Abstract
The present study was designed to evaluate the radioprotective effect of diethylcarbamazine (DEC) against oxidative stress and acute lung injury induced by total body radiation (TBI) in mice. For study the optimum dose for radiation protection of DEC, mice were administrated with three dose of DEC (10, 50 and 100 mg/kg), once daily for eight consecutive days. Animals were exposed whole body to 5 Gy X-radiation on the 9 day. The radioprotective potential of DEC in lung tissues was assessed using oxidative stress examinations at 24 h after TBI and histopathological assay also was analyzed one week after TBI. Results from biochemical analyses demonstrated increased malonyldialdehyde (MDA), nitric oxide (NO) and protein carbonyl (PC) levels of lung tissues in only irradiated group. Histopathologic findings also showed an increase in the number of inflammatory cells and the acute lung injury in this group. DEC pretreatment significantly mitigated the oxidative stress biomarkers as well as histological damages in irradiated mice. The favorable radioprotective effect against lungs injury was observed at a dose of 10 mg/kg of DEC in mice as compared with two other doses (50 and 100 mg/kg). The data of this study showed that DEC at a dose of 10 mg/kg with having antioxidant and anti-inflammatory properties can be used as a therapeutic candidate for protecting the lung from radiation-induced damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation therapy is generally considered as a key strategy in the cancer treatment. Lung is a sensitive tissue to ionizing radiation (IR). Radiation induced-lung injury (RILI) is a common complication in the total body irradiation (TBI) and upper body cancer radiotherapy, e.g. thoracic malignancies. For these concerns, the lung is the major dose limiting tissue that dictates the definitive dose, volume and technique of radiation (Abratt and Morgan 2002). RILI reveals sub-acute pneumonitis or late fibrosis, which are the most common causes of mortality after exposure to high dose of radiation therapy. It was reported that the incidence of interstitial pneumonitis (IP) in TBI has ranged from 29% to 55% with a fatality rate of up to 69% (Vujaskovic et al. 2000; Lee et al. 2003). These incidences are highly dependent on absorbed radiation to the lung. Pulmonary fibrosis, infiltration of inflammatory cells and collagen deposition in the extracellular matrix are complications of radiation (Chung et al. 2016). It was also reported that radiation by induction of lung injury can accelerate pulmonary metastasis in a passive metastatic breast cancer (Gong et al. 2015). Adverse effect of IR on humans’ lung and experimental animals have been recalled special interest among researchers. Experimental studies have indicated that several pharmacological agents able to reduce lung injury induced by ionizing radiation in animals (Molteni et al. 2000; Ozturk et al. 2004; Hunter et al. 2013). However, the discovery of effective therapy for lung injury without side effect has attracted great attention in recent years.
Diethylcarbamazine citrate (DEC) is an antifilarial drug that was used in the treatment of filariasis. In addition its antifilarial effect, it has anti-inflammatory, anti-fibrotic, anti-carcinogenic and antioxidative properties (Queto et al. 2010). DEC with reduction of lipoxygenase (LOX), cyclooxygenase (COX) enzymes and NO mitigates inflammation in the lung tissue (Queto et al. 2010). The protective effect of DEC has been seen on lung damage induced by carrageenan (Santos et al. 2014), myocardial infarction induced by isoproterenol (Jia et al. 2017), liver fibrosis induced by ethanol and liver injury induced by tetrachloride (El et al. 2017; Rocha et al. 2014). In the other hand it was reported that administration of DEC markedly improved asthma symptoms in patients with bronchial asthma (Wilson and McPhillips 1978; Florêncio et al. 2005). Researchers also showed that DEC can attenuate acute lung injury through its pro-apoptotic activity as well as it can significantly reduce inflammatory markers such as NO, NF-kb, MPO,COX2 and stress oxidative products in acute lung injury induced by lipopolysaccharide (LPS) (Fragoso et al. 2017) . The effect of DEC on IR-induced lung damage has not been reported yet. Therefore, the aim in present study was to evaluate the effect of DEC at different doses against radiation-induced lung injury with tissue biochemical and histological examination in mice model.
Materials and methods
Diethylcarbamazine citrate (DEC) was purchased from sigma Aldrich Company (USA) Thiobarbituric acid (TBA) (Sigma, USA), tricloroacetic acid (TCA) (Merck, Germany), mannitol (Sigma, USA), guanidine hydrochloride (99.5%) (Sigma, USA), dinitrophenyl hydrazine (DNPH) (Fluka, USA), n-butanol (Merck, Germany, 98%), 1,1,3,3-tetra methoxy-propane (Sigma, USA), coomasie brilliant blue (Merck, Germany) were provided for biochemical tests.
Experimental animals
The experiments were approved by the Ethical Committee of Mazandaran University of Medical Sciences (ID#IR.MAZUMS.REC.1397.58). Sixty male BALB/c mice (25–30 g) were obtained from the animal lab research center (Mazandaran University of Medical Sciences, Sari, Iran). The animals were housed under controlled temperature (22 ± 2 °C), lighting (12 h) and humidity (60 ± 10%) and provided with free access to food and water.
Experimental design
All the animal experiments (n = 60) were randomly divided into eight groups:
- I.
Control group: Mice only were treated with distilled water as vehicle via gavage (n = 8).
- II.
Only DEC (10 mg/kg): Mice were treated with 10 mg/kg of DEC (n = 8).
- III.
Only DEC (50 mg/kg): Mice were treated with 50 mg/kg of DEC (n = 8)
- IV.
Only DEC (100 mg/kg): Mice were treated with 100 mg/kg of DEC (n = 8)
- V.
IR: Mice only were exposed to irradiation (n = 9).
- VI.
IR + DEC (10 mg/kg): Mice were treated with 10 mg/kg of DEC and exposed to irradiation (n = 9)
- VII.
IR+ DEC (50 mg/kg): Mice were treated with 50 mg/kg of DEC and exposed to irradiation (n = 9)
- VIII.
IR+ (DEC 100 mg/kg): Mice were treated with 100 mg/kg of DEC and exposed to irradiation (n = 9)
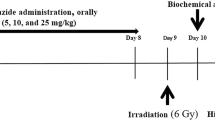
The dosages of the DEC were selected based on the previous studies to be 50 mg/kg and 100 mg/kg for gavage administration in animals (Santos et al. 2014; Jia et al. 2017; El et al. 2017; Rocha et al. 2014), in this study we also selected lower dose of DEC at 10 mg/kg as well as 50 and 100 mg/kg. DEC was dissolved in distilled water and administrated daily for 8 consecutive days via oral gavage (0.2 ml). One day after the last receipt of the drug, the mice were exposed to 5 Gy TBI (Le et al. 2010).
Radiation procedure
Two Plexiglas cages (26 × 26 × 4 cm) were designed with 18 individual homes that all of mice were placed individually in each home of these cages. Animals in IR and IR + DEC groups were exposed total body irradiation at doses of 5Gy from X-ray source (6-MeV X-ray beam generated by a clinical linear accelerator (Linear accelerator, Siemens, Primus, Germany) in maximum dose rate was 4Gy/min. The mice were not anesthetized during the procedure (Johnston et al. 2011) .
Biochemical analysis
The biochemical studies were performed at 24 h after irradiation, then 4–5 mice from each of groups were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg), and lung tissues were removed and then were washed in phosphate buffer saline (PBS) solution and homogenized with mannitol buffer. Homogenates were centrifuged at 1000×g for 10 min at 4 °C. The supernatant was collected for determination of biochemical parameters of lung tissue such as total protein, NO, MDA and PC (Shokrzadeh et al. 2015).
Total protein assay
Protein content of supernatant was determined by the coomassie blue protein binding method as described by Bradford (Spector 1978).
Measurement of NO level
The levels of NO in supernatant were quantified using colorimetric assay kit (nitric oxide colorimetric kit, Cib Biotech, Iran). Determination of NO was based on measuring the light absorbance of the red-violet diazo dye in 540 nm, which was the result of reaction between nitrite and sulphanilamide and N-(1-naphtyl)-ethylene-diamine dihydrochloride in the presence of enzyme nitrate reductase (Griess reaction). NO level of supernatant was expressed in μM (Shaki et al. 2016) .
Measurement of lipid peroxidation
In this study, MDA content of lung supernatant was determined according to the thiobarbituric acid (TBA) method. Pink color generated through the reaction between MDA and TBA under acidic conditions can be indication for secondary products of lipid peroxidation. Briefly, 200 μl from supernatant was added to a mixture containing 200 μl phosphoric acid 0.05 M and 25 μl TBA. The mixture was heated at 95 °C for 45 min. Then the mixture was cooled in ice bath for 10 min, 500 μl of n-butanol added to each mixture and centrifuged at 10000×g for 10 min and absorbance of supernatant was read at 532 nm on a plate reader and the concentrations of MDA in supernatant was concluded as μM using tetra methoxy propane as standard (Shokrzadeh et al. 2015).
Measurement of protein carbonyl
Protein carbonyl content in the supernatant was determined using the procedure was described by Dalle-Donne with slightly modification. In this protocol, 200 μl of supernatant was mixed with 300 μl of DNPH and was heated in bath for 30 min at 37 °C, then was centrifuged for 10 min at 10000×g. The precipitate was washed with ethanol-ethyl acetate and the mixture was centrifuged again. The precipitate was dissolved in 600 μl guanidine hydrochloride, heated for 15 min at 37 °C, and centrifuged for 3 min at 10000×g and absorbance of supernatant was read in 375 nm wavelength. Lung supernatant protein carbonyl concentration was expressed as mM (Dalle-Donne et al. 2003).
Histological preparation
Four mice from each group were followed for seven days after irradiation. Animals were sacrificed and the lungs were removed and fixed with 10% neutral-buffered formalin for 24 h. After dehydration through graded alcohols, embedded in paraffin, sections with 5 μm thickness were stained with hematoxylin and eosin according to the standard protocol. Then, the slides were observed by a histologist blindly under an optical microscope (Olympus, Tokyo, Japan). For semi-Quantitative scoring the extent of lung injury for each field was determined on a scale from 0 (normal lung), 1 (slight), 2 (mild), 3 (moderate), and 4 (severe) injury based on inflammatory cell infiltration (neutrophils, erythrocytes, macrophages, lymphocytes) in the alveolar sac or perivascular region, alveolar sac exudation, alveolar sac collapse, hyaline arteriosclerosis, thickened alveolar walls, edema, congestion and hemorrhage. The slides were evaluated in five fields of each slide and then, the average score was determined for each sample of lung (Tahamtan et al. 2015).
Statistical analysis
Statistical analysis of results was performed by using Graph Pad Prism 6 (USA) Software. All of the data are presented as mean ± standard error. One -way analysis of variance (ANOVA) was applied to compare variables between groups, followed by the Tukey’s multiple comparison tests for multiple comparisons. P value <0.05 was considered significant.
Results
Effect of DEC on radiation-induced lipid peroxidation
DEC mitigated the radiation-induced lipid peroxidation in lung tissue (Fig. 1a). The irradiated mice exhibited higher MDA levels than the control or DEC only (10, 50 and 100 mg/kg) in lung tissues of mice. Increase of 2.2-fold was observed in MDA level in IR group as compared with control mice (35.5 ± 8.5 μM vs 16.15 ± 0.3 μM, P < 0.0001). The MDA level concentration was significantly decreased in irradiated mice that pretreated with DEC at dose of 10 mg/kg (P < 0.0001).
Effect of DEC on radiation-induced NO levels
Nitric oxide is an important inflammatory mediator in the irradiation-induced pneumonitis. NO is a production of alveolar macrophages (AM) and alveolar epithelial cells after irradiation (Chen et al. 2016). NO levels significantly increased in lung tissues of irradiated mice in comparison to the control group (P < 0.0001). In contrast, the pretreatment of mice with DEC at dose of 10 mg/kg and 50 mg/kg displayed a reduction in NO levels in the lung tissue compared to irradiation only group (P < 0.0001) (Fig. 1b).
Effect of DEC on radiation-induced protein oxidation
Elevation of protein carbonyls is considered as biomarker of protein oxidation in lung injury and chronic obstructive pulmonary disease after exposure to IR (Kadiiska et al. 2013). Changes in protein carbonyls content in irradiated mice with and without pretreatment by DEC were assessed in this study (Fig. 1c). The irradiation alone mice showed significantly higher levels of protein carbonyl in comparison with control group (P < 0.0001). Significant decrements in protein carbonyl concentration were observed in all IR + DEC groups compared to the irradiation only group (P < 0.0001).
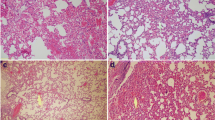
Effects of DEC on histopathologic features of RILI
The photomicrographs of lung tissue in all groups are shown in Fig. 2. In the control group, lung tissues had the normal pulmonary architecture. DEC administration at a dose of 10 mg/kg showed similar bronchi, alveolar sac and alveolar interval structure compared with the control group. In DEC administration at a dose of 50 mg/kg, pulmonary histology showed slight to mild of pneumonitis evidence. DEC-pretreated mice at dose of 100 mg/kg exhibited pulmonary hemorrhage, capillary hyperemia, neutrophils and mononuclear macrophage infiltration. Only irradiated mice displayed histological changes such as extensive erythrocytes and inflammatory cell infiltration into the alveolar sac and perivascular area, capillary hyperemia, alveolar septum thickening, alveolar sac collapse, edema and hemorrhage compared to control group (P < 0.0001). The higher doses of DEC had pneumonitis consistent with IR group. The IR + DEC (10 mg/kg) group exhibited a significant reduction in pulmonary cellular infiltration and alveolar septum thickness and hemorrhage compared with IR group (P < 0.0001). These findings indicating that DEC at a low dose (10 mg/Kg) can preserve pulmonary structure (Fig. 3).
Photomicrographs of effect of DEC pre-treatment on the histological architecture of lungs in mice. a: Control; b, c and d: DEC with 10, 50 and 100 mg/kg; respectively, e: irradiation; f, g and h: IR + DEC with 10, 50 and 100 mg/kg. Hemorrhage (Narrow arrow), inflammatory cell infiltration (thick arrow) and alveolar septum thickening show in irradiated treated mice. DEC with 10 mg/kg was reduced these damage. AS Alveolar Sac; Alveolar interval; AI. (H&E staining, Mag: ×400, scale bare: 100 μm), DEC (diethylcarbamazine)
The lung injury scores in IR and DEC + IR mice. The irradiation significantly increased injury score in the lung tissue as compared to the control group (P < 0.001). DEC (10 mg/kg) treatment significantly mitigated lung injury in irradiated treated mice compared with the irradiated group. (P < 0.0001). Also, no significant difference was seen between irradiation + DEC (100 mg/kg) (5.28 ± 0.10) and irradiation-alone (4.85 ± 0.11) groups, DEC (diethylcarbamazine), aP < 0.0001 vs control mice; cP < 0.0001 vs control mice; bP < 0.0001 vs irradiated mice; ns: non- significant
Discussion
Pulmonary pneumonitis and fibrosis following TBI are devastating conditions that are leading to limited treatment dose and subsequent reduced efficacy of cancer treatment. Hence, finding of a potential medicine for prevention and mitigation of these side effects are beneficial for cancer treatment. Several studies have reported the radioprotecive effects of medicines and natural products, which acted through antioxidant, anti-inflammatory and hematopoietic stimulation (Hosseinimehr et al. 2009; Salehifar and Hosseinimehr 2016; Hosseinimehr et al. 2006, 2015; Naeimi et al. 2017). Generation of stress oxidative and inflammation mediators are crucial parameters in radiation-induced lung injury (Li et al. 2016). In this study, TBI increased oxidative stress and inflammatory markers. Histological changes were also found after TBI. Pretreatment with DEC at dose of 10 mg/kg significantly decreased IR-induced oxidative stress and inflammatory response, and improved histopathological changes.
IR induces formation of MDA from cell membrane carbohydrates. MDA is produced during the peroxidation of the polyunsaturated fatty acids in a chain reaction “lipid peroxidation (LP)” and eventually resulting in loss of membrane integrity (Kamat et al. 2000). The results of this study showed a significant increase in MDA levels in lung tissue of irradiated animals, which revealed peroxidation of polyunsaturated fatty acids in the cell membrane of lung tissue. These results are consistent with previous studies (Kumar and Tiku 2016). In contrast, pre-administration of DEC at dose of 10 mg/kg significantly reduced the radiation-induced lipid peroxidation that was agreement with previous study that found treatment with DEC decreased MDA levels in alcoholic-induced hepatotoxicity in mice (Santos Rocha et al. 2012).
DEC as an antiflarial drug has anti-inflammatory property (Ribeiro et al. 2014). Pretreatment of irradiated mice with DEC ameliorated acute lung injury and oxidative stress induced by irradiation through decreasing the MDA, NO and PC levels in lung tissue. Enhanced MDA and PC as putative markers of oxidative stress accompany numerous pathologies with an inflammatory component in the irradiated lung. Several studies have demonstrated that the DEC blocks some enzymatic steps of the COX-2 pathways (Peixoto and Silva 2014). A possible molecular mechanism underlying radioprotective effect of DEC can be explained by dual COX-2 and NF-kB inhibitor role of DEC. It was reported that in first 24 h after exposure to 5 Gy radiation, COX-2 expressions increased more than 20-fold in lung tissue of irradiated mice relative to un-irradiated group (Chai et al. 2013). Induction of COX-2 expression by ionizing radiation can increase MDA and PC concentration 3-fold that was consistent with MDA and PC results of this study. Ionizing radiation also induces expression and activity of the NF-kB (Pordanjani and Hosseinimehr 2016). On the other hand, NF-kB regulates the expression of inducible nitric oxide synthase (iNOS) gene that catalyzes the generation of nitric oxide (NO) (Ruia et al. 2016). Thus, radioprotective effect of DEC against RILI may be suppressing COX-2 and NF-kB pathway. Protein damage following radiation has been demonstrated to have a significant effect on cellular viability and clonogenicity. Several studies have demonstrated that protein oxidation and increased PC lead to endoplasmic reticulum (ER) stress, that causing programmed cell death and accelerated senescence within 24 h post-irradiation (Panganiban et al. 2013). The findings of present study demonstrated that protein carbonyl oxidation 24 h after irradiation in the lungs of mice were significant higher in comparison with control group. However, highly reduced protein carbonyl content was observed in irradiated mice that were pretreated with DEC. These results confirmed the antioxidant activity of DEC in lung tissue.
NO is recognized as a signaling molecule that plays a key role in the pathogenesis of inflammation. Several findings reported that NO was produced in lung tissues of irradiated animals resulted to pneumonitis (Nozaki et al. 1997; Giaid et al. 2003). In a previous study, the anti-inflammatory effect of DEC was established on LPS-induced acute lung injury in mice (Fragoso et al. 2017). In this study, pretreatment mice with DEC at doses of 10 mg/kg and 50 mg/kg decreased the NO production in lungs tissue of irradiated mice. But there was no significant difference in NO levels between irradiated mice with irradiated mice pretreated with DEC at dose of 100 mg/kg. It is proved that DEC at dose of 100 mg/kg has not anti-inflammatory effect in irradiated mice.
Pneumonitis and fibrosis are the side effects of radiotherapy in the thoracic malignancies (Almeida et al. 2013). The severity of the RILI depends on dose of radiation. Histological changes in mice lungs have been reported at 1 week to 1 year after radiation (Rube et al. 2000). Infiltration of inflammatory cells, edema, Shedding of alveolar epithelial cells, and type II pneumocyte hyperplasia are histopathological features in patients or animal models that exposed to radiation. But alveolar fibrosis usually seen one to three months after irradiation (Collie et al. 2018). Since we performed histological evaluation one week after irradiation in this study, no fibrotic changes were seen in the lung tissue. Irradiated mice displayed histological changes such as extensive erythrocytes and inflammatory cell infiltration into the alveolar sac and perivascular area, capillary hyperemia, alveolar septum thickening, alveolar sac collapse, edema and hemorrhage that compared to control and IR + DEC groups. The lung injury score increased in the IR group. Lung injury may be attributed to IR-induced oxidative stress and lipid peroxidation. The pretreatment with DEC at a dose of 10 mg/kg inhibited the irradiation-induced activation and infiltration of macrophages and neutrophils in lung tissue. Santos et al. showed DEC is able to inhibit the activity of NF-kB in carrageenan-induced lung injury (Santos et al. 2014). They showed DEC in dose of 50 mg/Kg for three days before carrageenan administration led to a significant reduction in inflammatory markers. Also, DEC stimulates apoptosis in inflammatory cells and prevents the accumulation of these cells (Fragoso et al. 2017). In this study, we used three doses of DEC for 8 days and the results showed that the 10 mg/kg dose was more effective than the 50 mg/kg dose and 100 mg/kg dose had no protective role in this study.
The reuse of existing licensed drugs for new medical indications has known as drug repurposing, Repurposing drug offers a cost efficient way for new indication of radioprotective agent (Verma and Gangenahalli 2017). DEC is an anthelmintic drug that does not resemble other anti-parasitic compounds. It does not any toxic metallic elements (Ghai et al. 2017). For optimization dose of DEC to prevent RILI in mice, oxidative stress markers and histopathological assessments were investigated at different doses (10, 50, and 100 mg/kg). The current study, demonstrated for the first time that pretreatment with DEC at dose of 10 mg/kg can relieve radiation-induced pulmonary injury in mice. The limitations of this study were the evaluation of inflammatory markers such as NF-kb and COX-2 by immunohistochemical evaluation.
Conclusion
It was revealed that DEC at dose of 10 mg/kg with antioxidant property, effectively reduced the inflammation and oxidative stress parameters in the early stage of RILI. Of note, DEC at dose of 10 mg/kg carried no side effects while DEC at higher doses (50 and 100 mg/Kg) exhibited some side effects even in the lungs of healthy mice.
References
Abratt RP, Morgan GW (2002) Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer 35(2):103–109
Almeida C, Nagarajan D, Tian J, Leal SW, Wheeler K, Munley M, Blackstock W, Zhao W (2013) The role of alveolar epithelium in radiation-induced lung injury. PLoS One 8(1):e53628. https://doi.org/10.1371/journal.pone.0053628
Chai Y, Calaf G, Zhou H, Ghandhi S, Elliston C, Wen G et al (2013) Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice. Br J Cancer 108(1):91
Chen C, Yang S, Zhang M, Zhang Z, Hong J, Han D et al (2016) Triptolide mitigates radiation-induced pulmonary fibrosis via inhibition of axis of alveolar macrophages-NOXes-ROS-myofibroblasts. Cancer Biology & Therapy 17(4):381–389
Chung SI, Horton JA, Ramalingam TR, White AO, Chung EJ, Hudak KE et al (2016) IL-13 is a therapeutic target in radiation lung injury. Sci Rep 6:39714
Collie D, Murchison JT, Wright SH, McLean A, Howard L, del Pozo J et al (2018) Nebulisation of synthetic lamellar lipids mitigates radiation-induced lung injury in a large animal model. Sci Rep 8(1):13316
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329(1–2):23–38
El AE-DE-S, Sokar SS, Shebl AM, Mohamed DZ (2017) Antifibrotic effect of diethylcarbamazine combined with hesperidin against ethanol induced liver fibrosis in rats. Biomed Pharmacother 89:1196–1206
Florêncio M, Saraiva K, Peixoto C (2005) The effects of diethylcarbamazine on the ultrastructure of lung cells in vivo. Tissue Cell 37(3):241–246
Fragoso IT, Ribeiro EL, Gomes FO, Donato MA, Silva AK, Oliveira AC et al (2017) Diethylcarbamazine attenuates LPS-induced acute lung injury in mice by apoptosis of inflammatory cells. Pharmacol Rep 69(1):81–89. https://doi.org/10.1016/j.pharep.2016.09.021
Ghai D, Gulati M, Singh SK (2017) Herbal formulation for anthelmintic activity: design and evaluation. Lovely Professional University
Giaid A, Lehnert SM, Chehayeb B, Chehayeb D, Kaplan I, Shenouda G (2003) Inducible nitric oxide synthase and nitrotyrosine in mice with radiation-induced lung damage. Am J Clin Oncol 26(4):e67–e72. https://doi.org/10.1097/01.COC.0000077940.05196.86
Gong HY, Hu WG, Hu QY, Li XP, Song QB (2015) Radiation-induced pulmonary injury accelerated pulmonary metastasis in a mouse model of breast cancer. Oncol Lett 10(6):3613–3618. https://doi.org/10.3892/ol.2015.3810
Hosseinimehr SJ, Zakaryaee V, Froughizadeh M (2006) Oral oxymetholone reduces mortality induced by gamma irradiation in mice through stimulation of hematopoietic cells. Mol Cell Biochem 287(1–2):193–199. https://doi.org/10.1007/s11010-005-9111-5
Hosseinimehr SJ, Ahmadi A, Beiki D, Habibi E, Mahmoudzadeh A (2009) Protective effects of hesperidin against genotoxicity induced by (99m)Tc-MIBI in human cultured lymphocyte cells. Nucl Med Biol 36(7):863–867. https://doi.org/10.1016/j.nucmedbio.2009.06.002
Hosseinimehr SJ, Nobakht R, Ghasemi A, Pourfallah TA (2015) Radioprotective effect of mefenamic acid against radiation-induced genotoxicity in human lymphocytes. Radiat Oncol J 33(3):256–260. https://doi.org/10.3857/roj.2015.33.3.256
Hunter NR, Valdecanas D, Liao Z, Milas L, Thames HD, Mason KA (2013) Mitigation and treatment of radiation-induced thoracic injury with a cyclooxygenase-2 inhibitor, celecoxib. Int J Radiat Oncol Biol Phys 85(2):472–476. https://doi.org/10.1016/j.ijrobp.2012.04.025
Jia G, Zao M, Liu X (2017) Protective effect of diethylcarbamazine inhibits NF-κB activation in isoproterenol-induced acute myocardial infarction rat model through the PARP pathway. Mol Med Rep 16(2):1596–1602
Johnston CJ, Manning C, Hernady E, Reed C, Thurston SW, Finkelstein JN, Williams JP (2011) Effect of total body irradiation on late lung effects: hidden dangers. Int J Radiat Biol 87(8):902–913. https://doi.org/10.3109/09553002.2011.573439
Kadiiska MB, Basu S, Brot N, Cooper C, Csallany AS, Davies MJ et al (2013) Biomarkers of oxidative stress study V: ozone exposure of rats and its effect on lipids, proteins, and DNA in plasma and urine. Free Radic Biol Med 61:408–415
Kamat JP, Ghosh A, Devasagayam TP (2000) Vanillin as an antioxidant in rat liver mitochondria: inhibition of protein oxidation and lipid peroxidation induced by photosensitization. Mol Cell Biochem 209(1–2):47–53
Kumar S, Tiku AB (2016) Biochemical and molecular mechanisms of Radioprotective effects of Naringenin, a phytochemical from Citrus fruits. J Agric Food Chem 64(8):1676–1685. https://doi.org/10.1021/acs.jafc.5b05067
Le ON, Rodier F, Fontaine F, Coppe JP, Campisi J, DeGregori J et al (2010) Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 9(3):398–409
Lee SY, Kim YJ, Kim YJ (2003) Role of crosslinked protein in lung injury following total body irradiation and bone marrow transplantation. Exp Mol Med 35(6):565–571. https://doi.org/10.1038/emm.2003.74
Li X, Xu G, Qiao T, Yuan S, Zhuang X (2016) Effects of CpG oligodeoxynucleotide 1826 on acute radiation-induced lung injury in mice. Biol Res 49(1):8
Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, Wolfe LF, Brizio-Molteni L, Veno P (2000) Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol 76(4):523–532
Naeimi RA, Talebpour Amiri F, Khalatbary AR, Ghasemi A, Zargari M, Ghesemi M, Hosseinimehr SJ (2017) Atorvastatin mitigates testicular injuries induced by ionizing radiation in mice. Reprod Toxicol 72:115–121. https://doi.org/10.1016/j.reprotox.2017.06.052
Nozaki Y, Hasegawa Y, Takeuchi A, Fan Z, Isobe K, Nakashima I et al (1997) Nitric oxide as an inflammatory mediator of radiation pneumonitis in rats. American Journal of Physiology: Lung Cellular and Molecular Physiology 272(4):L651–L658
Ozturk B, Egehan I, Atavci S, Kitapci M (2004) Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys 58(1):213–219
Panganiban RA, Mungunsukh O, Day RM (2013) X-irradiation induces ER stress, apoptosis, and senescence in pulmonary artery endothelial cells. Int J Radiat Biol 89(8):656–667. https://doi.org/10.3109/09553002.2012.711502
Peixoto CA, Silva BS (2014) Anti-inflammatory effects of diethylcarbamazine: a review. Eur J Pharmacol 734:35–41
Pordanjani SM, Hosseinimehr SJ (2016) The role of NF-kB inhibitors in cell response to radiation. Curr Med Chem 23(34):3951–3963. https://doi.org/10.2174/0929867323666160824162718
Queto T, Xavier-Elsas P, Gardel MA, de Luca B, Barradas M, Masid D, E Silva PM, Peixoto CA, Vasconcelos ZM, Dias EP, Gaspar-Elsas MI (2010) Inducible nitric oxide synthase/CD95L-dependent suppression of pulmonary and bone marrow eosinophilia by diethylcarbamazine. Am J Respir Crit Care Med 181(5):429–437
Ribeiro EL, Barbosa KPDS, Fragoso IT, Donato MAM, dos Santos Gomes FO, da Silva BS et al (2014) Diethylcarbamazine attenuates the development of Carrageenan-induced lung injury in mice. Mediators of Inflammation. https://doi.org/10.1155/2014/105120
Rocha SWS, França MERD, Rodrigues GB, Barbosa, KPS, Nunes AKS, Pastor AF et al (2014) Diethylcarbamazine reduces chronic inflammation and fibrosis in carbon tetrachloride-(CCl4-) induced liver injury in mice. Mediators of Inflammation. https://doi.org/10.1155/2014/696383
Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A et al (2000) Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys 47(4):1033–1042
Ruia S, Saxena S, Prasad S, Sharma SR, Akduman L, Khanna VK (2016) Correlation of biomarkers thiobarbituric acid reactive substance, nitric oxide and central subfield and cube average thickness in diabetic retinopathy: a cross-sectional study. International Journal of Retina and Vitreous 2(1):8
Salehifar E, Hosseinimehr SJ (2016) The use of cyclooxygenase-2 inhibitors for improvement of efficacy of radiotherapy in cancers. Drug Discov Today 21(4):654–662. https://doi.org/10.1016/j.drudis.2016.02.019
Santos Rocha SW, Silva BS, Gomes FO, Soares e Silva AK, Raposo C, Barbosa KP et al (2012) Effect of diethylcarbamazine on chronic hepatic inflammation induced by alcohol in C57BL/6 mice. Eur J Pharmacol 689(1–3):194–203. https://doi.org/10.1016/j.ejphar.2012.05.044
Santos LAM, Ribeiro EL, Barbosa KPS, Fragoso IT, dos Santos Gomes FO, Donato MAM et al (2014) Diethylcarbamazine inhibits NF-κB activation in acute lung injury induced by carrageenan in mice. Int Immunopharmacol 23(1):153–162
Shaki F, Ashari S, Ahangar N (2016) Melatonin can attenuate ciprofloxacin induced nephrotoxicity: involvement of nitric oxide and TNF-alpha. Biomed Pharmacother 84:1172–1178. https://doi.org/10.1016/j.biopha.2016.10.053
Shokrzadeh M, Zamani E, Mehrzad M, Norian Y, Shaki F (2015) Protective effects of Propofol against methamphetamine-induced neurotoxicity. Toxicol Int 22(1):92–99. https://doi.org/10.4103/0971-6580.172250
Spector T (1978) Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem 86(1):142–146
Tahamtan R, Shabestani Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA et al (2015) Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J 17(1):111–120
Verma YK, Gangenahalli G (2017) Data mining for drug repurposing and new targets identification for radioprotection. Defence Life Science Journal 2(3):343–353
Vujaskovic Z, Marks LB, Anscher MS (2000) The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol 10(4):296–307
Wilson AF, McPhillips JJ (1978) Pharmacological control of asthma. Annu Rev Pharmacol Toxicol 18(1):541–561. https://doi.org/10.1146/annurev.pa.18.040178.002545
Acknowledgments
This work was supported by the Mazandaran University of Medical Sciences, Sari, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirmed that this article content has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farzipour, S., Amiri, F.T., Mihandoust, E. et al. Radioprotective effect of diethylcarbamazine on radiation-induced acute lung injury and oxidative stress in mice. J Bioenerg Biomembr 52, 39–46 (2020). https://doi.org/10.1007/s10863-019-09820-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-019-09820-9