Abstract
The aim of this study was to determine whether oxymatrine has a protective effect against acute pancreatitis (AP) in a rat model of l-arginine-induced AP. AP was induced by two intraperitoneal injections of l-arginine (250 mg/100 g) at a 1-h interval. Oxymatrine (50 mg/kg) was administered every 6 h after the induction of AP. Oxymatrine significantly reduced the plasma amylase, d-lactic acid and tumor necrosis factor alpha concentration, serum diamine oxidase and lipase activity, and pancreatic myeloperoxidase activity, which were increased in AP rats (P < 0.05). In addition, the pancreatic CD45 expression and the expression of claudin-1, but not zonula occludens-1 (ZO-1) and occludin, in the intestinal tissues were significantly reduced after the induction of AP. However, oxymatrine increased the expression of claudin-1 and CD45, but did not alter the expression of ZO-1 and occludin. In conclusion, our results demonstrated that oxymatrine is potentially capably of protecting against l-arginine-induced AP and attenuating AP-associated intestinal barrier injury by up-regulation of claudin-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute pancreatitis (AP), an acute inflammatory process of the pancreas, is a potentially life-threatening disease characterized by tissue edema, acinar necrosis, hemorrhage, and fat necrosis, as well as perivascular infiltration in the pancreas [1, 2]. Although there is continuing improvement in critical care, AP is still associated with substantial morbidity and mortality ranging from 15% to 40% in its severe form [3]. Infectious complication in the late phase is one of the major contributors to high the mortality in AP [4]. This complication is thought to be a result of the bacterial translocation and endotoxin translocation from the gastrointestinal tract, and intestinal barrier injury has been implicated in the mechanism [5]. The pathophysiology of AP remains poorly understood; however, it has been suggested that oxidative stress, inflammatory cytokines, and leukocytic infiltration play important roles in the pathogenesis of this disorder [6, 7]. At present, therapeutic efforts are limited to supportive treatments and aimed at ameliorating symptoms with anti-inflammatory agents, steroids, and analgesics [8, 9]. Due to the limitations of conventional therapy, various ethnobotanical agents are increasingly being pursued as alternative sources to develop novel and safe therapeutic agents to treat AP [10–12].
Oxymatrine is an alkaloid compound extracted from the root of Sophora flavescens Ait (kushen) [13]. It has been used to treat chronic hepatitis B patients in China for decades with confirmed safety [14]. Recent evidence has shown that oxymatrine possesses a wide variety of pharmacological effects, including anti-inflammatory, anti-apoptosis, anti-tumor, anti-hepatic fibrosis, and anti-arrhythmic functions [15–19]. In addition, oxymatrine has also been reported to exert a protective effect against end-organ (liver, heart, intestine, and brain) ischemia or ischemia/reperfusion injury [20–23]. To date, however, the possibility that oxymatrine may have the potential of protection against AP has not yet been clarified.
The aim of the present study, therefore, was to investigate whether oxymatrine has a protective effect against AP in a rat model of l-arginine-induced AP. Furthermore, we also examined the pancreatic CD45 expression and the expression of claudin-1, zonula occludens-1 (ZO-1), and occludin, as known components of tight junctions in the intestine, in an effort to elucidate the possible mechanism by which oxymatrine protects against AP.
MATERIALS AND METHODS
Animals
Adult Wistar rats weighting from 250 to 280 g were purchased from the Experimental Animal Centre of China Medical University. They were housed in plastic cages containing wood shaving and maintained in a room with a 12-h light cycle with free access to food and water. The animal study protocol was approved by the Ethics Committee for Animal Experiments of China Medical University.
Experimental Protocol
The following three groups of animals, each comprising of eight rats, are involved. Experimental AP was induced by two intraperitoneal (i.p.) injections of 250 mg/100 g body weight of l-arginine as a 20% solution in 0.15 M physiological saline, at an interval of 1 h (AP group), as previously described [24–26]. The control rats received an equal volume of 0.15 M physiological saline at the same times (control group). Rats in which AP was induced by the administration of l-arginine received i.p. injections of 50 mg/kg oxymatrine every 6 h after the induction of AP (oxymatrine group). After blood sampling, all rats were killed at 24 h after the second l-arginine or physiological saline injection. The pancreas and 5 cm of terminal ileums were removed and washed with phosphate-buffered saline (PBS) solution and immersed in liquid nitrogen until use. Blood samples were anticoagulated with heparin and centrifuged at 3,000×g for 10 min at 4°C, and the plasma was kept at −20°C until measurements.
Measurements of Plasma Amylase, d-Lactic Acid and TNF-α Concentration, Serum DAO and Lipase Activity, and Pancreatic MPO Activity
Plasma amylase was determined by using an amylase detection kit (Jiancheng Bioengineering Co. Ltd., Nanjing, China). The concentrations of amylase were expressed as units per liter. Plasma level of D-lactic acid was measured using a commercially available kit (Megazyme, Wicklow, Ireland). The concentration of tumor necrosis factor alpha (TNF-α) was also measured with a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) in accordance with the instructions of the manufacturer, and was detected spectrophotometrically at 450 nm with a correction wavelength set at 570 nm on a microplate reader. Plasma lipase activity was also determined using a commercial kit (Bioassay). These measurements were performed according to the respective manufacturer’s instructions. Plasma diamine oxidase (DAO) activity was assayed according to a colorimetric method described by Nobumichi et al. [27]. Pancreatic myeloperoxidase (MPO) activity, as a marker of tissue leukocyte infiltration, was assessed using the method of Kuebler et al. [28].
Morphological Examination
The pancreas and distal ileum tissues were harvested for morphological examinations. The 10% buffered formalin-fixed sample was embedded in paraffin, sectioned at 5-μm thickness with a microtome, and stained with hematoxylin and eosin (H&E) for light microscopic examination. Villous height (from the tip of the villus to the villus–crypt junction) was measured at three different sites in each rat.
Immunohistochemistry
Intestinal and pancreas tissues were fixed in 10% buffered formalin and processed for embedding in paraffin. After paraffin-embedded blocks had been cut into 5-μm sections and mounted onto slides, the sections were pretreated at 60°C for 1 h, then dewaxed in xylene, hydrated, and washed in 0.01 mol/L citrate buffer (pH 6.0). Following inhibition of endogenous peroxidase by 3% H2O2 in methanol, the sections were incubated with rabbit anti-CLDN1, anti-ZO-1, anti-OCL, or anti-CD45 polyclonal antibodies (all from Biosynthesis Biotechnology Co., Ltd, Beijing, China, 1:100 dilution) overnight at 4°C. After thoroughly washed with PBS solution, the corresponding secondary antibodies were applied and incubated at room temperature for 30 min. Reaction products were visualized by incubation with 3,3′-diaminobenzidine and then counterstained with hematoxylin. Negative controls were achieved by skipping the primary antibodies.
Quantitative Real-Time RT-PCR
Total RNA was isolated from intestinal tissues using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The concentration and purity of the RNA in each sample was determined using a spectrophotometer at 260 and 280 nm. Complementary DNA was synthesized from 1 μg of total RNA using a PrimeScript RT reagent kit (Tiangen Biotechnology Co., Ltd, Beijing, China). Quantitative real-time RT-PCR was performed using SYBR Green (Tiangen Biotechnology Co., Ltd, Beijing, China) on an Exicycler™ 96 real-time quantitative thermal block (Bioneer, Daejeon, Korea). The PCR primer sequences were designed according to the rat claudin-1, ZO-1, occludin, and β-actin gene sequences reported in GenBank and were chemically synthesized: claudin-1, forward 5′-GTGCATGAGGTGCTTAGAAG-3′ and reverse 5′-CACGTAGTCTTTCCCAGTAG-3′; ZO-1, forward 5′-GAAGGCTCATAGTTCCACAC-3′ and reverse 5′-GAGGATGCTATTGTCTCTGC-3′; occludin, forward 5′-GGACTGGCTCAGGGAATATC-3′ and reverse 5′-GCAACCAGCATCTGTCTAGG-3′; and β-actin, forward 5′-ACGTTGACATCCGTAAAGAC-3′ and reverse 5′-GAAGGTGGACAGTGAGGC-3′. The specificity of the amplified products was analyzed through dissociation curves generated by the equipment yielding single peaks. β-Actin was used as an internal control to normalize samples. PCR reactions of each sample were conducted in triplicate. Data were analyzed through the comparative threshold cycle (CT) method.
Western Blot Analysis
Total proteins were extracted from frozen intestinal tissues using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China), and protein concentrations were determined using a bovine serum albumin standard line. Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then electrotransferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% skim milk at room temperature for 2 h and then incubated overnight (4°C) with rabbit anti-claudin-1, anti-ZO-1, or anti-occludin polyclonal antibodies (all from Biosynthesis Biotechnology Co., Ltd, Beijing, China, 1:500 dilution), followed by horseradish peroxidase-conjugated secondary antibodies. Protein bands were visualized with ECL plus chemiluminescence kit (Millipore, Bedford, MA, USA).
Statistical Analysis
All data were expressed as means±SEM, and raw data were analyzed by the unpaired Student’s t test using SPSS 13.0 software. A P < 0.05 was considered to be statistically significant.
RESULTS
Plasma Amylase, D-Lactic Acid and TNF-α Concentration, Serum DAO and Lipase Activity, and Pancreatic MPO Activity
Plasma amylase levels, a specific marker for AP, were measured to confirm the successful induction of AP in rat models. As shown in Table 1, compared with the control group, the concentration of plasma amylase was significantly elevated in the AP group; however, this elevation was markedly reduced by oxymatrine treatment. In addition, after induction of AP, the d-lactic acid, TNF-α concentration, serum DAO and lipase activity, and pancreatic MPO activity in the AP group were significantly higher than those in the control group. Compared with the AP group, these levels significantly decreased in the oxymatrine group (Table 1, all P < 0.05).
Histopathology and Morphometry of the Pancreas and the Small Intestine
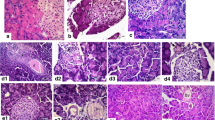
In the control rats, the histological features of the pancreas were typical of a normal architecture (Fig. 1a). Histological examination of pancreatic sections from AP rats revealed a tissue damage characterized by edema, inflammatory cell infiltrates, and acinar cell necrosis (Fig. 1b). Treatment of AP with oxymatrine resulted in a significant amelioration of pancreatic injury (Fig. 1c). The ileal villous heights of the AP rats were significantly shorter compared with those of the control and the oxymatrine-treated rats, demonstrating the injurious effects of AP and protective effects of oxymatrine on the intestinal mucosa (Fig. 1d–g).
Morphological changes of the pancreas and the small intestine stained with H&E. No histological alteration was observed in the pancreas collected from the control group (a). Histological examination (at 24 h after AP) of pancreatic sections from pancreatitic rats revealed edema and acinar cell necrosis, as well as inflammatory cells infiltration, and an important alteration of the pancreas was also present (b). A significant less histological alteration of the pancreas tissue was observed in pancreatitic rats, which received oxymatrine treatment (c). Representative H&E-stained section of the intestine was examined by light microscopy in the control rats (d), in the pancreatitic rats (e), and in the pancreatitic rats that received oxymatrine treatment (f). Original magnification, × 400. g Villous height was measured in different groups. *P < 0.05 vs the control group and # P < 0.05 vs the AP group.
Effects of Oxymatrine on Pancreatic CD45 Expression
We examined the expression of CD45 in pancreas by immunohistochemical staining. The photomicrographs of the immunohistochemical localization of CD45 in the pancreas are shown in Fig. 2. In the control pancreases, immunohistochemical expression of CD45 was prominent in acini and isolated acinar cells (Fig. 2a). The rats in the AP group exhibited a remarkable decrease in CD45 expression (Fig. 2b). However, oxymatrine treatment significantly reversed this change in pancreas (Fig. 2c).
Effects of Oxymatrine on the Expression of Tight Junction Associated Proteins in the Intestine
We examined ileal mucosa levels of claudin-1, ZO-1, and occludin to determine whether there were differences among groups with respect to expression of tight junction associated proteins. The photomicrographs of immunohistochemical localization of claudin-1 in ileum are illustrated in Fig. 3. Immunostaining of claudin-1 was mostly found in the membrane of epithelial cells along the entire length of the plasma membrane, and diffuse cytoplasmic staining was rarely observed. There was an apparent reduction in claudin-1 expression in tissues from AP group rats. However, after oxymatrine treatment, claudin-1 immunostaining was markedly increased in the intestine. The cellular localization of ZO-1 and occludin proteins had the same appearance as that of claudin-1. There was no significant difference in ZO-1 and occludin expression in ileum among the three groups (data not shown).
The expression of claudin-1, zonula occludens-1 (ZO-1) and occludin in intestinal tissues. (a) Immunohistochemical staining of claudin-1 intestinal tissues from the control group (a), the AP group (b), and the oxymatrine group (c) at 24 h after the animal model was established. Quantitative real-time RT-PCR analysis mRNA levels of claudin-1 in different groups. *P < 0.05 vs the control group and # P < 0.05 vs the AP group (d). Western blot analysis of protein levels of claudin-1 in different groups (e). Representative blots are shown, and protein size is expressed in kDa.
In order to further determine the expression of claudin-1, ZO-1 and occludin in ileal mucosa, messenger RNA (mRNA) and protein levels of these genes were measured by quantitative real-time RT-PCR and Western blot analysis, respectively. The mRNA level of claudin-1 in the intestine was significantly decreased at 24 h after the induction of AP. However, rat receiving oxymatrine demonstrated a significant elevation of claudin-1 mRNA level in the intestine. There were no significant differences of ZO-1 and occludin mRNA levels in the intestine among the three groups (Fig. 3d). Meanwhile, changes observed by Western blot analysis were in accordance with the findings in the quantitative real-time RT-PCR study (Fig. 3e).
DISCUSSION
Elevated serum amylase activity is a good marker for AP [25]. In the present study, the plasma amylase activity in the AP group was significantly higher than in the control group, indicating the successful induction of AP rat model. However, the plasma amylase activity was markedly decreased after oxymatrine treatment, suggesting a protective effect of oxymatrine against AP. It has been suggested that the activity of DAO is closely associated with villi height, nucleic acid, and protein synthesis of intestinal mucosal cells, and the high activity of plasma DAO indicates the impairment degree of the intestine [29, 30]. In the present study, we observed that the level of plasma DAO was increased markedly, and this elevation indicated that the intestine mucosal barrier function was damaged in AP rats. Furthermore, oxymatrine treatment significantly diminished the increase in plasma DAO activity in AP rats. However, the level of plasma DAO in oxymatrine-treated rats was still higher than in the control rats, suggesting that even if treatment of oxymatrine could lower plasma DAO level, it could not reverse the trend of mucosa injury.
Accumulating evidence indicates that activated pancreatic macrophages release proinflammatory cytokines, such as interleukin (IL)-1, IL-6, and TNF-α in response to local tissue damage in different AP animal models. These cytokines may act not only locally to aggravate AP but also both locally and systemically to increase the capillary permeability and promote leukocyte adherence and extravasation [31, 32]. In the present study, we consistently found that TNF-α plasma level was elevated following AP induction. Various published data have demonstrated that oxymatrine suppresses TNF-α in rats with traumatic brain injury, dextran sulfate sodium-induced colitis, and intestinal I/R injury [19, 22, 33]. In addition, oxymatrine has been reported to inhibit the quartz-induced secretion of TNF-α by pulmonary alveolar macrophages, thereby antagonizing the damage effect of quartz on the membrane of alveolar macrophages [34]. However, despite the anti-inflammatory effect of oxymatrine, there have been no previous reports linking oxymatrine with decreased inflammatory cytokines in AP model. We investigated this possibility in the present study and have demonstrated that oxymatrine decreased TNF-α in rats with l-arginine-induced AP. CD45, formerly called leukocyte-common antigen, is an abundant transmembrane glycoprotein expressed on the surface of all nucleated hematopoietic cells and their precursors. Traditionally, it has been identified as a leukocyte-specific receptor-like protein tyrosine phosphatase that plays an important role in regulating immune responses [35]. De Dios et al. have reported the down-regulation of CD45 in acinar cells during AP at both mRNA and protein level [36]. In the present study, we consistently observed the down-regulated pancreatic expression of CD45 in the AP rats, as evidenced by the immunohistochemical staining. However, this change was attenuated by oxymatrine treatment. Collectively, our data suggest that the protective effect of oxymatrine against AP may be mediated, at least in part, by the elevation of CD45 and the inhibition of inflammatory cytokines.
The intestinal epithelium forms a relatively impermeable barrier between the lumen and the submucosa. The function of this barrier is to prevent toxins in the lumen from spreading to distant tissues and organs, and the disruption of the intestinal barrier is known to be a predisposing factor for bacterial translocation during AP [37]. d-Lactate is the indigenous metabolic product of intestinal resident flora. Hence, measurement of elevated plasma d-lactate level may be useful for assessing the intestinal barrier injury [38]. In the present study, increased plasma d-lactate level was observed at 24 h after the induction of AP, indicating the intestinal barrier injury in AP rats. Furthermore, we found that oxymatrine decreased plasma d-lactate level in rats with l-arginine-induced AP, which suggests that oxymatrine may ameliorate the AP-induced intestinal barrier injury. The intestinal barrier function is maintained by a complex of proteins composing the tight junction that is located at the subapical aspect of the lateral membranes. The tight junction consists of numerous proteins, such as claudin-1, ZO-1, and occludin [39]. Previous studies have clearly demonstrated that these proteins are involved in the regulation of paracellular permeability. In this study, we found that the expression of claudin-1, but not ZO-1 and occludin, was significantly reduced in the intestinal tissues from l-arginine-induced AP rats. These results are consistent with previous findings from Yasuda et al. showing that ZO-1 and occludin were not changed but that apoptosis of intestinal epithelial cells was accelerated in an experimental AP model [40]. One of the major findings of our study is that claudin-1 was down-regulated in the intestinal tissues after 24 h induction of AP, and this reduction was reversed by oxymatrine, which suggests that down-regulation of claudin-1 is involved in the AP induced intestinal barrier injury, and oxymatrine may attenuate this barrier injury partly by up-regulation of claudin-1. However, the precise mechanism by which oxymatrine up-regulates claudin-1 in AP needs to be further studied.
In conclusion, our results demonstrated that oxymatrine is potentially capably of protecting against l-arginine-induced AP and attenuating AP-associated intestinal barrier injury by up-regulation of claudin-1. The findings of this study suggest that intervention with oxymatrine is worthy of more detailed experimental and clinical evaluation in AP.
REFERENCES
Whitcomb, D.C. 2006. Clinical practice. Acute pancreatitis. The New England Journal of Medicine 354(20): 2142–2150. doi:10.1056/NEJMcp054958.
Sand, J., and I. Nordback. 2009. Acute pancreatitis: risk of recurrence and late consequences of the disease. Nature Reviews Gastroenterology & Hepatology 6(8): 470–477. doi:10.1038/nrgastro.2009.106.
Raraty, M.G., S. Connor, D.N. Criddle, R. Sutton, and J.P. Neoptolemos. 2004. Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Current Gastroenterology Reports 6(2): 99–103.
Buter, A., C.W. Imrie, C.R. Carter, S. Evans, and C.J. McKay. 2002. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. The British Journal of Surgery 89(3): 298–302. doi:10.1046/j.0007-1323.2001.02025.x.
Zhang, X.P., J. Zhang, Q.L. Song, and H.Q. Chen. 2007. Mechanism of acute pancreatitis complicated with injury of intestinal mucosa barrier. Journal of Zhejiang University. Science B 8(12): 888–895. doi:10.1631/jzus.2007.B0888.
Bhatia, M., F.L. Wong, Y. Cao, H.Y. Lau, J. Huang, P. Puneet, and L. Chevali. 2005. Pathophysiology of acute pancreatitis. Pancreatology 5(2–3): 132–144. doi:10.1159/000085265.
Leung, P.S., and Y.C. Chan. 2009. Role of oxidative stress in pancreatic inflammation. Antioxidants & Redox Signaling 11(1): 135–165. doi:10.1089/ars.2008.2109.
Leach, S.D., F.S. Gorelick, and I.M. Modlin. 1992. New perspectives on acute pancreatitis. Scandinavian Journal of Gastroenterology Supplement 192: 29–38.
Garcia-Pagan, J.C., F. Feu, A. Castells, A. Luca, R.C. Hermida, F. Rivera, J. Bosch, and J. Rodes. 1994. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. Hepatology 19(3): 595–601.
Zeybek, N., S. Gorgulu, G. Yagci, M. Serdar, A. Simsek, N. Kaymakcioglu, S. Deveci, H. Ozcelik, and T. Tufan. 2003. The effects of gingko biloba extract (EGb 761) on experimental acute pancreatitis. The Journal of Surgical Research 115(2): 286–293.
Genovese, T., E. Mazzon, R. Di Paola, C. Muia, C. Crisafulli, M. Menegazzi, G. Malleo, H. Suzuki, and S. Cuzzocrea. 2006. Hypericum perforatum attenuates the development of cerulein-induced acute pancreatitis in mice. Shock 25(2): 161–167. doi:10.1097/01.shk.0000188326.82641.b7.
Pandol, S.J., A.K. Saluja, C.W. Imrie, and P.A. Banks. 2007. Acute pancreatitis: bench to the bedside. Gastroenterology 132(3): 1127–1151. doi:10.1053/j.gastro.2007.01.055.
Ling, J.Y., G.Y. Zhang, Z.J. Cui, and C.K. Zhang. 2007. Supercritical fluid extraction of quinolizidine alkaloids from Sophora flavescens Ait. and purification by high-speed counter-current chromatography. Journal of Chromatography A 1145(1–2): 123–127. doi:10.1016/j.chroma.2007.01.080.
Lu, L.G., M.D. Zeng, Y.M. Mao, J.Q. Li, M.B. Wan, C.Z. Li, C.W. Chen, Q.C. Fu, J.Y. Wang, W.M. She, X. Cai, J. Ye, X.Q. Zhou, H. Wang, S.M. Wu, M.F. Tang, J.S. Zhu, W.X. Chen, and H.Q. Zhang. 2003. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World Journal of Gastroenterology 9(11): 2480–2483.
Liu, J., Y. Liu, and C.D. Klaassen. 1994. The effect of Chinese hepatoprotective medicines on experimental liver injury in mice. Journal of Ethnopharmacology 42(3): 183–191.
Dong, Y., H. Xi, Y. Yu, Q. Wang, K. Jiang, and L. Li. 2002. Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the anti-hepatitis B virus mechanism of oxymatrine. Journal of Gastroenterology and Hepatology 17(12): 1299–1306.
Chen, X., R. Sun, J. Hu, Z. Mo, Z. Yang, D. Liao, and N. Zhong. 2008. Attenuation of bleomycin-induced lung fibrosis by oxymatrine is associated with regulation of fibroblast proliferation and collagen production in primary culture. Basic & Clinical Pharmacology & Toxicology 103(3): 278–286. doi:10.1111/j.1742-7843.2008.00287.x.
Zhang, Y., B. Piao, B. Hua, W. Hou, W. Xu, X. Qi, X. Zhu, Y. Pei, and H. Lin. 2010. Oxymatrine diminishes the side population and inhibits the expression of beta-catenin in MCF-7 breast cancer cells. Medical Oncology. doi:10.1007/s12032-010-9721-y.
Dong, X.Q., W.H. Yu, Y.Y. Hu, Z.Y. Zhang, and M. Huang. 2010. Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflammation Research. doi:10.1007/s00011-010-0300-7.
Jiang, H., F. Meng, J. Li, and X. Sun. 2005. Anti-apoptosis effects of oxymatrine protect the liver from warm ischemia reperfusion injury in rats. World Journal of Surgery 29(11): 1397–1401. doi:10.1007/s00268-005-7885-y.
Hong-Li, S., L. Lei, S. Lei, Z. Dan, D. De-Li, Q. Guo-Fen, L. Yan, C. Wen-Feng, and Y. Bao-Feng. 2008. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytotherapy Research 22(7): 985–989. doi:10.1002/ptr.2452.
Zhao, J., S. Yu, L. Tong, F. Zhang, X. Jiang, S. Pan, H. Jiang, and X. Sun. 2008. Oxymatrine attenuates intestinal ischemia/reperfusion injury in rats. Surgery Today 38(10): 931–937. doi:10.1007/s00595-008-3785-8.
Liu, Y., X.J. Zhang, C.H. Yang, and H.G. Fan. 2009. Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-kappaB expression. Brain Research 1268: 174–180. doi:10.1016/j.brainres.2009.02.069.
Takacs, T., L. Czako, E. Morschl, F. Laszlo, L. Tiszlavicz, Z. Rakonczay Jr., and J. Lonovics. 2002. The role of nitric oxide in edema formation in l-arginine-induced acute pancreatitis. Pancreas 25(3): 277–282.
Naito, Z., T. Ishiwata, Y.P. Lu, K. Teduka, T. Fujii, K. Kawahara, and Y. Sugisaki. 2003. Transient and ectopic expression of lumican by acinar cells in l-arginine-induced acute pancreatitis. Experimental and Molecular Pathology 74(1): 33–39.
Hegyi, P., Z. Rakonczay Jr., R. Sari, C. Gog, J. Lonovics, T. Takacs, and L. Czako. 2004. l-arginine-induced experimental pancreatitis. World Journal of Gastroenterology 10(14): 2003–2009.
Hosoda, N., M. Nishi, M. Nakagawa, Y. Hiramatsu, K. Hioki, and M. Yamamoto. 1989. Structural and functional alterations in the gut of parenterally or enterally fed rats. The Journal of Surgical Research 47(2): 129–133.
Kuebler, W.M., C. Abels, L. Schuerer, and A.E. Goetz. 1996. Measurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assay. International Journal of Microcirculation, Clinical and Experimental 16(2): 89–97.
Bieganski, T., J. Kusche, W. Lorenz, R. Hesterberg, C.D. Stahlknecht, and K.D. Feussner. 1983. Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochimica et Biophysica Acta 756(2): 196–203.
Luk, G.D., T.M. Bayless, and S.B. Baylin. 1980. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. The Journal of Clinical Investigation 66(1): 66–70. doi:10.1172/JCI109836.
Norman, J.G., G.W. Fink, W. Denham, J. Yang, G. Carter, C. Sexton, J. Falkner, W.R. Gower, and M.G. Franz. 1997. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Digestive Diseases and Sciences 42(8): 1783–1788.
Norman, J. 1998. The role of cytokines in the pathogenesis of acute pancreatitis. American Journal of Surgery 175(1): 76–83.
Zheng, P., F.L. Niu, W.Z. Liu, Y. Shi, and L.G. Lu. 2005. Anti-inflammatory mechanism of oxymatrine in dextran sulfate sodium-induced colitis of rats. World Journal of Gastroenterology 11(31): 4912–4915.
Li, S., L. Yang, J. Li, and D. Zhang. 2006. Inhibitory effect of oxymatrine on quartz-induced secretion of TNF-alpha by the pulmonary alveolar macrophages in the fibroblast proliferation. Journal of Huazhong University of Science and Technology 26(6): 644–646.
Penninger, J.M., J. Irie-Sasaki, T. Sasaki, and A.J. Oliveira-dos-Santos. 2001. CD45: new jobs for an old acquaintance. Nature Immunology 2(5): 389–396. doi:10.1038/8768787687.
de Dios, I., L. Ramudo, A.C. Garcia-Montero, and M.A. Manso. 2006. Redox-sensitive modulation of CD45 expression in pancreatic acinar cells during acute pancreatitis. The Journal of Pathology 210(2): 234–239. doi:10.1002/path.2037.
Nejdfors, P., M. Ekelund, B. Jeppsson, and B.R. Westrom. 2000. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: species- and region-related differences. Scandinavian Journal of Gastroenterology 35(5): 501–507.
Smith, S.M., R.H. Eng, and F. Buccini. 1986. Use of D-lactic acid measurements in the diagnosis of bacterial infections. The Journal of Infectious Diseases 154(4): 658–664.
Steed, E., M.S. Balda, and K. Matter. 2010. Dynamics and functions of tight junctions. Trends in Cell Biology 20(3): 142–149. doi:10.1016/j.tcb.2009.12.002.
Yasuda, T., Y. Takeyama, T. Ueda, M. Shinzeki, H. Sawa, T. Nakajima, and Y. Kuroda. 2006. Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis. The Journal of Surgical Research 135(1): 18–26. doi:10.1016/j.jss.2006.02.050.
ACKNOWLEDGMENTS
This study was supported by grants from The National Natural Science Foundation of China (grant number 30840076) and The Natural Science Foundation of Liaoning Province (grant number 20082058).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Wang, Y., Dong, M. et al. Oxymatrine Ameliorates l-Arginine-Induced Acute Pancreatitis in Rats. Inflammation 35, 605–613 (2012). https://doi.org/10.1007/s10753-011-9352-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9352-2