
Some researchers argue that ASA can exert its therapeutic potential as a free radical scavenger and as an antiischemic substance and that it raises the plasma antioxidant activity [9, 10, 22]. The study by Mantena et al. showed that both Cu-5ASA and Zn-5ASA reduced the deleterious effect of radiation and hence could be useful agents in reducing the side effects of therapeutic radiation [10]. In a study, it is argued that 5-ASA, an amino derivative of salicylic acid, prevents irradiation-induced inflammatory processes as well as expression of tumor necrosis factor, monocyte chemotactic protein-1, inducible nitric oxide synthase, and macrophage infiltration in rat colon [7]. In the present study, we observed that ASA treatment might be of therapeutic value in radiotherapy (RT) induced oxidative stress and inflammation in the lung tissue.
Neutrophils are the most abundant inflammatory cells at the earliest stages of wound healing. They also contribute to the inflammatory response and trigger fibroblast proliferation and recruitment [5]. MPO is an essential enzyme for normal neutrophil function, and when neutrophils are stimulated by various stimulants, MPO increases, as other cellular-tissue-damaging substances do [25]. It is interesting that increased levels of MPO and inflammatory cells are contained in the alveolar epithelial lining fluid of most patients with idiopathic pulmonary fibrosis and that they spontaneously release increased amounts of H2O2. This agrees with the MPO system with a role in the epithelial cell injury found in this disorder in human beings [13, 26, 27]. In the present study, it is shown that ASA, unlike WR-2721, decreased the MPO level in the lung tissues when compared to the R group. It may be required for time-dependent research to observe the possible alteration in the oxidative and antioxidative parameters like MPO.
In the initial exudative phase, capillary endothelial cells and epithelial lining cells are the most susceptible cells [3–5]. Injury to small vessels and capillaries and development of vascular congestion and increased capillary permeability represent early radiation damage. An exudate rich in fibrin accumulates in the alveolar spaces at this stage. Additionally, an inflammatory infiltrate occurs. This might cause a second course of increased permeability [3–5]. In spite of the presence of no statistical significance among the groups, clues which supported the biochemical findings were available in the histopathological examination of the tissue specimens in our study. Congestion, collapse, and hemorrhage were evident microscopic findings in the RT group, respectively, as compared with the groups to which ASA and WR-2721 were administered (Fig. 1). There are scarce studies to investigate the property of ASA that protects against radiation damage to lung or the other tissue. In their study, Van Kleef et al. assessed the renal function and histological damage in mice following high-dose radiation [28]. In their study, post-RT renal dysfunction decreased with the long-term use of ASA; however, similar to our study, no statistical difference was observed histopathologically. The findings of a study suggested the association of overexpression of heat shock protein 60 with the reduction of histopathological damage in ASA radioproprotected rat submandibular salivary gland [29].
On the other hand, the capability of reducing oxidative injury and genocytotoxicity of ASA may have a place in a cancer prevention strategy [22, 23]. In their study on the Huntington’s disease, Khoshnan et al. observed that sodium salicylate increased neuronal resistance to DNA damage caused by DNA-damaging agents (e.g., etoposide and gamma irradiation) [30]. In the studies on [31, 32] skin carcinogenesis, the abilities of ASA and the other NSAIDs to suppress tumor formation have been accounted for by the inhibition of ultraviolet-B-induced activator proteini1 and/or COX-2 activation [33].
Oxidative stress with a fundamental role in the pathogenesis of radiation damage is the characteristic of inflammatory intestinal diseases included in the cancerous colon tissue and etiology [34]. Although the mechanism of ASA to prevent colon cancer could not be clearly understood, salicylic acid, its main metabolite, was observed to reduce the elevated indices of oxidative stress and the synthesis of prostaglandins with proinflammatory and potential neoplastic effects [35, 36]. In a clinical study, Mennie et al. applied a high dose of ASA to the patients with diarrhea who were irradiated due to gynecological malignity and who gave no response to conventional treatment. They reported that ASA treatment might be of therapeutic value in RT-induced diarrhea, significantly reduced the number of bowel movements, and eliminated abdominal complaints [37].
Optimum radiotherapy for cancer includes a balanced approach to normal tissue prevention and appropriate treatment. There are numerous factors that contribute to normal tissue radioprotection. In conclusion, although the findings of this study are limited to biochemical and histopathological parameters, this study gives clues to the possible radioprotective effect of ASA and is parallel with the few related studies on this issue. Despite some arguments on effects and side effects, due to the considerable history of clinical use for ASA, further experimental studies are needed to rule out its potential radioprotector role.
References
Marks, L.B., X. Yu, Z. Vujaskovic, et al. 2003. Radiation-induced lung injury. Seminars in Radiation Oncology 13: 333–345.
Hill, R.P. 2005. Radiation effects on the respiratory system. The British Institute of Radiology 27: 75–81.
Yarnold, J., and M.C. Brotons. 2010. Pathogenetic mechanisms in radiation fibrosis. Radiotherapy Oncology 97: 149–161.
Davis, S.D., D.F. Yankelevitz, and C.I. Henschke. 1992. Radiation effects on the lung: clinical features, pathology, and imaging findings. American Journal of Roentgenology 159: 1157–1164.
Wynn, T.A. 2011. Integrating mechanisms of pulmonary fibrosis. Journal of Experimental Medicine 208: 1339–1350.
Weiss, J.F. 1997. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environmental Health Perspectives 105: 1473–1478.
Linard, C., O. Grémy, and M. Benderitter. 2008. Reduction of peroxisome proliferation-activated receptor gamma expression by gamma-irradiation as a mechanism contributing to inflammatory response in rat colon: modulation by the 5-aminosalicylic acid agonist. Journal of Pharmacology and Experimental Therapeutics 324: 911–920.
Demirel, C., S. Kilçiksiz, O.I. Ay, S. Gürgül, M.N. Ay, and N. Erdal. 2009. Effect of N-acetylcysteine on radiation-induced genotoxicity and cytotoxicity in rat bone marrow. Journal of Radiation Research 50: 43–50.
Warner, T.D., and J.A. Mitchell. 2002. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum? Proceeding of the Nationall Academy of Sciences U S A 99: 13371–13373.
Mantena, S.K., and M.K. Chandrasekharan. 2008. Radioprotection by copper and zinc complexes of 5-aminosalicylic acid: a preliminary study. Journal of Environmental Pathology, Toxicology and Oncology 27: 123–134.
Chan, A.T., E.L. Giovannucci, J.A. Meyerhardt, E.S. Schernhammer, G.C. Curhan, and C.S. Fuchs. 2005. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 294: 914–923.
Moysich, K.B., R.J. Menezes, A. Ronsani, et al. 2002. Regular aspirin use and lung cancer risk. BMC Cancer 2: 31.
Kilciksiz, S., C. Demirel, N. Erdal, S. Gürgül, L. Tamer, L. Ayaz, and Y. Ors. 2008. The effect of N-acetylcysteine on biomarkers for radiation-induced oxidative damage in a rat model. Acta Medica Okayama 62: 403–409.
Capton, G.D., J.C. Cook, and M.E. Hurtt. 2003. Relationship between cyclooxygenase 1 and 2 selective inhibitors and fetal development when administered to rats and rabbits during the sensitive periods for heart development and midline closure. Birth Defects Research Part B Reproductive Toxicology 68: 47–56.
Beutler, E., and T. Gelbart. 1986. Improved assay of the enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase and glutathione synthetase. Clinica Chimica Acta 158: 115–123.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265–275.
Yagi, K. 1994. Lipid peroxides and related radicals in clinical medicine. In: Free radicals in diagnostic medicine, ed. Armstrong, D. p. 1–15 New York, Plenum
Golowich, S.P. 1955. Methods in enzymology, vol. IIth ed, 769. New York: Academic Press.
Molteni, A., J.E. Moulder, E.F. Cohen, et al. 2000. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. International Journal of Radiation Biology 76: 523–532.
Hong, J.H., C.S. Chiang, C.Y. Tsao, P.Y. Lin, C.J. Wu, and W.H. McBride. 2001. Can short-term administration of dexamethasone abrogate radiation-induced acute cytokine gene response in lung and modify subsequent molecular responses? International Journal of Radiation Oncology Biology Physics 51: 296–303.
Vujaskovic, Z., Q.F. Feng, Z.N. Rabbani, M.S. Anscher, T.V. Samulski, and D.M. Brizel. 2002. Radioprotection of lungs by amifostine is associated with reduction in profibrogenic cytokine activity. Radiation Research 157: 656–660.
Shi, X., M. Ding, Z. Dong, et al. 1999. Antioxidant properties of aspirin: characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-kappaB activation, and TNF-alpha production. Molecular and Cellular Biochemistry 199: 93–102.
Asanuma, M., S. Nishibayashi-Asanuma, I. Miyazaki, M. Kohno, and N. Ogawa. 2001. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. Journal of Neurochemistry 76: 1895–1904.
Verheij, M., F.A. Stewart, Y. Oussoren, J.J. Weening, and L. Dewit. 1995. Amelioration of radiation nephropathy by acetylsalicylic acid. International Journal of Radiation Biology 67: 587–596.
Drew, J.E., J.R. Arthur, A.J. Farquharson, W.R. Russell, P.C. Morrice, and G.G. Duthie. 2005. Salicylic acid modulates oxidative stress and glutathione peroxidase activity in the rat colon. Biochemical Pharmacology 70: 888–893.
Cantin, A.M., S.L. North, G.A. Fells, R.C. Hubbard, and R.G. Crystal. 1987. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis Journal Of Clinical Investigation 79: 1665–1673.
Hoggatt, J., P. Singh, K.N. Stilger, P.A. Pelt, et al. 2013. Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells Molecules Diseases 50: 147–153.
Van Kleef, E.M., J.A. Te Poele, Y.G. Oussoren, A. Van der Wal, L.G. Dewit, and F.A. Stewart. 2000. Influence of acetylsalicylic acid on development of radiation-induced nephropathy. International Journal of Radiation Biology 76: 1565–1573.
Mohamed D.G., and R. M. Amin, 2015. Involvement of heat shock proteins 60 in acetylsalicylic acid radioprotection of Albino rat submandibular salivary gland Journal of Radiation Research and Applied Sciences (article in press).
Khoshnan, A., J. Ko, S. Tescu, P. Brundin, and P.H. Patterson. 2009. IKKalpha and IKKbeta regulation of DNA damage-induced cleavage of huntingtin. PloS One 4: e57–68.
Huang, C., W.Y. Ma, D. Hanenberger, M.P. Cleary, G.T. Bowden, and Z. Dong. 1997. Inhibition of ultraviolet B-induced activator protein-1 (AP-1) activity by aspirin in AP-1-luciferase transgenic mice. Journal of Biological Chemistry 272: 26325–26331.
Dong, Z., C. Huang, R.E. Brown, and W.Y. Ma. 1997. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. Journal Of Biological Chemistry 272: 9962–9970.
Bair, W.B., N. Hart, J. Einspahr, et al. 2002. Inhibitory effects of sodium salicylate and acetylsalicylic acid on UVB-induced mouse skin carcinogenesis. Cancer Epidemiol Biomarkers Prevention 11: 1645–1652.
Awtry, E.H., and J. Loscalzo. 2000. Aspirin. Circulation 101: 1206–1218.
Weitz, J., M. Koch, J. Debus, T. Hohler, P.R. Galle, and M.W. Buchler. 2005. Colorectal cancer. Lancet 365: 153–165.
Niho, N., T. Kitamura, M. Takahashi, et al. 2006. Suppression ofazoxymethane-induced colon cancer development in rats by a cyclooxygenase-1 selective inhibitor, mofezolac. Cancer Science 97: 1011–1014.
Mennie, A.T., V.M. Dalley, L.C. Dinneen, and H.O. Collier. 1975. Treatment of radiation-induced gastrointestinal distress with acetylsalicylate. Lancet 2: 942–943.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demirel, C., Kilciksiz, S.C., Gurgul, S. et al. Inhibition of Radiation-Induced Oxidative Damage in the Lung Tissue: May Acetylsalicylic Acid Have a Positive Role?. Inflammation 39, 158–165 (2016). https://doi.org/10.1007/s10753-015-0234-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0234-x