Abstract
Mastitis, an inflammatory reaction of the mammary gland, is recognized as one of the most costly diseases in dairy cattle. Oxymatrine, one of the alkaloids extracted from Chinese herb Sophora flavescens Ait, has been reported to have many biological activities, such as anti-inflammatory, anti-virus, and anti-hepatic fibrosis properties. The aim of this study was to investigate the protective effect and the anti-inflammatory mechanism of oxymatrine on lipopolysaccharide (LPS)-induced mastitis in mice. The mouse mastitis was induced by 10 μg of LPS for 24 h. Oxymatrine was intraperitoneally administered with the dose of 30, 60, and 120 mg/kg 1 h before and 12 h after LPS induction. The results showed that oxymatrine significantly attenuated the damage of the mammary gland induced by LPS. Oxymatrine inhibited the phosphorylation of NF-κB p65 and IκB in NF-κB signal pathway and reduced the phosphorylation of p38, ERK, and JNK in mitogen-activated protein kinase (MAPKs) signal pathway. The results showed that oxymatrine had a protective effect on LPS-induced mastitis, and the anti-inflammatory mechanism of oxymatrine was related to the inhibition of NF-κB and MAPKs signal pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Mastitis, a highly prevalent and costly infectious disease mainly caused by bacteria, is defined as an inflammation of the mammary gland [1]. Escherichia coli is one of the most important pathogens that often causes clinical mastitis [2]. Although the efficacy of antibiotic treatment in mastitis is remarkable, the residues of antibiotic in the milk and meat are harmful to the health of human.
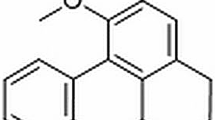
Sophora flavescens Ait is a traditional Chinese medicine, and it has been used for the treatment of many diseases for thousands of years [3]. Oxymatrine (Fig. 1), the main component of Chinese herb Radix S. flavescens Ait, had been reported to have many pharmacological effects, such as anti-inflammation, protecting hepatocytes, and inhibiting immune reaction [4]. In previous studies, the protective effects of oxymatrine on traumatic rat brain injury, colitis of rats, acute pancreatitis in rats, and acute lung injury in mice have been confirmed [5–7]. Considering the extensive effects of oxymatrine, we investigate whether oxymatrine has a protective effect on mastitis in a lipopolysaccharide (LPS)-induced mouse mastitis model and elucidates the potential anti-inflammatory mechanism.
MATERIALS AND METHODS
Reagents
Oxymatrine was purchased from the National Institute for the Control of Pharmaceutical and Biological Products. LPS (Escherichia coli 055:B5) was purchased from Sigma (St. Louis, MO, USA). Mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit was purchased from Biolegend (USA), IL-1β and IL-6 ELISA kits were obtained from eBioscience (USA). All the monoclonal antibodies which were used in Western blot were obtained from Cell Signaling Technology Inc (Beverly, MA, USA).
Animals
Seventy-two female and 36 male BALB/c mice (6-8w; the Center of Experimental Animals of the Baiqiuen Medical College of Jilin University) were used in the present study. All female and male mice were housed together respectively for 7 days to adapt themselves to the surroundings. Then, two female and one male mice were randomly divided in each cage supplied with sufficient water and forages. All cages had been washed carefully and conducted with autoclave sterilization. The house had been sterilized thoroughly with disinfectants. When female mice were pregnant, they were separated from others, and every pregnant mouse was housed in a single cage. All experiments are in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Experiment Model and Experimental Protocol
The LPS-induced mouse mastitis model was established as described in our previous research [8]. Briefly, the teat duct was infused with 50 μL of 20 % LPS made up of 10 μg of LPS and 40 μL of nonpyrogenic phosphate-buffered saline (PBS) by a 100-uL syringe equipped with a 30-gauge blunt needle. The female mice after parturition were divided into six groups, including blank control group, LPS group, LPS + oxymatrine groups (30, 60, 120 mg/kg) group. Each group contains 12 mice. The treatment groups were respectively administered intraperitoneally with 30, 60, 120 mg/kg of oxymatrine at 1 h before and 12 h post LPS infusion based on our preliminary experiment. The blank control group and LPS group were supplied with an equal volume of PBS i.p. At a 24-h post LPS inoculation, the mice were killed with CO2 inhalation, and then the 4 th pairs of MGs were collected and stored at −80 °C until analysis.
Histopathologic Analysis
After 24 h of LPS induction, the mammary tissues were collected and immediately fixed into 10 % formaldehyde solution for 48 h. Then the mammary tissues were dehydrated through gradients of ethanol. Through a series of processing, the mammary tissues were embedded in paraffin. The sections were stained with hematoxylin and eosin. The histopathologic changes were examined under light microscopy.
MPO Immunohistochemistry
Five-micrometer thick paraffin-embedded sections were cut for myeloperoxidase (MPO) immunohistochemistry. The sections were treated with 3 % H2O2 for 10 min to block the endogenous peroxidase and then incubated into the rabbit anti-mouse MPO antibody (1:200, Thermo, RB 373-A0) at 4 °C overnight after washing with PBS three times. The sections were washed three times and incubated with HRP-conjugated secondary antibody. All the sections were analyzed after diaminobenzidine (DAB) staining.
Detection of the Levels of Inflammatory Cytokines in the Homogenate of the Mammary Gland
The mammary gland tissues were weighted and homogenized with PBS (1:9, w/v). The homogenates were centrifuged twice. The supernatants were collected. All the processes were operated as described in our previous research [8]. The levels of TNF-α, IL-1β, and IL-6 in the homogenate of the mammary gland were measured by ELISA in accordance with the instructions of the manufacturer.
Western Blot Analysis
The proteins were extracted from the mammary gland tissues using the T-PER Tissue Protein Extraction Reagent (Thermo, 78510). Protease inhibitors and protein phosphatase inhibitors were added in the protein extraction buffer. Concentrations of the protein were assayed by BCA protein assay kit. The activation of NF-κB and MAPKs signal pathways were analyzed through measuring the phosphorylation of NF-κB p65 and IκB in NF-κB signal pathway and p38, ERK, and JNK in MAPKs signal pathway. Proteins 60 μg were subjected to 10 % SDS-PAGE. The nitrocellulose membranes were blocked with 5 % (w/v) BSA in Tris–Tween-buffered saline (TBST) for 2 h followed by transferred the proteins. The membranes were incubated into antibodies (1:1,000) overnight at 4 °C and then washed with TBST three times. The membranes were incubated into the second antibodies for 1 h. These proteins were detected by Super-Signal West Pico Chemiluminescent Substrate after another three-time wash.
Statistical Analysis
All of the data in this study were expressed as means ± SEM and analyzed by one-way ANOVA. A value of P < 0.05 was considered significant (P < 0.05, P < 0.01).
RESULT
Oxymatrine Improved the LPS-Induced Histopathologic Changes
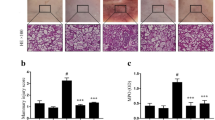
Histopathologic changes of the mammary gland tissues from each experimental group were examined after hematoxylin and eosin staining. There is no inflammatory reaction in the control group (Fig. 2a). Compared with the control group, apparent histopathologic changes could be seen in the LPS group, represented by the thickening of the alveolus’ wall, interstitial patchy hemorrhage, hyperemia, edema, and the extensive existence of inflammatory cells in alveolus spaces (Fig. 2b). In the LPS + oxymatrine groups with the dose of 30 (Fig. 2d), 60 (Fig. 2e), and 120 mg/kg (Fig. 2f), the LSP-induced histopathologic changes were markedly attenuated in a dose-dependent manner.
Effect of oxymatrine on mammary gland tissue damage in mice at 24 h after LPS induction. Representative photomicrographs showing hematoxylin and eosin staining: a the black control group, b the LPS group, d the LPS + oxymatrine (30 mg/kg) group, e the LPS + oxymatrine (60 mg/kg) group, f the LPS + oxymatrine (120 mg/kg) group.
Oxymatrine Reduced the Activity and Distribution of MPO
The activation of MPO, reflected the level of inflammation and oxidative stress, is a functional biomarker of neutrophils. The results of immunohistochemistry were shown in Fig. 3, LPS challenge resulted in a significant increase of the activity and distribution of MPO in the LPS group (Fig. 3b) compared with the control group (Fig. 3a). Treatment with oxymatrine at the dose of 30 (Fig. 3d), 60 (Fig. 3e), and 120 mg/kg (Fig.3f) markedly reduced the LPS-induced increases of MPO activity and distribution in the mammary gland tissues.
Effect of oxymatrine on immunohistochemistry of MPO in mammary gland tissues. At 24 h after LPS induction, the activity and distribution of MPO significantly increased in the LPS group (b) than the black control group (a). The LPS + oxymatrine groups with the dose of 30 mg/kg (d), 60 mg/kg (e), 120 mg/kg (f) markedly decreased the activity and distribution of MPO.
Oxymatrine Decreased the Levels of Pro-inflammatory Cytokines
The levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 were measured by ELISA. As shown in Fig. 4, compared with the control group, LPS instillation significantly increased the levels of TNF-α, IL-1β, and IL-6. In contrast, these increases induced by LPS were significantly decreased in LPS + oxymatrine groups.
Effects of oxymatrine on the levels of TNF-α (a), IL-1β (b) and IL-6 (c) in the homogenate of LPS-induced mice mammary gland tissues. The levels of TNF-α, IL-1β, and IL-6 were determined by ELISA. Compared with the black control group, the levels of TNF-α, IL-1β, and IL-6 were significantly increased compared with the LPS group; levels of TNF-α, IL-1β, and IL-6 were declined in a dose-dependent manner. The data was expressed as means ± SEM. # P < 0.01 versus the control group. *P < 0.05, **P < 0.01 versus the LPS group.
Oxymatrine Downregulated the Activation of NF-κB and MAPKs Signal Pathways
To investigate the possible molecular mechanism of oxymatrine inhibiting the LPS-induced inflammatory response, the activation of NF-κB and MAPKs signal pathways were evaluated. The levels of the phosphorylation of NF-κB p65 and IκB in NF-κB signal pathway and p38, ERK, and JNK in MAPKs signal pathway were markedly increased after LPS induction compared with the control group. However, treatment with oxymatrine significantly inhibited these upregulations by LPS. All the results were shown in Figs. 5 and 6.
Effect of oxymatrine on the levels of the phosphorylation of NF-κB p65 and IκB in NF-κB signal pathway. The levels of the phosphorylation of NF-κB p65 and IκB were increased in the LPS group, # P < 0.01, versus the black control group. Oxymatrine inhibited the LPS-induced increase of the phosphorylation of NF-κB p65 and IκB. *P < 0.05, **P < 0.01 versus the LPS group.
Effect of oxymatrine on the levels of the phosphorylation of p38, ERK, and JNK in MAPKs signal pathway. The levels of the phosphorylation of p38, ERK, and JNK were increased in the LPS group, # P < 0.01 versus the black control group. Oxymatrine inhibited the LPS-induced increase of the phosphorylation of p38, ERK, and JNK. *P < 0.05, **P < 0.01 versus the LPS group.
DISCUSSION
Bovine mastitis, an inflammation reaction of the mammary gland, is one of the most costly diseases in dairy industry. Despite the intensive research and prevention measures have been carried out for decades, the mastitis is still an intractable disease. Approximately 80 % of all intramammary infections by coliform bacteria result in clinical mastitis [9, 10]. LPS is the main component of the cell wall of Gram-negative bacteria such as E. coli. LPS-induced mastitis model in mice was considered as a valuable tool to study the effects of mastitis without bacteria infection [11].
Oxymatrine, which has been used to treat chronic hepatitis B patients for decades in China, is an alkaloid compound extracted from the root of S. flavescens Ait [12]. It has been proven that oxymatrine has many pharmacological activities, such as protecting ischemic and reperfusion injury in the liver, intestine, and heart via anti-inflammation and anti-apoptosis [13–15]. Many previous researches have confirmed that there is no toxic effect with an intraperitoneal injection of oxymatrine at a dose of 120 mg/kg [16]. In order to find the better compound to treat mastitis, we detected the effects of emodin, salidroside, curcumin, cyaniding-3-β-glucoside, and glycyrrhizin on LPS-induced mastitis in mice in our previous studies [17–21]. In this study, we investigated the effects of oxymatrine in LPS-induced mouse mastitis. The results of our study showed that oxymatrine could reduce the inflammatory response of LPS-induced mastitis in mice.
Histopathologic changes were an index of the response to the inflammation reactions. Polymorphonuclear neutrophilic leukocytes (PMN) are the most important natural defense mechanism against the acute bacterial infection of mastitis [22]. Leukocytes transmigrated from circulating pool into the mammary gland was a striking feature of acute mastitis [23]. In this study, there is no histopathologic change that can be seen in the black control group. However, lots of PMN were present in mammary alveolus; the walls of mammary alveolus were edematous, interstitial patchy hemorrhage in LPS group. As shown in Fig. 2, treatment with oxymatrine reduced the histopathologic changes induced by LPS. The activity and distribution of MPO, which reflects the number and distribution of neutrophils in the tissue, were significantly elevated after LPS induction. Administered with oxymatrine markedly decreased the activity and distribution of MPO in the mammary gland. The data was consistent with the histopathologic changes.
Furthermore, mastitis is associated with the increased secretion of pro-inflammatory cytokines. Significant productions of TNF-α, IL-1β, and IL-6 have been observed in many different types of inflammatory processes, including mastitis [24]. An increase of TNF-α, IL-1β, and IL-6 were also detected in the infected bovine mammary gland [25]. Many previous researches have suggested that pro-inflammatory cytokines, such as TNF-α and IL-1, might play an important role in the pathogenesis of mastitis [26, 27]. Moreover, TNF-α, IL-1β, and IL-6 are pro-inflammatory cytokines which also have been improved directing the migration of neutrophils (PMNs) to the site of infection [24]. In the current study, we found that oxymatrine inhibited TNF-α, IL-1β, and IL-6 production induced by LPS.
To further illuminate the possible molecular mechanism of oxymatrine suppressing the production of pro-inflammatory cytokines, the activations of NF-κB and MAPKs signal pathways were detected. NF-κB is a pleiotropic regulator of many genes involved in immunity and inflammation [28]. NF-κB is sequestered in the cytoplasm through physical association with inhibitory proteins of the IκB family, which maintains the transcription factor in an inactive state [29]. The MAPKs signal pathway, another extracellular signal transduction pathway stimulated by inflammatory mediators, includes three members: p38, ERK, and JNK. The results of Western blot suggested that the activation of NF-κB and MAPKs signal pathways were augmented by increasing the levels of phosphorylation of p65, IκB, p38, ERK, and JNK. However, treatment with oxymatrine could significantly inhibit the activations of NF-κB and MAPKs signal pathways.
In conclusion, our study revealed that treatment with oxymatrine significantly ameliorated the histopathologic changes, the activity and distribution of MPO, and the production of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the mammary gland tissues which is possibly linked with the inhibition of the activations of NF-κB and MAPKs signal pathways. Therefore, these data strongly suggested that oxymatrine could be a promising therapeutic medicine in the treatment of mastitis. To clarify the exact target of oxymatrine as well as further molecular mechanism, more work should be done.
References
Petrovski, K.R., M. Trajcev, and G. Buneski. 2006. A review of the factors affecting the costs of bovine mastitis. Journal of the South African Veterinary Association 77: 52–60.
Zhao, X., and P. Lacasse. 2008. Mammary tissue damage during bovine mastitis: causes and control. Journal of Animal Science 86: 57–65.
Xu, G.L., L. Yao, S.Y. Rao, Z.N. Gong, S.Q. Zhang, and S.Q. Yu. 2005. Attenuation of acute lung injury in mice by oxymatrine is associated with inhibition of phosphorylated p38 mitogen-activated protein kinase. Journal of Ethnopharmacology 98: 177–183.
Yuan, X.P., Y.Y. Wang, D.R. Du, Z. Hu, M.X. Xu, M.B. Xu, and Z.F. Liu. 2012. The effects of the combination of sodium ferulate and oxymatrine on lipopolysaccharide-induced acute lung injury in mice. Inflammation 35: 1161–1168.
Dong, X.Q., W.H. Yu, Y.Y. Hu, Z.Y. Zhang, and M. Huang. 2011. Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflammation Research 60: 533–539.
Zheng, P., F.L. Niu, W.Z. Liu, Y. Shi, and L.G. Lu. 2005. Anti-inflammatory mechanism of oxymatrine in dextran sulfate sodium-induced colitis of rats. World Journal of Gastroenterology 11: 4912–4915.
Zhang, Z.Q., Y.Q. Wang, M. Dong, J.C. Cui, D.Q. Rong, and Q. Dong. 2012. Oxymatrine ameliorates l-arginine-induced acute pancreatitis in rats. Inflammation 35: 605–613.
Li D, Fu Y, Zhang W, Su G, Liu B, Guo M, Li F, Liang D, Liu Z, Zhang X, et al. 2012. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflammation Research.
Bannerman, D.D., A. Chockalingam, M.J. Paape, and J.C. Hope. 2005. The bovine innate immune response during experimentally-induced Pseudomonas aeruginosa mastitis. Veterinary Immunology and Immunopathology 107: 201–215.
Wang, Y., D.S. Zarlenga, M.J. Paape, and G.E. Dahl. 2002. Recombinant bovine soluble cd14 sensitizes the mammary gland to lipopolysaccharide. Veterinary Immunology and Immunopathology 86: 115–124.
Lee, J.W., M.J. Paape, T.H. Elsasser, and X. Zhao. 2003. Elevated milk soluble CD14 in bovine mammary glands challenged with Escherichia coli lipopolysaccharide. Journal of Dairy Science 86: 2382–2389.
Lu, L.G., M.D. Zeng, Y.M. Mao, J.Q. Li, M.B. Wan, C.Z. Li, C.W. Chen, Q.C. Fu, J.Y. Wang, W.M. She, et al. 2003. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World Journal of Gastroenterology 9: 2480–2483.
Xiang, X.X., G.J. Wang, X. Cai, and Y.L. Li. 2002. Effect of oxymatrine on murine fulminant hepatitis and hepatocyte apoptosis. Chinese Medical Journal 115: 593–596.
Hong-Li, S., L. Lei, S. Lei, Z. Dan, D. De-Li, Q. Guo-Fen, L. Yan, C. Wen-Feng, and Y. Bao-Feng. 2008. Cardioprotective effects and underlying mechanisms of oxymatrine against ischemic myocardial injuries of rats. Phytotherapy Research 22: 985–989.
Zhao, J.P., S.J. Yu, L.Q. Tong, F. Zhang, X. Jiang, S. Pan, H.C. Jiang, and X.Y. Sun. 2008. Oxymatrine attenuates intestinal ischemia/reperfusion injury in rats. Surgery Today 38: 931–937.
Liu, Y., X.J. Zhang, C.H. Yang, and H.G. Fan. 2009. Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-kappa B expression. Brain Research 1268: 174–180.
Li, D., Y. Fu, W. Zhang, G. Su, B. Liu, M. Guo, F. Li, D. Liang, Z. Liu, X. Zhang, et al. 2013. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflammation Research 62: 9–15.
Li, D., N. Zhang, Y. Cao, W. Zhang, G. Su, Y. Sun, Z. Liu, F. Li, D. Liang, B. Liu, et al. 2013. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-kappaB and MAPKs signal pathways. European Journal of Pharmacology 705: 79–85.
Fu Y, Wei Z, Zhou E, Zhang N, Yang Z. 2014. Cyanidin-3-O-beta-glucoside inhibits lipopolysaccharide-induced inflammatory response in mouse mastitis model. Journal of Lipid Research.
Fu Y, Zhou E, Wei Z, Liang D, Wang W, Wang T, Guo M, Zhang N, Yang Z. 2014. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and mouse mastitis model. FEBS Journal.
Fu, Y., R. Gao, Y. Cao, M. Guo, Z. Wei, E. Zhou, Y. Li, M. Yao, Z. Yang, and N. Zhang. 2014. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-kappaB signaling pathway in lipopolysaccharide-induced mastitis in mice. International Immunopharmacology 20: 54–58.
Mehrzad, J., L. Duchateau, and C. Burvenich. 2004. Viability of milk neutrophils and severity of bovine coliform mastitis. Journal of Dairy Science 87: 4150–4162.
Prin-Mathieu, C., Y. Le Roux, G.C. Faure, F. Laurent, M.C. Bene, and F. Moussaoui. 2002. Enzymatic activities of bovine peripheral blood leukocytes and milk polymorphonuclear neutrophils during intramammary inflammation caused by lipopolysaccharide. Clinical and Diagnostic Laboratory Immunology 9: 812–817.
Miao, J.F., Y.M. Zhu, B.B. Gu, X.B. Wang, S.X. Zou, and Y.E. Deng. 2007. Evaluation of the changes of immune cells during lipopolysaccharide-induced mastitis in rats. Cytokine 40: 135–143.
Alluwaimi, A.M. 2004. The cytokines of bovine mammary gland: prospects for diagnosis and therapy. Research in Veterinary Science 77: 211–222.
Waller, K.P., I.G. Colditz, S. Lun, and K. Ostensson. 2003. Cytokines in mammary lymph and milk during endotoxin-induced bovine mastitis. Research in Veterinary Science 74: 31–36.
Peli, A., A. Scagliarini, D. Britti, and A. Boari. 2003. Detection of proinflammatory and regulatory cytokines in bovine milk using RT-PCR. Veterinary Research Communications 27: 779–781.
Collins, T., M.A. Read, A.S. Neish, M.Z. Whitley, D. Thanos, and T. Maniatis. 1995. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB Journal 9: 899–909.
Viatour, P., S. Legrand-Poels, C. van Lint, M. Warnier, M.P. Merville, J. Gielen, J. Piette, V. Bours, and A. Chariot. 2003. Cytoplasmic I kappa B alpha increases NF-kappa B-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. Journal of Biological Chemistry 278: 46541–46548.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 31272622, 31201926).
Conflict of Interests
The authors declare that they have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Z., Yin, R., Cong, Y. et al. Oxymatrine Lightened the Inflammatory Response of LPS-Induced Mastitis in Mice Through Affecting NF-κB and MAPKs Signaling Pathways. Inflammation 37, 2047–2055 (2014). https://doi.org/10.1007/s10753-014-9937-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9937-7