Abstract
River–floodplain systems are characterized by high connectivity, which favours the spread of non-native species. In floodplain, floods increase connectivity, which increases the similarity of abiotic conditions among environments. High connectivity and low environmental variability may favour the establishment of non-native species such as Limnoperna fortunei, but this has not yet been tested. We sampled L. fortunei larvae in nine connected lakes and nine isolated lakes to rivers in the upper Paraná River floodplain to evaluate how spatial (connection) and abiotic (environmental variability) factors affect the larvae density of L. fortunei. We considered the rivers as propagule source of L. fortunei because this invasive species has successfully established in rivers, but not in lakes. Our findings revealed that connected lakes had a high larval density of L. fortunei, while isolated lakes had a low density. Isolated lakes presented a high multi-environmental variability, which was strong negatively related with the larval density of L. fortunei. However, the connectivity decreased the multi-environmental variability, indirectly increasing the larval density of L. fortunei. Our study illustrates that permanent connectivity with invaded environments increase the larvae density of L. fortunei in non-invaded environments, which occurs both directly (through propagule dispersion) and indirectly (by decreasing multi-environmental variability).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-native species are a global driver of biodiversity loss and ecosystem change. A central issue to understanding species invasion is explaining why the success of the establishment of non-native species varies so dramatically in different sites (Duncan et al., 2019). Non-native species present traits that facilitate their establishment and dispersal among habitats (McMahon, 2002), promoting successful invasions (Sousa et al., 2014). Also, there are ecosystems more susceptible to invasions than others (i.e., increased invasibility), wherein biological invasion may be facilitated (Duncan et al., 2019; Redding et al., 2019). Additionally, after arriving in a new environment, non-native species deal with environmental and biological conditions that may prevent their establishment (Redding et al., 2019). Then, increasing the propagule pressure may be an efficient strategy to maintain a viable population and increase the success of biological invasions (Lockwood et al., 2005).
River–floodplain systems are complex aquatic systems composed of a myriad of different types of environments (Agostinho et al., 2004), which may be connected seasonally (few times during the year) or permanently (Junk et al., 1989; Thomaz et al., 2007). In these systems, the flood pulse is the trigger that increases the connectivity among environments, particularly between non-connected lakes and other aquatic habitat types (Elosegi et al., 2010). The increased connectivity favours organism dispersions, including invaders (Espínola et al., 2014; Meghan et al., 2018). Thus, in river–floodplain systems, the potential for the dispersion of organisms is directly related to the hydrological connectivity of environments (Warfe et al., 2013). High levels of connectivity may lead to the increased similarity of environmental conditions between habitats (i.e., environmental homogenization), which reduces the abiotic variability of each environment, favouring the establishment of non-native species (Lopes et al., 2014; Bozelli et al., 2015; Havel et al., 2015). Moreover, in permanently connected environments, regardless of the flood pulse, the non-native species dispersion occurs constantly (Pringle, 2001; Elosegi et al., 2010). Conversely, in isolated environments, the dispersion of non-native species depends on a flood pulse able to connect the environments. Therefore, the spread of non-native species may occur more easily among permanently connected environments than isolated ones.
The Limnoperna fortunei (Dunker, 1857) is a nuisance non-native species in South America (Boltovskoy et al., 2006) able to grow rapidly and tolerate different environmental conditions (Nakano et al., 2011; Bonel et al., 2013) and has caused many ecosystem impacts on newly invaded environments (Boltovskoy, 2015). The biological characteristics of adult fouling and planktonic larvae facilitate L. fortunei dispersion (Nakano et al., 2010). In the larval stage, water currents facilitate their dispersion, while during the adult stage, the traffic of colonised vessels may also facilitate dispersion (Darrigran & Damborenea, 2009). However, the larval stages are the main propagule source of this species (Oliveira et al., 2004).
In the upper Paraná River floodplain, L. fortunei is successfully established in rivers, in which the larval stages are constantly found (Ernandes-Silva et al., 2016a). Although L. fortunei has started expanding to environments adjacent to these rivers, such as lakes, they still failed to successfully establish in these environments. Furthermore, as L. fortunei dispersion depends on the water, their density is predicted to spatially differ between connected and isolated environments. Finally, L. fortunei reproduction occurs during the flood period when more larvae are found (Ernandes-Silva et al., 2016b).
In this study, we aimed to evaluate how spatial and environmental factors affect the larval density of L. fortunei in connected and isolated lakes. We considered the presence of connection with rivers (presence of canals connecting lakes to rivers) and distance from the rivers as spatial factors. We also aimed to measure the multiple single abiotic variables within each lake and calculate the coefficient of variation (CV) among these variables (multi-environmental variability) as environmental factors. We predicted that (i) permanent connection to the rivers is the main positive driver of the increase on the density of L. fortunei on the lakes; conversely, that (ii) the multi-environmental variability within each lake is the main negative driver of the decrease on the density of L. fortunei in lakes; and that (iii) such multi-environmental variability should be higher in isolated lakes because permanent connection decrease the environmental variability within environments.
Methods
Study area
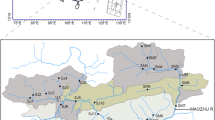
We focused on the large spatial scale and sampled 18 shallow lakes in the upper Paraná River floodplain, all of them adjacent to three rivers (Fig. 1). Nine lakes have a permanent connection with the river (which is defined by canal presence), while the other nine lakes are isolated and may temporarily become connected to the river during floods strong enough to connect them. We considered the three rivers (Paraná River, Ivinhema River, and Baía River) as propagule sources of L. fortunei to the lakes because these rivers have well-established populations of L. fortunei (see Ragonha et al., 2015; Ernandes-Silva et al., 2016a, b). The Ivinhema River has populations with lesser larvae density (mean ± SD = 499 ± 535) than those of the Baia River (4,279 ± 8,326) and the Paraná River (4,590 ± 5,862), but L. fortunei has been found in these rivers since 2000 (Ernandes-Silva et al., 2016a, b). The upper Paraná River floodplain presents a tropical and subtropical climate, with an annual average precipitation of 1,500 mm (Instituto Brasileiro de Geografia e Estatística, IBGE, 2012). This floodplain presents annual connections between rivers and adjacent isolate lakes, which occurs in the rainy season, beginning in the last quarter of the year and extending through the end of the summer (i.e., March to April). Importantly, connected lakes exchange water and materials (e.g., nutrients, suspended particles, and biota) with the river regardless of floods, while isolated lakes exchange water and material with the river during floods.
Map of the upper Parana River floodplain with the 18 sampled lakes. 1: Lake Clara (isolated); 2: Lake Pau Velho (connected); 3: Lake Garças (connected); 4: Lake Osmar (isolated); 5: Lake Leopoldo (connected); 6: Lake Preto (isolated); 7: Lake Pousada das Garças (connected); 8: Lake Porcos (connected); 9: Lake Eucalipto (isolated); 10: Lake Aurélio (isolated); 11: Lake Fechada (isolated); 12: Lake Guaraná (connected); 13: Lake Finado Raimundo (connected); 14: Lake Sumida (connected); 15: Lake Cervo (isolated); 16: Lake Capivara (isolated); 17: Lake Ventura (isolated); and 18: Lake Patos (connected). Blue and red circles represent connected and isolated lakes, respectively
Larvae sampling
Flood events are the main drivers of propagule pressure of L. fortunei (Ernandes-Silva et al., 2016b) and during the floods, the environments are connected and exchange water and materials (e.g., Thomaz et al., 2007) as well as species (e.g., Padial et al., 2014). Therefore, increasing the occurrence and density of L. fortunei larvae stages between the connected and isolated lakes during flood events. Flood events also cause homogenization of the environmental conditions in isolated and permanently connected lakes, making them similar to rivers (Thomaz et al., 2007). Additionally, after floods, the environmental variables may start to act (physiological filter) on the survival of larval stage, performing a filtering process (e.g., Rahel, 2002) in both types of lakes. Therefore, we sampled the L. fortunei larvae 2 months after the flood event finished. In each lake, 100 l of subsurface water was filtered in the central and two marginal regions of the lake using a plankton net (30-μm mesh), totalling 300 l of filtered water. These samples were preserved in 80% alcohol for a later screening of the larvae in the laboratory using an optical microscope in magnifications of five and 10 times. Larval stages were counted and measured according to the methods of Kasyanov et al. (1998).
Environmental variables
Concomitant to the larvae samples, we measured the limnological variables (water temperature, °C; dissolved oxygen, mg/l; conductivity, µS/cm; pH; turbidity, NTU) and took the water samples for analysis of the chlorophyll-a, phosphorous, and nitrogen concentrations. Limnological variables were measured using the multi-parameter equipment (HORIBA). Water samples for chlorophyll-a analysis were filtered in a glass fibre membrane (Whatman™ GF 52-C), and the pigments were extracted using acetone with later measurement using the spectrophotometer, according to the methods of Golterman et al. (1978). Total nitrogen was measured according to the persulphate method (Bergamin et al., 1978) and was analysed using a spectrophotometer in the presence of cadmium through a flow-injection system (Giné et al., 1980). Total phosphorous was measured according to the acid digestion method (Golterman et al., 1978). These variables were selected according to Cataldo et al. (2005), Oliveira et al. (2011), and Ernandes-Silva et al. (2016b), who highlighted that these variables are determinants for the distribution pattern of the L. fortunei.
Spatial connectivity and distance from the propagule source
To estimate the spatial connectivity between lakes and rivers, we used the connectivity presence or absence. Connected lakes have canals that connect them permanently with the river, while isolated lakes have no canals and only connect with the river seasonally during floods. However, some floods do not surpass the levee, and the connection between the river and the isolated lakes may not exist during these weak floods. Also, the distance may be considered a proxy of connectivity in a landscape (Kindlmann & Burel, 2008), and in the upper Paraná River floodplain, many connected lakes have long canals that separate them from rivers, while isolated lakes may be found closer to the rivers, although unconnected to them (Supplementary Material). The distance was measured in metres using high-resolution satellite imagery from Google Earth (https://earth.google.com/web/). For the connected lakes, we measured the distance of the channels that connected to the rivers, whereas for the isolated lakes, we measured the distance of the river to the lake, considering the lower altitudinal area between the lake and river.
Data analysis
We evaluated the effect of each one of the environmental variables on the density of L. fortunei; nevertheless, we know that these variables do not act in isolation on organisms, and variance among environmental variables within the environment may influence species presence and density (Thomaz et al., 2007). Thus, we also calculated the multi-environmental variability index, which was estimated by calculating the coefficient of variation (CV), the ratio between the standard deviation (SD) and the mean µ (CV = SD/µ) of all the single environmental variables measured within each lake. Before calculating the multi-environmental variability index, we standardized each of the eight single environmental variables, namely, the water temperature, dissolved oxygen, conductivity, pH, turbidity, chlorophyll-a, phosphorous, and nitrogen, using the maximum transformation as follows: f(x) = xi/maxf0(x), in which each transformed variable had a minimum value of zero and a maximum value of 1. These standardized single environmental variables were then used to calculate the CV. We made this prior transformation to put all the environmental variables on the same scale, otherwise, this would have given higher weighting to the variables with high values. For example, nitrogen had values above 2,000 μg/l−1, whereas phosphorous had values below 200 μg/l−1. The CV may reflect the average change among multiple environmental variables and, thus, has been used as a measure of environmental variability (Naeem & Li, 1997; Wang et al., 2019). We expected that the highest multi-environmental variability within lakes could have negative effects on the non-native species, and this multi-environmental variability would be higher in isolated lakes.
We applied t-tests to compare the larvae density (as a proxy of propagule pressure), distance from rivers, limnological variables (temperature, dissolved oxygen, pH, conductivity, chlorophyll-a, turbidity, total phosphorus, and total nitrogen), and multi-environmental variability between connected and isolated lakes, considering a 95% confidence interval.
To predict the larvae density of the L. fortunei across floodplain lakes as a function of connectivity and distance from the propagule source and limnological factors inherent to each lake, we fitted a piecewise structural equation model (Lefcheck, 2016). Specifically, we modelled the density of L. fortunei across 18 lakes against connectivity and distance from the rivers, individual limnological variables (temperature, dissolved oxygen, pH, conductivity, chlorophyll-a, turbidity, total phosphorus and total nitrogen), and multi-environmental variability within each lake. The model was created using a generalized linear model (GLM) for connectivity path, which was used as a presence/absence (1/0) value, and assuming a binomial error with the “logit” link function, and linear effect model (LM) for other paths. When needed, the variables were log-transformed. The structural equation model was performed using the piecewiseSEM package in the R software (Lefcheck, 2016).
We performed a full model with all predictors; i.e., connection, distance, single limnological variables, and multi-environmental proxy. We checked the multicollinearity of each component of the model by calculating the variance inflation factor (VIF) for each predictor. A VIF > 3 indicates possible collinearity. We excluded the dissolved oxygen, temperature, and total phosphorous of the full model due to collinearity. Then, we reduced the number of variables using Akaike Information Criteria corrected for small sample size (AICc) implemented in the piecewiseSEM package (Lefcheck, 2016). This allowed us to identify a minimally adequate model that predicts the spatial L. fortunei establishment. We compared the full model (including all spatial predictors, except removed variables due to collinearity) with reduced, nested models in the piecewiseSEM using their AICc values. We removed those variables that had a null effect in L. fortunei (AICfullmodel − AICfinalmodel) (Table 1). We considered ΔAICc = 2 units to differentiate the models. Note that the full and reduced final models differed in ΔAICc = 49.02 units from the full model (Table 1).
We presented the standardized coefficient for each path and estimated the indirect effects through coefficient multiplication. Due to the small sample size (n = 18), we used the bootstrap method to obtain the significance of the path coefficients in the final model (1,000 randomisation) in the lavaan package (Rosseel, 2012). However, contrary to piecewiseSEM, lavaan does not fit a generalised linear model. We compared the significance of the bootstrapped results with the significance of the maximum likelihood from piecewiseSEM (Supplementary Material) and found similar results, confirming model robustness. Model fit was evaluated using Shipley´s test of d-separation through Fisher’s C statistic (Table 1), where P > 0.05 indicates an adequate model fit. All the analyses were conducted using the R software (R Core Team, 2016).
Results
The t-test evidenced that larvae density (t = 9.17, P < 0.001), chlorophyll-a (t = − 2.55, P = 0.033), total phosphorous concentration (t = − 2.372, P = 0.045), multi-environmental variability (t = − 4.724, P = 0.001), and distance from the propagule source (t = 5.96, P < 0.001) differed significantly between connected and isolated lakes. The larvae density (propagule pressure) and distance from the propagule source were significantly higher in connected than isolated lakes (Fig. 2A, E), while chlorophyll-a, phosphorous level, and multi-environmental variability were higher in isolated lakes (Fig. 2B–D). Other environmental variables did not differ significantly between the connected and isolated lakes (Supplementary Material), but it was possible to observe a higher environmental variability in the isolated lakes.
The best and most parsimonious model for the density of the L. fortunei across floodplain lakes (i.e., selected by AICc) was the model with connectivity, distance from the propagule source, and multi-environmental variability (Table 1). These three factors explained more than 86% of the variance in the larval density of L. fortunei (Fig. 3). The structural equation model (SEM) fitted data well (Supplementary Material), and revealed that connectivity with propagule source was the most important direct positive predictor to the larval density of the L. fortunei (β = 0.706), whereas multi-environmental variability was the main direct negative predictor (β = − 0.502) (Fig. 3). Additionally, the SEM revealed that spatial connectivity increased the density of the L. fortunei by decreasing the multi-environmental variability (r = 0.235), and distance from the propagule source increased the density of the L. fortunei by increasing the spatial connectivity (r = 0.677) (Fig. 3).
Structural equation model of the golden mussel density in functions of best predictor according to the Akaike Information Criteria corrected for small sample size (AICc), which were the presence of connection of lakes with invaded rivers, distance from lakes to rivers (m), and multi-environmental variability. Solid black and red arrows indicate significant positive and negative paths, respectively (P ≤ 0.05 piecewiseSEM). Arrows for non-significant paths (P ≥ 0.05 piecewiseSEM) are in light grey. The thickness of the significant paths represents the magnitude of the standardized regression coefficient or effect sizes, given on the arrows. The asterisk represents the P significance (*P < 0.05, **P 0.01, ***P < 0.001). The R2 for the component model is given on the boxes
Discussion
Understanding how and why the distribution of non-native species varies across spatial scales has been a central topic in ecology over the past decades (Elton, 1958). Emergent tourism and global commerce have consistently increased the transport of new non-native species across the globe (Ricciardi et al., 2017), threatening global biodiversity. By analysing the spatial distribution of one of the most obnoxious non-native species in tropical floodplains, we found that the permanent connectivity with invaded rivers has increased the density of L. fortunei in connected lakes. Conversely, we found that L. fortunei had low density in isolated lakes, which our results have suggested to be due to the high multi-environmental variability within these lakes that had a strong negative effect on the density of L. fortunei.
Effects of connectivity on the spatial establishment of L. fortunei
The connectivity among habitats facilitates the exchange of materials and the species movement across the landscape (Elosegi et al., 2010). Our results have demonstrated that connectivity may also favour the dispersal of non-native species. Thus, connectivity increases the propagule pressure of L. fortunei in lakes permanently connected with rivers, which was confirmed because the density of L. fortunei was higher in connected than in isolated lakes (Fig. 2A). Furthermore, connectivity leads to the homogenisation of the environmental conditions in the lakes because the multi-environmental variability decreased as the connectivity increased. Indeed, in the study site, connectivity increased the similarity between environments in terms of the environmental conditions (Thomaz et al., 2007), and as L. fortunei is established in rivers, this could facilitate their establishment also in lakes. Isolated lakes are those that connect to rivers only during high water periods, while connected lakes have canals that connect them permanently to the rivers (Agostinho et al., 2007). Although L. fortunei may reach both types of lakes (isolated and connected) (Ernandes-Silva et al., 2016b), our results have illustrated that their density is higher in connected lakes because propagule pressure is higher and environmental variability is lower in these lakes. Additionally, although not measured in this study, the intensity of repeated introduction events overtime could likely be much higher in permanently connected lakes, which favour L. fortunei reach in these lakes, increasing their density. Such constant arrivals of L. fortunei propagule likely increases their density in these connected lakes.
Effects of environmental filtering on the spatial establishment of L. fortunei
Invasibility varies spatially (Melbourne et al., 2007; Duncan et al., 2019), and propagule pressure is a key factor to the successful establishment of invasive species (Von Holle & Simberloff, 2005; Catford et al., 2012). Propagule pressure may overcome environmental filters, increasing the density of non-native species (Simberloff, 2009; Miller et al., 2014). However, in our study, the overcoming of environmental filters did not occur in isolated lakes, wherein larvae densities were much lower than those found in connected lakes. Importantly, L. fortunei was introduced in the upper Paraná River floodplain around 2000 and became established in rivers (Ernandes-Silva et al., 2016b), but has failed to establish on any floodplain lakes, mainly those isolated where the density of the species was much lower. Our analysis has illustrated that the possible explanation for the low density of L. fortunei larvae in isolated lakes is the great environmental variability within these lakes, which had a strong negative effect on the density of L. fortunei (Oliveira et al., 2011). Isolated lakes presented a high multi-environmental variability, with lower dissolved oxygen, higher turbidity, and higher nutrient levels than those of connected lakes. L. fortunei is tolerant to extreme environmental conditions such as low oxygen, low pH, and high temperature conditions (Karatayev et al., 2007), however, extreme environmental oscillations such as oxygen depletion, high turbidity and temperature may promote the mass mortality of L. fortunei (McDowell & Sousa, 2019), which are frequently observed in isolated lakes.
Isolated lakes in the upper Paraná River floodplain seasonally face great disturbances, mainly related to droughts, which have been more frequent due to the damming of the Paraná River upstream of the floodplain area (Moi et al., 2020). Furthermore, damming reduces the connectivity between rivers and adjacent isolated lakes (Agostinho et al., 2004), disfavouring the L. fortunei dispersion across these lakes. As L. fortunei has a high tolerance and no environmental variable has significantly affected its density, this result suggests that abiotic factors alone could not prevent the persistence of the L. fortunei. However, other factors that were not measured here, such as competition with native species, predation, and extreme droughts might be acting synergistically with abiotic factors and, thus, preventing L. fortunei establishment in isolated lakes. During larval development, L. fortunei normally presents high mortality rates, which increase in highly variable environmental conditions (Boltovskoy, 2015), associated with low inputs of new individuals due to limited dispersion that are probably primary factors for the low density of L. fortunei in the isolated lakes.
Relationship between distance from and connectivity with the propagule source
The relationship between the distance from and connectivity with the propagule source are other key factors that influence the establishment of non-native species (King & Howeth, 2019). Contrary to the expected negative relationship between distance and connectivity, we observed that connected lakes were more distant from the rivers than isolated lakes. Such pattern occurs because some connected lakes have long canals that separate them from the rivers, while some isolated lakes are closer to the rivers, but only connect to them during overbank floods. Most importantly, the strong positive relationship between distance and connectivity and the positive effect of connectivity on the density of L. fortunei demonstrate that the existence of a permanent connection is the most important factor increasing the density of L. fortunei (likely favouring the L. fortunei establishment) in the Upper Paraná River floodplain lakes, and even overcome the distance from the propagule source.
Final remarks
The higher the propagule pressure, the greater the likelihood of individuals being resistant to certain environmental conditions (Oliveira et al., 2011), which may favour the adaptation of non-native populations to new selective pressures and its establishment in new environments (Lockwood et al., 2005). Our findings suggest that the presence of permanent spatial connectivity between invaded and non-invaded habitats increases the intensity (density) of non-native propagules, which can cause L. fortunei larvae to colonise and potentially be established in connected lakes even under unfavourable environmental conditions. Moreover, flooding events in the Upper Paraná River floodplain ecosystem are vital for non-native dispersion through promotion of propagule arrivals at the isolated lakes; nevertheless, this propagule pressure caused by flooding occurs with a lower frequency than those caused by permanent spatial connectivity. This difference likely contributes to the high multi-environmental variability in the isolated lakes, which have a negative effect on the density of L. fortunei larvae. We highlight some important issues that emerged from our findings: (i) how much L. fortunei propagule pressure (via flooding) should succeed in their establishment against environmental variability in isolated lakes? (ii) extreme climates events (e.g., floods or droughts extremes) are expected to increase in tropical floodplains (IPCC, 2014); thus, how could these events favour or disfavour L. fortunei dispersion?
Our study is one of the first to integrate multiples spatial and environmental predictors that may influence the density of L. fortunei across tropical floodplains system, particularly using larvae. Our study demonstrates that connectivity is the vital factor that increases the density of the L. fortunei across the upper Paraná River floodplain lakes. We highlight that connectivity is an important characteristic that drives floodplains (Agostinho et al., 2004). However, as our results showed, connectivity also increases the propagule pressure of non-native species in environments that are permanently connected with a propagule source. Therefore, we suggest that management actions for the control of invasive species should focus on the connectivity because it could even overcome the distance from the propagule source. This finding illustrates that permanently connected environments with propagule sources are more prone to successful invasions of L. fortunei, whereas, in isolated environments, the high environmental variability in association with low propagule pressure are responsible for the lack of establishment. Therefore, we suggest that ecosystems permanently connected with invaded ecosystems should be prioritised in monitoring programmes and management plans related to the invasion.
References
Agostinho, A. A., F. M. Pelicice, A. C. Petry, L. C. Gomes & H. F. Júlio Jr, 2007. Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquatic Ecosystem Heath and Management 10:174-186.
Agostinho, A. A., S. M. Thomaz & L. C. Gomes, 2004. Threats for biodiversity in the floodplain of the Upper Paraná River: effects of hydrological regulation by dams. Ecohydrology and Hydrobiology 4:255-268.
Bergamin, H., B. F. Reis & E. A. G. Zagatto, 1978. A new device for improving sensitivity and stabilization in flow injection analysis. Analytica Chimica Acta 97:427-431.
Boltovskoy, D., N. Correa, D. Cataldo & F. Sylvester, 2006. Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Río de la Plata watershed and beyond. Biological Invasions 8:947-963.
Boltovskoy, D. 2015. Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Cham: Springer.
Bonel, N., L. C. Solari & J. Lorda, 2013. Differences in density, shell allometry and growth between two populations of Limnoperna fortunei (Mytilidae) from the Río De La Plata basin, Argentina. Malacologia 56:43-58.
Bozelli, R. L., S. M. Thomaz, A. A. Padial, P. M. Lopes & L. M. Bini, 2015. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 753:233-241.
Cataldo, D., D. Boltovskoy, J. L. Hermosa & C. Canzi, 2005. Temperature dependent larval development rates of Limnoperna fortunei (Mollusca, Bivalvia). Journal of Molluscan Studies 71:41-46.
Catford, J. A., P. A. Vesk, D. M. Richardson & P. Pysek, 2012. Quantifying levels of biological invasion: towards the objective classification of invaded and invasible ecosystems. Global Change Biology 18:44-62.
Darrigran G. & C. Damborenea, 2009. Características da espécie. In Darrigran, G. & C. Damborenea (eds), Introdução a Biologia das Invasões. O mexilhão dourado na América do Sul: biologia, impacto, prevenção e controle. Cubo editora, São Carlos: 43-60.
Duncan, R. P., P. Cassey, A. L. Pigot & T. M. Blackburn, 2019. A general model for alien species richness. Biological Invasions 21:2665-2677.
Elosegi, A., J. Díez & M. Mutz, 2010. Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiologia 657:199-215.
Elton, C., 1958. The Ecology of Invasions by Animals and Plants. Methuen, London.
Espínola, L. A., M. L. Amsler, A. R. Paira, E. E. Drago, M. C. M. Blettler & A. A. Agostinho, 2014. Effects of decadal changes in the hydrological regime of the middle reach of the Paraná River (Argentina) on fish densities. Environmental Biology of Fishes 97:757-771.
Ernandes-Silva, J., F. H. Ragonha, S. Jati & A. M. Takeda, 2016a. Limnoperna fortunei Dunker, 1857 larvae in different environments of a Neotropical floodplain: relationships of abiotic variables and phytoplankton with different stages of development. Brazilian Journal of Biology 76:154-161.
Ernandes-Silva, J., F. H. Ragonha, L. C. Rodrigues & R. P. Mormul, 2016b. Freshwater invasibility level depends on the population age structure of the invading mussel species. Biological Invasions 18:1421-1430.
Giné, M. F., F. H. Bargamin, E. A. G. Zagatto & B. F. Reis, 1980. Simultaneous determination of nitrate and nitrite by flow injection analysis. Analytica Chimica Acta 114:191-197.
Golterman, H. L., R. S. Clymo & M. A. M. Ohmstad, 1978. Methods for Physical and Chemical Analysis of Freshwater. Blackwell Scientific, Oxford.
Havel, J. E., K. E. Kovalenko, S. M. Thomaz, S. Amalfitano & L. B. Kats, 2015. Aquatic invasive species: challenges for the future. Hydrobiologia 750:147-170.
Instituto Brasileiro de Geografia e Estatística, IBGE, 2012. Manual técnico da vegetação brasileira, Vol. 1, 2ª ed. Manuais técnicos em Geociências. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro: 271p.
IPCC, 2014. In Core Writing Team, Pachauri, R. K. and L. A. Meyer (eds), Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva: 151 pp.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river–floodplain systems. Canadian Journal of Fisheries Aquatic Sciences 106:110-127.
Kasyanov, V. L., G. A. Kryuchkova, V. A. Kulikova & L. A. Medvedeva, 1998. Larvae of Marine Bivalves and Equinoderms. Smithsonian Institution Libraries, Washington, DC.
Karatayev, A. Y., D. Boltovskoy, D. K. Padilla & L. E. Burlakova, 2007. The invasive bivalves Dreissena polimorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. Journal of Shellfish Research 26:205-213.
King, G. E. & J. G. Howeth, 2019. Propagule pressure and native community interact to influence invasion success in metacommunities. Oikos 128:1549-1564.
Kindlmann, P. & F. Burel, 2008. Connectivity measures: a review. Landscape Ecology 23:879-890.
Lefcheck, J. S., 2016. PiecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution 7:573-579.
Lockwood, J. L., P. Cassey & T. Blackburn, 2005. The role of propagule pressure in explaining species invasions. Trends in Ecology Evolution 20:223-228.
Lopes, P. M., L. M. Bini, S. A. J. Declerck, V. F. Farjalla, L. C. G. Vieira, C. C. Bonecker, F. A. Lansac-Toha, F. A. Esteves & R. L. Bozelli, 2014. Correlates of zooplankton beta diversity in tropical lake systems. PLoS ONE 9: e109581.
McDowell, W. G. & R. Sousa, 2019. Mass mortality events of invasive freshwater bivalves: current understanding and potential directions for future research. Frontiers in Ecology and Evolution 7:1-12.
McMahon, R. F., 2002. Evolutionary and physiological adaptations of aquatic invasive animals: r selection versus resistance. Canadian Journal of Fisheries and Aquatic Sciences 59:1235-1244.
Meghan, J., S. Thomason, C. D. McCort, M. D. Netherland & B. J. Grewell, 2018. Temporal and nonlinear dispersal patterns of Ludwigia hexapetala in a regulated river. Wetlands Ecology and Management 26:751-762.
Melbourne, B. A., H. V. Cornell, K. F. Davies, C. J. Dugaw, S. Elmendorf, A. L. Freestone, R. J. Hall, S. Harrison, A. Hastings, M. Holland, M. Holyoak, J. Lambrinos, K. Moore & H. Yokomizo, 2007. Invasion in a heterogeneous world: resistance, coexistence or hostile takeover? Ecology Letters 10:77-94.
Miller, A. L., J. M. Diez, J. J. Sullivan, S. R. Wangen, S. K. Wiser, R. Meffin & R. P. Duncan, 2014. Quantifying invasion resistance: the use of recruitment functions to control for propagule pressure. Ecology 95:920-929.
Moi, D. A., J. Silva-Ernandes, M. T. Baumgarthner & R. P. Mormul, 2020. The effects of river-level oscillations on the macroinvertebrates community in a river–floodplain system. Limnology 21:219-232.
Naeem, S. & S. Li, 1997. Biodiversity enhances ecosystem reliability. Nature 390:507-509.
Nakano, D., T. Kobayashi & I. Sakaguchi, 2010. Differences in larval dynamics of golden mussel Limnoperna fortunei between dam reservoirs with and without an aeration system. Landscape and Ecological Engineering 6:53-60.
Nakano, D., T. Kobayashi, N. Endo & I. Sakaguchi, 2011. Growth rate and settlement of Limnoperna fortunei in a temperate reservoir. Journal of Molluscan Studies 77: 142-148.
Oliveira, M. D., L. A. Pellegrin, R. R. Barreto, C. L. Santos & I. G. Xavier, 2004. Área de Ocorrência do Mexilhão Dourado na Bacia do Alto Paraguai entre os anos de 1998 e 2004. Embrapa Pantanal-Corumbá: 19 p (Documentos/Embrapa Pantanal, ISSN 1517–1973; 64).
Oliveira, M. D., D. F. Calheiros, C. M. Jacobi & S. K. Hamilton, 2011. Abiotic factors controlling the establishment and abundance of the invasive golden mussel Limnoperna fortunei. Biological Invasions 13:717-729.
Padial, A. A., F. Ceschin, S. A. J. Declerck, L. De Meester, C. C. Bonecker, F. A. Lansac-Tôha, L. Rodrigues, L. C. Rodrigues, S. Train, L. F. M. Velho & L. M. Bini, 2014. Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE 9(10): e111227.
Pringle, C. M., 2001. Hydrologic connectivity and the management of biological reserves: a global perspective. Ecological Applications 11:981–98.
R Core Team, 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [available on internet at https://www.R-project.org/]. Accessed 20 August 2020.
Ragonha, F. H., R. P. Tramonte & A. M. Takeda, 2015. Colonizing behavior of Limnoperna fortunei druses on the Corbicula fluminea (Mollusca: Bivalvia) on his start of invasion in the Upper Paraná River floodplain. Arquivos do Mudi 19:1-5.
Rahel, F. J., 2002. Homogenization of freshwater faunas. Annual Review of Ecology and Systematics 33:291-315.
Redding, D. W., A. L. Pigot, E. E. Dyer, C. H. Sekercioğlu, S. Hark & T. M. Blackburn, 2019. Location-level processes drive the establishment of alien bird populations worldwide. Nature 571:103-106.
Ricciardi, A., T. M. Blackburn, J. T. Carlton, J. T. A. Dick, P. E. Hulme, J. C. Iacarella, J. M. Jeschke, A. M. Liebhold, J. L. Lockwood, H. J. Maclsaac, P. Pysek, D. M. Richardson, G. M. Ruiz, D. Simberloff, W. J. Sutherland, D. A. Wardle & D. C. Aldridge, 2017. Invasion science: a horizon scan of emerging challenges and opportunities. Trends in Ecology and Evolution 32:464-474.
Rosseel, Y., 2012. Lavaan: an R Package for structural equation modeling. Journal of Statistical Software 48:1-36.
Simberloff, D., 2009. The role of propagule pressure in biological invasions. Annual Review of Ecology Evolution and Systematics 40:81-102.
Sousa, R., A. Novais, R. Costa & D. L. Strayer, 2014. Invasive bivalve in fresh water: impacts from individuals to ecosystems and possible control strategies. Hydrobiologia, 735:233-251.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river–floodplain systems. Hydrobiologia 579:1-13.
Von Holle, B. & D. Simberloff, 2005. Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212-3218.
Wang, Y., M. W. Cadottem, Y. Chen, L. H. Fraser, Y. Zhang, F. Huang, S. Luo, N. Shi & M. Loreau, 2019. Global evidence of positive biodiversity effects on spatial ecosystem stability in natural grasslands. Nature Communications 10:1-9.
Warfe, D. M., N. E. Pettit, R. H. Magierowski, B. J. Pusey, P. M. Davies, M. M. Douglas & S. E. Bunn, 2013. Hydrological connectivity structures concordant plant and animal assemblages according to niche rather than dispersal processes. Freshwater Biology 58:292-305.
Acknowledgements
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES): Finance Code 001. Vanessa Ernandes Amo, Jéssica Ernandes-Silva, and Dieison André Moi are thankful for the CAPES Scholarships. Roger Paulo Mormul acknowledges the National Council for Scientific and Technological Development (CNPq) for providing continuous funding through a Scientific Productivity Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Katya E. Kovalenko, Fernando M. Pelicice, Lee B. Kats, Jonne Kotta & Sidinei M. Thomaz / Aquatic Invasive Species III
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Amo, V.E., Ernandes-Silva, J., Moi, D.A. et al. Hydrological connectivity drives the propagule pressure of Limnoperna fortunei (Dunker, 1857) in a tropical river–floodplain system. Hydrobiologia 848, 2043–2053 (2021). https://doi.org/10.1007/s10750-021-04543-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04543-8