Abstract
Radiation-induced lung injury (RILI) is a potentially life-threatening complication of radiotherapy. In the current study, we examined the potential protective effects of URB937, an inhibitor of fatty acid amide hydrolase using a mouse model of RILI. Briefly, male C57BL/6 mice received 16Gy irradiation to the thoracic region and then intraperitoneal injection of either URB937 (1 mg/kg) or vehicle every 2 days for 30 days. The extent of the lung injury was evaluated histologically at the end of the drug treatment as well as 3 months after the cessation of the treatment. The data showed URB937 attenuated radiation-induced lung injury and increased endocannabinoid concentration in lung tissue. Treatment with URB937 decreased leukocyte migration and inflammatory cytokines in bronchoalveolar lavage fluid and plasma at day 30. Histopathological examination revealed URB937 could restore lung structure and restrain inflammatory cell and fibroblast accumulation caused by irradiation in lung tissue. URB937 also decreased radiation-induced pro-inflammatory (e.g., interleukin-1β, interleukin-6, tumor necrosis factor-α) and pro-fibrotic cytokines (e.g., transforming growth factor-β1) level in lung tissue, as well as lipid peroxidation in the lungs. Mouse survival examined in a separate group of experimental subjects indicated that URB937 could prolong animal survival. Experiments using a mouse bearing Lewis lung carcinoma cells showed that URB937 does not affect irradiation-induced inhibition of tumor growth. These results suggest that inhibiting fatty acid amide hydrolase could ameliorate RILI without compromising the efficacy of irradiation on tumor control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Irradiation is a major treatment modality for thoracic cancer but could damage healthy tissue through complex mechanisms that include reactive oxygen species (ROS), excessive inflammation, and fibrosis. Radiation-induced lung injury (RILI) is a major concern in the use of thoracic radiation [1, 2].
Acute RILI, typically manifesting as pneumonitis at 1–6 months after thoracic radiation [3], occurs in 9.4~28% of the patients receiving stereotactic thoracic irradiation [4]. Pulmonary fibrosis is the key feature of chronic RILI and evolves during a longer period of time [5, 6]. Exposure of lung tissue to irradiation leads to lung structure destruction, alveolar edema, fibroblast proliferation, and collagen deposition [7]. Ionizing radiation continuously induces inflammatory cascade and the resulting free radicals further promote activation of fibroblasts in proliferation [8]. Previous studies have demonstrated that RILI could be alleviated by blocking ROS, pro-inflammatory cytokines, e.g., tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6), as well as the pro-fibrotic cytokine transforming growth factor β1 (TGF-β1) [9, 10]. New strategies are needed to attenuate/prevent inflammatory and fibrosis without affecting the efficacy of radiotherapy.
Endocannabinoids (e.g., anandamide [AEA] and 2-arachidonoyl glycerol [2-AG]) are endogenous lipid-signaling molecules in many cells in a variety of tissues. Endocannabinoids have a broad range of biological properties that resemble the action of Δ-9-tetra-hydroxycannabinol [11]. Endocannabinoids have been shown to protect the heart from ischemia–reperfusion injury [12] and reduce the level of biomarkers for inflammation and fibrosis in pancreatic stellate cells [13]. Accumulating evidence suggests that increasing endocannabinoids, by inhibiting fatty acid amide hydrolase (FAAH), could inhibit tumor angiogenesis and decrease cancer cell migration [14,15,16].
We hypothesized that URB937 could attenuate RILI by suppressing pulmonary inflammation and lung fibrosis cascades, without affecting the efficacy of irradiation on tumor control. In the current study, we used a mouse model to examine whether URB937, a potent and selective peripherally-acting FAAH inhibitor [17], could attenuate RILI. Potential effects on tumor growth were also examined.
MATERIAL AND METHODS
Experimental Procedures
Adult male specific-pathogen-free C57BL/6J mice (8 weeks of age; from the Center of Experimental Animals, the Academy of Military Medical Sciences, Beijing, China) were maintained under a 12 h light/dark cycle with unlimited access to food and water. All animal experiments were performed in accordance with the guidelines set by the Care and Use of Laboratory Animals published by the US National Institutes of Health, and approved by the Animal Care and Use Committee of Sichuan University.
Mice received a single dose of X-ray irradiation (16Gy; 297.43 cGy/min) to the whole thorax under intraperitoneal (ip.) pentobarbital anesthesia (40 mg/kg). The head, abdomen, and extremities were shielded with lead strips. Starting from 2 h after the irradiation, mouse received ip. injection of URB937 (1 mg/kg in 1:4 DMSO/PEG300; synthesized and characterized in our lab; chemical structure shown in Fig.1d, synthetic route shown in supplementary Fig.1) or vehicle, once every 2 days for 30 days. A group of mice receiving anesthesia but no irradiation was included as an additional control. At the end of the treatment, mice were sacrificed for histological examination. A separate group of mice was sacrificed 3 months after the cessation of the drug treatment to evaluate the extent of fibrosis. Mouse survival was monitored in a separate group of mice. In the survival experiments, mice were euthanized when losing 20% bodyweight compared to the baseline.
Malondialdehyde
Plasma malondialdehyde (MDA) concentration was determined using a commercial kit (Nanjing Jiancheng Bio-engineering Institute) based on thiobarbituric acid reactivity.
RNA Isolation and Real-Time PCR
Total RNA was extracted from the lungs, and reverse transcribed to cDNA using MMLV reverse transcriptase (Invitrogen). Primer sequence is listed in supplementary Fig. 2. Real-time quantitative PCR was carried out using the SYBR Premix Ex Taq™ II real-time PCR kit (Takara). Results were normalized against the internal GAPDH control. All experiments were performed in triplicate.
Histological Evaluation
Removed lungs were perfused routinely and immersed in 4% paraformaldehyde for 24 h, dehydrated through grade series of ethanol, and embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin (H&E) or Masson’s triple stain and examined under a light microscope (Olympus CX41RF) [18, 19]. The extent of fibrosis was assessed by evaluating the thickness of alveolar and bronchiolar walls, as previously reported [20].
Bronchoalveolar Lavage Fluid Analysis
This set of experiments was conducted in a separate group of mice. Briefly, upon sacrifice of the mice, a small plastic tube was inserted into the trachea. The preparation was irrigated with 1-ml physiological saline for 3 times. The bronchoalveolar lavage fluid (BALF) was centrifuged at 400 g for 15 min. The pellet was suspended in 0.5 ml 1% glacial acetate to dissolve red blood cells and then subjected to Wright-Giemsa staining. Inflammatory cells were counted by smear microscope.
Western Blot Analysis
Tissue samples were homogenized as previously described [3]. Samples containing equal amount of proteins were separated by 10% SDS-PAGE and transferred onto a PVDF membrane (Millipore). After blocking with 5% skim milk for 1 h, membranes were incubated with a rabbit TGF-β1 antibody (1:500, Cell Signaling Technology) overnight at 4 °C. After extensive washing, membranes were incubated with HRP-conjugated anti-rabbit secondary antibody (1:5000, Santa Cruz) before visualization using an enhanced chemiluminescence kit (Millipore). β-Actin (1:1000, Santa Cruz) was used as the internal control. Protein concentration was determined using a Bradford assay.
Plasma Cytokines
Plasma concentration of IL-1β, IL-6, TNF-α, and TGF-β1 was measured using Luminex® Screening (R&D Systems).
Liquid Chromatography Quantification for Endocannabinoids
Tissue samples were homogenized as previously described [21]. Homogenate (20 μl) samples were added to a polypropylene plastic tube containing 2.0 mL chloroform and 1.0 mL methanol. After adding aliquots of 10 pmol d5-2-arachidonoylglycerol and 5 pmol d8-arachidonoyl-ethanolamide, the mixture was vortexed for 30 s, and centrifuged at 1400g for 10 min at 10 °C. The aqueous phase was dried using a stream of nitrogen gas. The extraction was repeated twice. The product was re-constituted in 0.1 ml 2:1 CHCl3:CH3OH and purified on silica columns. The eluate from the column with 9:1 CHCl3:CH3OH was dried for analysis of AEA with a liquid chromatography coupled with an atmospheric pressure-chemical ionization-single quadrupole mass spectrometer using positive ion analysis mode. The amount of endocannabinoids was determined against deuterated internal standards.
Xenograft Assays
Lewis lung carcinoma cells (LLCs; from American Type Culture Collection) were cultured in RPMI-1640 medium (Hyclone) supplemented with 10% fetal bovine serum (Hoffmann-La Roche) and 1% penicillin/streptomycin at 37 °C in 5% CO2. Mice were subcutaneously injected with 1 × 106 LLC cells into the right proximal hind leg. When the tumor volume reached 70 mm3, mice received irradiation (16Gy at 297.43 cGy/min; directed to the lesions), followed by URB937 (1 mg/kg, ip., once every 2 days) or vehicle until sacrifice (when tumor volume reached 2000 mm3). Tumour volume was calculated according to the following formula: volume (V) = length × width × width × 0.5.
Statistical Analyses
Data are represented as mean ± SEM, with at least three independent experiments. All variables were analyzed by one-way ANOVA followed by Dunnett’s t test for pairwise comparisons. Statistical significance was defined at p < 0.05.
RESULTS
URB937 Prolonged the Survival of Irradiated Mice and Increased Pulmonary Endocannabinoid Concentration
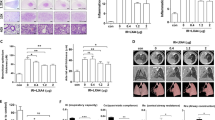
Figure1a is a schematic illustration of the in vivo experiments. URB937 increased AEA in mice receiving irradiation (Fig.1b). URB937 increased the mouse survival: 70 vs. 30% in the vehicle control at 32 weeks (Fig.1c).
URB937 Attenuated Inflammatory Infiltration to the Lungs and Decreased Plasma Concentration of Representative Pro-inflammatory Cytokines
URB937 attenuated irradiation-induced increase of inflammatory cells in the BALF (Fig.2a) and plasma concentration of representative cytokines, including IL-1β, IL-6, TNF-α, and active TGF-β1 (Fig.2b).
URB937 Attenuated Acute RILI (30 Days After Irradiation)
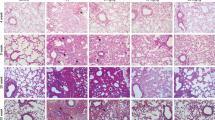
Irradiation increased the number of inflammatory cells outside the alveolar septa and alveolar thickness. Interstitial edema was also apparent. Such change was attenuated by URB937. Masson’s triple stain did not show a difference in fibrosis of alveolar walls at day 30 (Fig.3a, b).
Effects of URB937 at day 30. a Representative H&E (upper panel) and Masson staining (lower panel). Bar = 50 μm. b Summary histopathologic alterations. c Plasma MDA concentration. d Cytokine mRNA levels. e Western bolt analysis of TGF-β protein expression. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
URB937 decreased MDA content by 31% compared with radiation only group (Fig.3c). Irradiation increased messenger RNAs (mRNAs) for pro-inflammatory and pro-fibrotic cytokines, including IL-1β, IL-6, TNF-α, and TGF-β1 in the lungs. URB937 significantly reduced lung concentration of these cytokines (Fig.3d). Western blot showed a decrease of TGF-β1 in the lungs (Fig.3e).
URB937 Alleviated Lung Fibrosis
Histopathologic examination at 120 days after radiation revealed significant fibrosis of the lungs, including dramatic interstitial hyperplasia, vacuolization of the blood vessels, and irregular thickening of the muscular layer. Masson’s trichrome staining revealed collagen deposition in alveolar septa and bronchiolar tissue. URB937 attenuated these changes (Fig.4a, b).
URB937 attenuated the elevation of MDA levels by 41% at day 120 (Fig.4c). Irradiation-induced increases in mRNA level of pro-inflammatory cytokines and pro-fibrotic cytokines in lung tissues were also attenuated by URB937 (Fig.4d). Western blot analysis demonstrated that irradiation-induced increase of TGF-β1 in the lungs was decreased by URB937 (Fig.4e).
URB937 Did Not Affect the Antitumor Effects of Irradiation
Irradiation resulted in a dramatic decrease in tumor growth. URB937 did not alter the effects of the irradiation (Fig.5).
DISCUSSION
Radiotherapy is recommended by the current guidelines for patients with thoracic carcinoma. However, RILI not only affects the life quality of patients but also limit the doses of radiotherapy [22]. To improve tumor locoregional control and patient survival, new strategies that could prevent this severe side effect without interfering with anticancer treatments are needed.
Cannabinoids have been used clinically for a variety of disease conditions, for example to alleviate nausea and vomitting induced by chemotherapy. Cannabinoids have been shown to attenuate oxidative stress, inflammation, cell death, and fibrosis in both preclinical and clinical researches [23, 24]. Cannabis-based pharmacological agents, however, face legal and ethical concerns, but also generate physical and psychological dependence symptom. Targeting endocannabinoids clearly represents a strategy that could circumvent such barriers.
The present study showed that inhibiting fatty acid amide hydrolase with URB937 could attenuate lung injury in both acute and chronic stages of RILI. URB937 also significantly prolonged the survival time of mice with RILI. Mechanistic experiments showed that URB937 decreased inflammatory cell infiltration in BALF and cytokine expression in plasma at early RILI and decreased oxidative stress and pro-inflammatory/fibrotic cytokines in lung tissue at the entire process of radiation-induced lung injury. Importantly, URB937 did not alter tumor growth inhibition caused by irradiation.
The observed increase of endocannabinoids upon URB937 treatment in the current study supported the notion that FAAH is the pharmacological target of URB937. Radiation-induced lung injury is typified by lung structure pathological changes such as pulmonary interstitial edema and excessive collagen deposition [7, 25]. Pathologic examination in the current study showed that the severity of lung structure damage induced by irradiation could be attenuated by URB937, supporting the beneficial action of URB937 in the radiation lung injury.
Tissues exposed to irradiation increase cytokine production at early stage, which in turn induces inflammatory cells in BLAF. In the current study, URB937 decreased inflammatory cells in BLAF and cytokines in plasma. Such a finding is consistent with the effects of URB937 in mouse staphylococcal enterotoxin B-induced lung injury model [26].
Irradiation increases lipid peroxidation which in turn produces a variety of damages to tissues and cells [27]. Decreasing lipid peroxidation product (e.g., MDA) could protect lung tissue from radiation injury [28]. A previous study showed that endocannabinoids could decrease the MDA level in the gastric mucosa and colitis [29]. Our study suggests that the protective effect of URB937 on RILI involves reduction of the plasma lipid peroxidation.
Cytokines are essential for both inflammation establishment and phagocyte activation. Previous studies have demonstrated the critical role of inflammatory cytokines in the radiation injury process [30]. The cannabinoid-2 (CB2) receptors are expressed in immune cells and have been shown to reduce IL-1β and TNF-α RNA expression in lung tissue [31, 32], whereas AEA has been shown to decrease IL-6 expression and protect against the development of intestinal radiation injury [33]. In the current study, we observed a reduction in pro-inflammatory cytokine (IL-1β, IL-6, TNF-α) in irradiated mice receiving URB937; such a reduction might have contributed to attenuated radiation pneumonia and fibrosis.
Irradiation accelerates the production of pro-fibritic cytokine TGF-β1 in lung tissue, forming a perpetual cascade of inflammatory cytokines. TGF-β1 overexpression after radiation injury promotes the formation and inhibits the breakdown of connective tissue [34, 35]. Recently, Gonzalez and colleagues showed that endocannabinoids could downregulate TGF-β1 and restrain fibrogenesis by stimulating PPAR-γ signaling [36]. Our study showed that increasing endocannabinoids by URB937 could suppress radiation-induced elevation of TGF-β1 expression.
Ideal radioprotective agents should alleviate radiation pneumonia and fibrosis without compromising the antitumor effects of radiotherapy. The anti-inflammatory and analgesic therapeutic effects of FAAH inhibitors have been shown in some clinical test [37]. Inhibiting FAAH attenuate tumor proliferation, migration, and invasiveness and was used in preclinical cancer treatment [38, 39]. In current the study, we found URB937 did not alter tumor growth inhibition caused by irradiation, confirming the selectivity of the protective effects to healthy but not tumor cells.
The current study has several limitations. First, URB937 is a chemical agent with limited selectivity. We are now planning experiments using molecular approaches such as gene knockout. Also, despite a preventive role of endocannabinoids in early inflammation in most disease models [40, 41], endocannabinoids have been shown to produce opposite effects (either pro- or anti-fibrogenic action), possibly due to involvement of distinct receptor types [42, 43].
CONCLUSIONS
Increasing endogenous endocannabinoids by inhibiting FAAH with URB937 could alleviate RILI in a mouse model. These findings implicated endocannabinoids in controlling inflammation and fibrosis. The results also encourage future study of inhibiting FAAH as a target in the treatment of RILI.
Abbreviations
- RILI:
-
Radiation-induced lung injury
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- TGF-β:
-
Transforming growth factor
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- AEA:
-
Anandamide
- FAAH:
-
Fatty acid amide hydrolase
- H&E:
-
Hematoxylin and eosin
- BLAF:
-
Bronchoalveolar lavage fluid
- LLCs:
-
Lewis lung carcinoma cells
References
Xue, J., X. Li, Y. Lu, L. Gan, L. Zhou, Y. Wang, J. Lan, et al. 2013. Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Molecular Therapy 21 (2): 456–465. doi:10.1038/mt.2012.183.
Graves, P.R., F. Siddiqui, M.S. Anscher, and B. Movsas. 2010. Radiation pulmonary toxicity: from mechanisms to management. Seminars in Radiation Oncology 20 (3): 201–207. doi:10.1016/j.semradonc.2010.01.010.
Xue, J., L. Gan, X. Li, J. Li, G. Qi, Y. Wu, X. Fu, et al. 2010. Effects of lysophosphatidic acid and its receptors LPA(1/3) on radiation pneumonitis. Oncology Reports 24 (6): 1515–1520.
Ueki, N., Y. Matsuo, Y. Togashi, T. Kubo, K. Shibuya, Y. Iizuka, T. Mizowaki, K. Togashi, M. Mishima, and M. Hiraoka. 2015. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. Journal of Thoracic Oncology 10 (1): 116–125. doi:10.1097/JTO.0000000000000359.
Gan, L., J.X. Xue, X. Li, D.S. Liu, Y. Ge, P.Y. Ni, L. Deng, Y. Lu, and W. Jiang. 2011. Blockade of lysophosphatidic acid receptors LPAR1/3 ameliorates lung fibrosis induced by irradiation. Biochemical and Biophysical Research Communications 409 (1): 7–13. doi:10.1016/j.bbrc. 2011.04.084.
Ding, N.H., J.J. Li, and L.Q. Sun. 2013. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Current Drug Targets 14 (11): 1347–1356.
Heinzelmann, F., V. Jendrossek, K. Lauber, K. Nowak, T. Eldh, R. Boras, R. Handrick, et al. 2006. Irradiation-induced pneumonitis mediated by the CD95/CD95-ligand system. Journal of the National Cancer Institute 98 (17): 1248–1251. doi:10.1093/jnci/djj335.
Boothe, D.L., S. Coplowitz, E. Greenwood, C.L. Barney, P.J. Christos, B. Parashar, D. Nori, K.S. Chao, and A.G. Wernicke. 2013. Transforming growth factor beta-1 (TGF-beta1) is a serum biomarker of radiation induced fibrosis in patients treated with intracavitary accelerated partial breast irradiation: preliminary results of a prospective study. International Journal of Radiation Oncology, Biology, Physics 87 (5): 1030–1036. doi:10.1016/j.ijrobp.2013.08.045.
Tsoutsou, P.G., and M.I. Koukourakis. 2006. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. International Journal of Radiation Oncology, Biology, Physics 66 (5): 1281–1293. doi:10.1016/j.ijrobp.2006.08.058.
Terasaki, Y., I. Ohsawa, M. Terasaki, M. Takahashi, S. Kunugi, K. Dedong, H. Urushiyama, et al. 2011. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. American Journal of Physiology. Lung Cellular and Molecular Physiology 301 (4): L415–L426. doi:10.1152/ajplung.00008.2011.
Rodriguez-Manzo, G., and A. Canseco-Alba. 2015. Biphasic effects of anandamide on behavioural responses: emphasis on copulatory behaviour. Behavioural Pharmacology 26 (6): 607–615. doi:10.1097/FBP.0000000000000154.
Li, Q., M. Shi, and B. Li. 2013. Anandamide enhances expression of heat shock protein 72 to protect against ischemia-reperfusion injury in rat heart. The Journal of Physiological Sciences 63 (1): 47–53. doi:10.1007/s12576-012-0228-5.
Michalski, C.W., M. Maier, M. Erkan, D. Sauliunaite, F. Bergmann, P. Pacher, S. Batkai, et al. 2008. Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells. PloS One 3 (2): e1701. doi:10.1371/journal.pone.0001701.
Ravi, J., A. Sneh, K. Shilo, M.W. Nasser, and R.K. Ganju. 2014. FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway. Oncotarget 5 (9): 2475–2486. doi:10.18632/oncotarget.1723.
Portella, G., C. Laezza, P. Laccetti, L. De Petrocellis, V. Di Marzo, and M. Bifulco. 2003. Inhibitory effects of cannabinoid CB1 receptor stimulation on tumor growth and metastatic spreading: actions on signals involved in angiogenesis and metastasis. The FASEB Journal 17 (12): 1771–1773. doi:10.1096/fj.02-1129fje.
Velasco, G., S. Hernandez-Tiedra, D. Davila, and M. Lorente. 2016. The use of cannabinoids as anticancer agents. Progress in Neuro-Psychopharmacology & Biological Psychiatry 64: 259–266. doi:10.1016/j.pnpbp.2015.05.010.
Moreno-Sanz, G., O. Sasso, A. Guijarro, O. Oluyemi, R. Bertorelli, A. Reggiani, and D. Piomelli. 2012. Pharmacological characterization of the peripheral FAAH inhibitor URB937 in female rodents: interaction with the Abcg2 transporter in the blood-placenta barrier. British Journal of Pharmacology 167 (8): 1620–1628. doi:10.1111/j.1476-5381.2012.02098.x.
Penney, D.P., D.W. Siemann, P. Rubin, and K. Maltby. 1994. Morphological correlates of fractionated radiation of the mouse lung: early and late effects. International Journal of Radiation Oncology, Biology, Physics 29 (4): 789–804.
Travis, E.L. 1980. The sequence of histological changes in mouse lungs after single doses of x-rays. International Journal of Radiation Oncology, Biology, Physics 6 (3): 345–347.
Ashcroft, T., J.M. Simpson, and V. Timbrell. 1988. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. Journal of Clinical Pathology 41 (4): 467–470.
Dincheva, I., A.T. Drysdale, C.A. Hartley, D.C. Johnson, D. Jing, E.C. King, S. Ra, et al. 2015. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature Communications 6: 6395. doi:10.1038/ncomms7395.
Huang, K., D.A. Palma, and Iaslc Advanced Radiation Technology Committee. 2015. Follow-up of patients after stereotactic radiation for lung cancer: a primer for the nonradiation oncologist. Journal of Thoracic Oncology 10 (3): 412–419. doi:10.1097/JTO.0000000000000435.
Rajesh, M., P. Mukhopadhyay, S. Batkai, V. Patel, K. Saito, S. Matsumoto, Y. Kashiwaya, et al. 2010. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. Journal of the American College of Cardiology 56 (25): 2115–2125. doi:10.1016/j.jacc.2010.07.033.
Ahmed, W., and S. Katz. 2016. Therapeutic use of cannabis in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 12 (11): 668–679.
Brickey, W.J., I.P. Neuringer, W. Walton, X. Hua, E.Y. Wang, S. Jha, G.D. Sempowski, et al. 2012. MyD88 provides a protective role in long-term radiation-induced lung injury. International Journal of Radiation Biology 88 (4): 335–347. doi:10.3109/09553002.2012.652723.
Rao, R., P.S. Nagarkatti, and M. Nagarkatti. 2015. Delta(9) tetrahydrocannabinol attenuates staphylococcal enterotoxin B-induced inflammatory lung injury and prevents mortality in mice by modulation of miR-17-92 cluster and induction of T-regulatory cells. British Journal of Pharmacology 172 (7): 1792–1806. doi:10.1111/bph.13026.
Lin, L., L. Zhang, L. Yu, L. Han, W. Ji, H. Shen, and Z. Hu. 2016. Time-dependent changes of autophagy and apoptosis in lipopolysaccharide-induced rat acute lung injury. Iran J Basic Med Sci 19 (6): 632–637.
Kang, S.K., Z.N. Rabbani, R.J. Folz, M.L. Golson, H. Huang, D. Yu, T.S. Samulski, M.W. Dewhirst, M.S. Anscher, and Z. Vujaskovic. 2003. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. International Journal of Radiation Oncology, Biology, Physics 57 (4): 1056–1066.
Warzecha, Z., A. Dembinski, P. Ceranowicz, M. Dembinski, J. Cieszkowski, P. Kownacki, and P.C. Konturek. 2011. Role of sensory nerves in gastroprotective effect of anandamide in rats. Journal of Physiology and Pharmacology 62 (2): 207–217.
Rube, C.E., D. Uthe, F. Wilfert, D. Ludwig, K. Yang, J. Konig, J. Palm, et al. 2005. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation. International Journal of Radiation Oncology, Biology, Physics 61 (5): 1482–1492. doi:10.1016/j.ijrobp.2004.12.072.
Buckley, N.E. 2008. The peripheral cannabinoid receptor knockout mice: an update. British Journal of Pharmacology 153 (2): 309–318. doi:10.1038/sj.bjp.0707527.
Liu, Z., Y. Wang, H. Zhao, Q. Zheng, L. Xiao, and M. Zhao. 2014. CB2 receptor activation ameliorates the proinflammatory activity in acute lung injury induced by paraquat. BioMed Research International 2014: 971750. doi:10.1155/2014/971750.
Wang, J., J. Zheng, A. Kulkarni, W. Wang, S. Garg, P.L. Prather, and M. Hauer-Jensen. 2014. Palmitoylethanolamide regulates development of intestinal radiation injury in a mast cell-dependent manner. Digestive Diseases and Sciences 59 (11): 2693–2703. doi:10.1007/s10620-014-3212-5.
Anscher, M.S. 2010. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. The Oncologist 15 (4): 350–359. doi:10.1634/theoncologist.2009-S101.
Liu, Y., T. Xia, W. Zhang, Y. Zhong, L. Zhang, X. Wang, and H. Yu. 2013. Variations of circulating endothelial progenitor cells and transforming growth factor-beta-1 (TGF-beta1) during thoracic radiotherapy are predictive for radiation pneumonitis. Radiation Oncology 8: 189. doi:10.1186/1748-717X-8-189.
Gonzalez, E.G., E. Selvi, E. Balistreri, A. Akhmetshina, K. Palumbo, S. Lorenzini, P.E. Lazzerini, et al. 2012. Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. Annals of the Rheumatic Diseases 71 (9): 1545–1551. doi:10.1136/annrheumdis-2011-200314.
Kerbrat, A., J.C. Ferre, P. Fillatre, T. Ronziere, S. Vannier, B. Carsin-Nicol, S. Lavoue, et al. 2016. Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. The New England Journal of Medicine 375 (18): 1717–1725. doi:10.1056/NEJMoa1604221.
Jonsson, K.O., S. Holt, and C.J. Fowler. 2006. The endocannabinoid system: current pharmacological research and therapeutic possibilities. Basic & Clinical Pharmacology & Toxicology 98 (2): 124–134. doi:10.1111/j.1742-7843.2006.pto_376.x.
Li, H., J.T. Wood, K.M. Whitten, S.K. Vadivel, S. Seng, A. Makriyannis, and H.K. Avraham. 2013. Inhibition of fatty acid amide hydrolase activates Nrf2 signalling and induces heme oxygenase 1 transcription in breast cancer cells. British Journal of Pharmacology 170 (3): 489–505. doi:10.1111/bph.12111.
Ribeiro, A., V. Ferraz-de-Paula, M.L. Pinheiro, L.B. Vitoretti, D.P. Mariano-Souza, W.M. Quinteiro-Filho, A.T. Akamine, et al. 2012. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. European Journal of Pharmacology 678 (1–3): 78–85. doi:10.1016/j.ejphar.2011.12.043.
Hoyer, F.F., M. Khoury, H. Slomka, M. Kebschull, R. Lerner, B. Lutz, H. Schott, et al. 2014. Inhibition of endocannabinoid-degrading enzyme fatty acid amide hydrolase increases atherosclerotic plaque vulnerability in mice. Journal of Molecular and Cellular Cardiology 66: 126–132. doi:10.1016/j.yjmcc.2013.11.013.
Teixeira-Clerc, F., B. Julien, P. Grenard, J. Tran Van Nhieu, V. Deveaux, L. Li, V. Serriere-Lanneau, C. Ledent, A. Mallat, and S. Lotersztajn. 2006. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nature Medicine 12 (6): 671–676. doi:10.1038/nm1421.
Akhmetshina, A., C. Dees, N. Busch, J. Beer, K. Sarter, J. Zwerina, A. Zimmer, O. Distler, G. Schett, and J.H. Distler. 2009. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis and Rheumatism 60 (4): 1129–1136. doi:10.1002/art.24395.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81472808, No.81301935, No.81472196).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The use of C57BL/6J mice was approved by Animal Care and Use Committee of Sichuan University.
Conflict of Interest
The authors declare that they have no conflicts of interests.
Additional information
Rui Li and Guo Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, R., Chen, G., Zhou, L. et al. The Fatty Acid Amide Hydrolase Inhibitor URB937 Ameliorates Radiation-Induced Lung Injury in a Mouse Model. Inflammation 40, 1254–1263 (2017). https://doi.org/10.1007/s10753-017-0568-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0568-7