Abstract
Classical biological control for the management of floating invasive plants has been highly successful in South Africa. However, restoring ecosystem services has been compromised by a new suite of submerged invasive plants. This study proposes that biological control of floating invasive macrophytes acts as a catalyst in a regime shift between floating and submerged invasive plant dominance. Regime shifts are large and sudden changes in the structure and functioning of ecosystems. The proposed shift is driven by the rapid decomposition of floating plants and subsequent increase in availability of nutrients and light. A mesocosm experiment explored the effect of biological control on floating Pistia stratiotes L. (Araceae) upon the growth of invasive submerged Egeria densa Planch. (Hydrocharitaceae), and native submerged plant species of the same family; Lagarosiphon major (Ridl.) Moss (Hydrocharitaceae). The results revealed a cascade effect of biological control of P. stratiotes on the availability of nitrogen, resulting in increased relative growth rates and invasive capacity for E. densa. In contrast, the native L. major could not compete with healthy or damaged P. stratiotes. These findings highlight the vulnerability of South African freshwater systems to submerged plant invasions and demonstrate the importance of a more holistic approach to invasive plant management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecosystems are defined by the interactions and internal processes that occur within them (DeAngelis & Waterhouse, 1987; Biggs et al., 2009). These processes maintain an equilibrium that can be fluid in its response to external pressures and maintain varying degrees of stability. When internal processes such as competition and nutrient cycling are altered by external pressures, such as nutrient loading or species removal, the result can be a shift from one stable state to another (Rietkerk & Van de Koppel, 1997; Schefffer & Carpenter, 2003; Suding et al., 2004; Viaroli et al., 2008; Scheffer, 2009). These regime shifts can occur even when the change in environmental conditions is relatively small, but it passes a critical threshold causing the dominant system feedbacks to weaken or break driving the trajectory of the system to change towards a new regime (Walker & Meyers, 2004; Kinzig et al., 2006; Biggs et al., 2009). New feedback mechanisms are then created and maintained, allowing the new regime to become stable (Beisner et al., 2003; Schefffer & Carpenter 2003; Folke et al., 2004; Walker & Meyers, 2004). Regime shifts have been documented in a wide range of systems, but bridging the gap between the theoretical side of regime shift research with the more applied aspects of system management and conservation is difficult and complex (Andersen et al., 2009; Conversi et al., 2015).
A widely accepted regime shift between stable states is that between floating and submerged plant dominance in freshwater lakes (Scheffer et al., 2003; Netten et al., 2010). Depending on environmental conditions, both submerged and floating macrophytes can be superior competitors for different resources. This is due to an asymmetry in competitive abilities; submerged plants can access nutrients in the sediment as well as removing nutrients from the water column, limiting the growth of floating macrophytes, whereas floating plants have more access to light and are able to shade out submerged species (Scheffer, 2009). Stable states characterized by the presence of floating invasive plants have degraded numerous freshwater ecosystems worldwide (Mitchell, 1985; Center, 1994; Gaertner et al., 2014). South Africa has been particularly hard hit, because the region’s topography and climate has resulted in very few natural lakes, resulting in a lack of evolutionary history in South Africa’s native flora for species adapted to thrive in slow moving or still waters (Basson et al., 1997). Nutrient pollution as a result of heavy urbanization (from industry, agricultural run-off, and sewage treatment), as well as previously poorly regulated water management has led to the eutrophication of the majority of these water bodies (Oberholster & Ashton, 2008; Van Ginkel, 2011). The waters are consequently heavily loaded with ammonium and nitrates, and in combination with a lack of naturally occurring native macrophytes, the freshwater ecosystems in South Africa are particularly vulnerable to invasive alien plant establishment (Hood & Naiman, 2000; Odume et al., 2016).
Dense mats of invasive floating macrophytes such as water hyacinth [Eichhornia crassipes Mart. Solms (Pontederiaceae)] and water lettuce [Pistia stratiotes L. (Araceae)] that form on the water’s surface reduce the quality of freshwater, increase the siltation of rivers, dams and wetlands, reduce biodiversity and ecosystem functioning, drown livestock and threaten irrigation canals and pumps (Janse & Van Puijenbroek, 1998; Scheffer et al., 2003; Caraco et al., 2006). Floating macrophyte invasions in South Africa have been well studied and the majority are now regarded as being under varying degrees of control through strategies including mechanical, chemical and, more successfully, the release of multiple classical biological control agents (Hill, 2003; McConnachie et al., 2004; Coetzee et al., 2011; Hill & Coetzee, 2017). However, restoration of degraded systems can be successional, but it is not always linear (Suding et al., 2004). It is important to understand the full implications of current management options for floating plants on ecosystems and what that means for the sustainability and provision of South Africa’s freshwater services. Whilst increased control of floating plants is promising both ecologically and economically, the past decade has witnessed an increase in the establishment of submerged invasive macrophytes in South Africa, with species such as Myriophyllum spicatum L. (Haloragaceae), Hydrilla verticillata (L.F.) Royle (Hydrocharitaceae) and Egeria densa Planch. (Hydrocharitaceae) recorded as established across the country (Madeira et al., 2007; Martin & Coetzee, 2011; Coetzee et al., 2011; Weyl & Coetzee, 2014).
As submerged plant invasions are often not identified until individual plants have reached the water’s surface, chances of an early response for control and management are small. Although the presence of submerged plants can initially improve water quality through increasing levels of dissolved oxygen in the water column (Brix & Shierup, 1989; Jha et al., 2015; Kelly et al., 2015), when populations explode, the dense monoculture stands have negative implications similar to those of floating invasive plants. These include damage to hydro-electrical equipment, decreased water quality and biodiversity, reduction in flow rate, limitations to water access, as well as altering nutrient regimes and sedimentation which may increase flood risk (Chen & Barko, 1988; Barko et al., 1988; Vermaat et al., 2000; Bickel & Closs, 2008; Yarrow et al., 2009; Stiers et al., 2011).
This study proposes that in the systems dominated by floating invasive plants, the application of biological control agents rapidly diminishes the dense mats, and their decay results in a sudden influx of nutrients and increased light levels in the water column (Jewell, 1971; Hill, 1979; Shilla et al., 2006; Chimney & Pietro, 2006; Longhi et al., 2008). This process, further fueled by external nutrient loading, creates a resource-rich and poorly occupied habitat that is vulnerable to plant colonization.
The fundamental processes involved in biological control result in community level trophic cascade effects, yet understanding of the long-term and wider scale consequences that this has on systems is limited (Polis et al., 2000; Carvalheiro et al., 2008; Simberloff, 2014; Nofemela, 2013; López-Núñez et al., 2017). Historically, biological control research stems from a bi-trophic perspective; exploring interactions between a target weed and its potential agent. This species-level approach to managing invasive plants may help with immediate issues related to their establishment. However, exploring multi-trophic cascade effects would paint a more holistic picture of the impacts control can have, thus saving time and resources as well as increasing system sustainability.
A major review of regime shift research identified that shifts are more likely to occur when anthropogenic pressures have reduced resilience by actions such as removing whole functional groups of species (Folke et al., 2004). The biological control programmes targeting floating invasive species in South Africa aim to do exactly that; remove a functional species. Even though the ‘function’ may not be a desired one, the ability of such species to dominate and alter their environments indicates that just as their presence has ecological impacts (Hill, 2003; Midgley et al., 2006; Téllez et al., 2008), so too will their removal. Whilst biological control has effectively reduced populations of floating invasive plants, the effect this has on the submerged plant community structure has not previously been explored. Thus, this study proposes that freshwater ecosystems may experience a regime shift, from floating invasive to submerged invasive plant dominance, driven by the application of biological control agents on the floating plants (Fig. 1).
Causal relationships between key factors and processes of the regime shift, and the internal feedback mechanisms that reinforce (R) and balance (B) the system. Created within STELLA® (iSEE systems Inc., Version 1.0.3). The strength of interactions is depicted by the thickness of the arrows connecting the variables that are positively (+) or negatively (−) related
To explore the proposed regime shift of this study, two mesocosm experiments were conducted to compare how the growth of native submerged Lagarosiphon major (Roxb.) (Ridley) Moss (Hydrocharitaceae) and invasive submerged E. densa was (independently) affected by the biological control of the floating invasive macrophyte, P. stratiotes, under a range of nitrogen concentrations and initial planting densities. Whilst the mesocosms cannot replicate the highly complex web of interactions and processes that occur within whole, natural ecosystems, the competitive interactions between the floating and submerged species are easier to interpret in an experimental setting, and findings can be used to guide understanding of observations in the field (Benton et al., 2007; Spivak et al., 2011; Stewart et al., 2013).
Methods
Mesocosm experiments were conducted inside greenhouse tunnels at the Waainek Research Facility at Rhodes University in Grahamstown, South Africa. The first experiment (P. stratiotes and E. densa) was completed between 8 April and 17 June 2015, while the second experiment (P. stratiotes and L. major) ran from 4 April to 23 May 2016. Individual P. stratiotes plants were sourced from insect-free stock plants maintained at the Waainek Research Facility. Adult Neohydronomus affinis Hustache (Coleoptera: Curculionidae), a weevil routinely used in the biological control of P. stratiotes (Cilliers, 1991), were supplied by the South African Sugarcane Research Institute (SASRI). Stock plants for each of the submerged plant species were collected 6 months before the start of their respective experiments and cultivated at the research facility. The E. densa culture was collected from the Kouga River, near Patensie, Eastern Cape, (33°44′54.6″S, 24°38′07.6″E), and the L. major culture was collected from a population in a quarry dam near Stutterheim, Eastern Cape, (32°35′14.4″S, 27°27′48.4″E). All plants were cultivated at the Waainek Research Facility where they grew in a flow through system, which comprised of eight connected tanks of spring water that passed through a UV light and filter. During cultivation, plants were grown under 80% shade cloth, with no artificial light sources (typical light readings inside the polytunnel were ~ 1000 Einstein/m2/s) and were supplied with initial optimum nutrients according to Smart et al. (1994). One month prior to both experiments, all submerged plants were treated with Malathion (Kombat), an organophosphate insecticide (according to manufacturer’s guidelines) to kill any phytophagous insects associated with the plants collected from the field. The floating plants were not subjected to this treatment so as not to harm the biocontrol agents.
Both experiments were conducted in mesocosms constructed from black plastic 75 L bins (55 cm diameter), each filled with 65 L of spring water. In the bottom of each bin, a 10 L planting container was filled with a 10 cm layer of pond sediment topped with a 2 cm layer of silica sand to prevent clouding of the water and to limit algal growth. Sediment was collected from Jameson Dam, Eastern Cape, South Africa (33°19′07.2″S, 26°26′24.0″E). Thirty apical shoots (20 cm in length) of the relevant submerged species were planted in each container, and left for 3 weeks to acclimatise and grow to a starting density of 100% cover.
There were three initial planting density treatments following the De Wit replacement series (De Wit & Van den Bergh, 1965), each a different ratio of floating to submerged plants: 90:10, 50:50 and 10:90% respectively. After the acclimation phase, either 27, 15 or 3 shoots (approximately 50 cm in length) were removed from each container to represent the 10, 50 and 90% densities of the submerged plant, respectively. To achieve the planting densities of the floating P. stratiotes (90, 50 and 10%), either nine, five or one rosette(s) each of a similar size class (12–16 cm diameter) were added to the mesocosms.
Treatments
There was a total of 24 treatment combinations arranged in a full factorial design, and replicated three times, resulting in a total of 72 mesocosms. The mesocosms were set up in a random block design. The treatment combinations consisted of the three initial planting densities, in the presence or absence of the host specific biological control agent N. affinis on P. stratiotes, growing at four initial nitrogen concentrations of the water. For the biological control treatments, two mating weevil pairs per water lettuce rosette were used to ensure plant control would be achieved (Diop et al., 2010). There were four nitrogen treatments; very low, low, high and very high (0.0, 0.1, 1.0 and 10.0 mg N/L respectively). Nitrogen was added at the start of the experiment in the form of ammonium nitrate, to help quantify the role nitrogen loading plays within the proposed regime shift. Although high levels of ammonium are documented as toxic to some submerged plants (Wang et al., 2008; Cao et al., 2009), the range of concentrations are representative of numerous eutrophic systems across South Africa (Morrison et al., 2001; Odume et al., 2016). Phosphorous (and other micronutrients) was provided to all plants at the start of the experiment as part of a standard nitrogen-free Hoagland’s solution (5.2 mg/L) (Hoagland & Arnon, 1938) which ensures sufficient micronutrients for optimal plant growth. Once the experiment was set up, fine white gauze was secured over each mesocosm to prevent N. affinis movement between containers.
Measurements
Digital thermometers (Thermochron iButtons used with Climastats Environmental Monitoring software, version 4) were placed in waterproof vials and were used to record daily temperatures throughout the experiments. Temperature was not significantly different between the two experiments and was therefore ruled out as a significant variable to explain contrasting results (F(1,33) = 0.551, P = 0.46).
Light levels (PAR) were recorded fortnightly, five centimetres below water surface in the centre of each mesocosm, using an Apogee MQ-200 Quantum Meter. pH was also measured fortnightly using a Eutech PCTEST35 multi-parameter pen. Neither light nor pH (which ranged between 7.2 and 7.4) were significantly correlated with the growth of either E. densa (light: F(1,70) = 0.658, P = 0.42; pH: F(1,70) = 1.795, P = 0.185) or L. major (light: F(1,63) = 0.103, P = 0.749, pH: F(1,53) = 0.922, P = 0.341). These variables were subsequently removed from further analysis. Soil chemistry of Jameson Dam sediment (analysed at Bemlab Laboratory, Strand, Western Cape, South Africa) can be found in Table 1. Every fortnight, dissolved oxygen (mg/L) was measured using a Sper Scientific 850045 DO pen.-Weekly ammonium [NH4+] (mg/L) and nitrate [NO3−] (mg/L) levels were measured in each mesocosm, using Vernier Software and Technology™ ion specific electrodes (ISE) and a LabQuest® 2 interface.
The shoots of the submerged plants that were removed at the beginning of each experiment (to create the three planting densities) were dried, weighed and the average shoot weight was used to calculate the remaining above-ground biomass in each mesocosm at the start of the experiments. Upon completion of the experiments, the above and below-ground biomass for the submerged plants and the floating plants were harvested, dried in drying ovens at 96°C for 72 h and weighed to calculate the relative growth rate (RGR) over the experimental period.
The RGRs of all plants were calculated using the standard formula (Evans, 1972):
where W1 and W2 (g) are the start and end dry weights respectively at t1 and t2, are the start and end time in days.
Data analyses
General linear model (GLM) full factorial analyses of variance (ANOVA), followed by Tukey HSD post hoc tests were performed to investigate interactions between initial planting densities, biological control and total aquatic nitrogen levels on RGR and plant biomass across treatments. Linear regression models tested for significant correlations between both plant biomass and growth rates of each species and all measured variables within the mesocosms. Homogeneity of slopes was analysed using an analysis of co-variance (ANCOVA), to determine interactions between P. stratiotes biomass and biological control on submerged plant biomass. All statistical analyses were conducted in the R environment (version 3.2.3; R Development Core Team, 2014; available at http://cran.r-project.org) using R Studio (version 0.98.1103).
Results
Biomass
At the end of the experiment, an increase in the biomass of P. stratiotes significantly reduced the biomass of L. major and E. densa, both in the presence and absence of biological control agents on P. stratiotes (Fig. 2). The relationships between the biomass of L. major and P. stratiotes in the presence and absence of biological control were significant (F(1,60) = 16.12, P < 0.001). However, the slopes of each relationship did not differ (F(59,60) = 0.18, P = 0.67). In contrast, the biomass of E. densa was significantly affected by the biomass of P. stratiotes (F(63,64) = 7.67, P = 0.0074), and in the presence of biocontrol agents, the slope was significantly steeper (F(1,63) = 4.18, P = 0.0449).
The relationship between the dry biomass of Pistia stratiotes and a Lagarosiphon major and b Egeria densa at the end of each experiment (submerged biomass scales differ). Each marker represents a mesocosm, in the presence (+ BC) or absence (− BC) of Neohydronomus affinis, the biological control agent of P. stratiotes. Asterisk indicates statistical significance
As levels of total nitrogen (mg N/L) in the water column increased, the final biomass of E. densa significantly increased (Fig. 3). There was no significant relationship between levels of ammonium and the biomass of E. densa (F(1,69) = 0.05, P = 0.83) although nitrate levels had a significantly positive effect (F(1,70) = 9.18, P < 0.01). In contrast there was a significant negative effect of total nitrogen on the biomass of L. major, furthermore there was a significant negative effect of ammonium (F(1,63) = 7.76, P < 0.01), and positive effect of nitrate (F(1,63) = 38.5, P < 0.00) on the native species biomass. Throughout the experiment, despite starting N levels, average ammonium levels were never recorded to be higher than 0.6 mg/L for the L. major experiment, and 0.4 mg/L for the E. densa experiment, while nitrate levels were more variable (with considerably more outliers), ranging from 0–38.7 mg/L for L. major and 0.1–38 mg/L for the E. densa experiment. Across both experiments, the biomass of P. stratiotes decreased significantly as concentrations of total nitrogen in the water increased (F(1,133) = 8.7, P < 0.01) (Fig. 4).
At the end of each experiment, the levels of dissolved oxygen (mg/L) in the water column significantly increased with higher biomass of both submerged plants, although the relationship between biomass and dissolved oxygen was much weaker for L. major (Fig. 5). The correlation differed significantly between the two species (F(2,134) = 16.18, P < 0.001), with a significantly steeper slope for E. densa than for L. major (F(1,133) = 105.81, P < 0.001). This suggests increased levels of photosynthesis for E. densa compared to L. major which makes sense as there was more biomass recorded for E. densa, therefore, more plants to do photosynthesis.
Growth rates
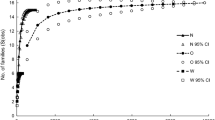
The different concentrations of nitrogen treatments of the mesocosms did not significantly affect the RGR of either submerged plant species, at any planting density, either in the presence or absence of biological control and was subsequently removed from further analyses (Table 2). The RGRs of L. major were significantly lower than those of E. densa in both the absence (F(1,64) = 14.91, P < 0.001) and presence (F(1,64) = 73.90, P < 0.001) of biocontrol agents on P. stratiotes (Fig. 6). In addition, the effect of initial planting density on RGR was significantly different between the two species (F(2,64) = 3.77, P = 0.03), with lower submerged plant densities exhibiting increased growth rates.
The mean relative growth rates (± SE, n = 3), at the three initial submerged planting densities, of a Egeria densa in the absence of biocontrol agents (Neohydronomus affinis) on Pistia stratiotes, b E. densa in the presence of biocontrol agents, c Lagarosiphon major in the absence of biocontrol agents and d L. major in the presence of biocontrol agents. The four data series represent the four initial nitrogen treatments (VL very low, L low, H high, VH very high)
There was no significant highest order interaction between biological control treatments, initial planting density and the different concentrations of nitrogen for both E. densa (F6,42 = 0.05, P = 0.77) and L. major (F6,40 = 2.04, P = 0.08). However, the biological control treatment of P. stratiotes significantly increased the RGRs of E. densa (F(2,42) = 16.46, P≤0.001), but not L. major (F(1,40) = 2.988, P = 0.09). Similarly, the initial planting density also significantly affected the RGRs of E. densa (F(2,42) = 10.98, P < 0.001), but not L. major (F(1,40) = 1.58, P = 0.23). RGRs of E. densa were significantly higher at the lowest initial submerged planting density (10%) compared to 90 and 50%.
Discussion
The presence of non-controlled P. stratiotes significantly reduced the biomass of both submerged species across all treatments. However, over time, the invasive E. densa and the native L. major each responded differently to the presence of P. stratiotes, which was subjected to biological control. The biomass results indicate that as P. stratiotes is controlled (and biomass reduced), the biomass of E. densa plants increased; supporting the theory that E. densa can capitalise on the resources made available through the biological control of P. stratiotes. The presence of controlled P. stratiotes significantly increased the RGRs of E. densa; an expected outcome as more resources (e.g., nutrients, light) were made available through the degradation of the damaged floating plants (Chimney & Pietro, 2006; Shilla et al., 2006; Longhi et al., 2008). Speculatively, these higher RGRs of E. densa (relative to L. major) may indicate a shift from floating invasive to submerged invasive plant dominance, when the floating plant community is reduced due to weevil herbivory. Within the context of these experiments, competitive ability is defined as the ability to outcompete other species under the same set of environmental conditions, using RGR as a proxy, which indicates a superior capacity to utilize available resources. For example, a strong positive association between a species’ RGR and its competitive ability and invasiveness has been reported elsewhere (Kolar & Lodge, 2001; Grotkopp et al., 2002; Dawson et al., 2011). This association suggests that trophic cascades induced by the application of classical biological control may result in increased competitive ability of submerged alien plants, compared to submerged native plants. In contrast to E. densa however, a shift between floating and submerged plant dominance did not occur between P. stratiotes and the native L. major. RGRs of L. major, even when starting at a much higher initial density than that of P. stratiotes, were below zero, demonstrating plant mortality in almost all treatments. This trend occurred despite the biological control of P. stratiotes, supporting the conclusion that L. major is a poor competitor for resources in the presence of P. stratiotes, regardless of whether P. stratiotes is subject to control or not. The decline of L. major across the variety of treatments indicates that this species will not thrive in systems that are eutrophic and light-limited. These are two key characteristics of systems dominated by floating invasive plants across South Africa, suggesting that they are simultaneously vulnerable to colonization from invasive submerged plants (such as E. densa), and less likely to promote colonization of native plants such as L. major. This study provides quantifiable evidence for the proposed regime shift between invasive floating and invasive submerged plant dominant states driven by the application of biological control of floating plant communities, and highlights the cascading effects that the biological control of floating plants can have on submerged plant communities.
Upon identifying biological control as a potential catalyst for the regime shift, it is essential to understand the underlying mechanisms driving the transition. No relationship between measured light levels and the RGR or biomass of E. densa was observed in the current study, whereas a strong correlation between biomass and nitrate levels in the water was apparent. This lack of relationship between light availability and plant growth was not surprising, as there is some evidence to suggest that the competitive advantage of E. densa increases in low-light conditions (Bini et al., 1999; Carrillo et al., 2006), and cultivations of E. densa populations for prior to the study produced more plants in better condition under 80% shade cloth compared to 40% or no shade cloth (E. Strange personal observation). These observations suggest that the observed shift in plant dominance, while potentially influenced by light availability, was driven primarily by the nutrients made available from the degradation of the floating P. stratiotes subject to weevil herbivory. This is supported by strong evidence that nutrient loading is a key factor which influences species assemblages and increases the establishment of invasive species in aquatic plant communities (Davis et al., 2000; Daehler, 2003; Tyler et al., 2007; Früh et al., 2012; Sharip et al., 2012; Uddin & Robinson, 2017).
Unexpectedly low levels of dissolved oxygen were recorded across all treatments which may be a product of the experimental time frame. Reduced levels of dissolved oxygen are often recorded below mats of floating plants due to a reduction in gas exchange between the air and water, limited photosynthetic opportunities for submerged plants and increased microbial decomposition activity (Pokorný & Rejmánková, 1983; Janes et al., 1996; Morris et al., 2004; Netten et al., 2010). Despite this, there was still a positive relationship between the growth of E. densa and dissolved oxygen levels, an overall trend that supports the assumption that as E. densa acquires more resources for growth, its photosynthetic rates increase and subsequently releases more dissolved oxygen into the water column (Cook & Urmi-König, 1984).
The results of this study not only help further the exploration of a potential regime shift occurring in South Africa, they also provide important information for the management of E. densa, an invasive species of growing global concern (Champion & Wells, 2014; Matthews et al., 2014). In this experiment, when E. densa populations started at the lowest planting density of 10% (paired with 90% P. stratiotes cover), the largest increase in submerged plant RGR was observed, a trend supported in the wider literature (Henry-Silva et al., 2008; Pistori et al., 2004). This is presumed to occur because as plant density increases, access to resources from individuals are reduced, thus following the conventions of intraspecific competition (Schoener, 1973). These results, combined with the knowledge that E. densa autofragments and reproduces vegetatively (Cabrera-Walsh et al., 2013), suggest that immediately post-establishment, this species can rapidly become problematic. Further still, the growth of E. densa was not affected by the presence of ammonium in the water column which did have a negative impact on the growth of L. major. This suggests an increased sensitivity of the native species to the degraded conditions that exist in many of South Africa’s systems (Oberholster & Ashton, 2008). Biological control can effectively reduce floating invasive plants; however, one possible outcome is that it helps pave the way for submerged invasive plant dominance. To spend time and resources effectively controlling a symptom (floating invasive plants) without addressing the problem (water quality) is inefficient for long-term sustainability. Further field testing is required to establish whether the proposed shift is occurring following successful nationwide biological control of floating plants.
Clearly there are limitations within any mesocosm experiment, and to extrapolate the findings to definitively explain observations in the field would be a great over-simplification. However, increased complexity of model systems does not always correlate with rates of success in proving bi-stability and it has been suggested that future regime shift research should primarily focus on the specific mechanisms behind switches in ecological states (Schroder et al., 2005). Manipulation experiments may be bound by spatial and temporal constraints, but small-scale experiments can be crucial to help explain large-scale patterns, and can be a powerful way to show that a system has alternate attractors (Schefffer & Carpenter 2003; Stewart et al., 2013; Fordham, 2015). This study highlights the benefits of including multi-trophic considerations for future invasive plant management, as well as research into the mechanisms of submerged plant invasions and the vulnerabilities of native macrophyte communities in South Africa, and beyond.
Conclusion
The aim of biological control initiatives against floating invasive plants has always been to reduce their populations and restore access to vital ecosystem systems such as potable water and the recovery of biodiversity (Hill & Coetzee, 2017). However, we conclude that in the presence of submerged invasive species, regime shifts from an invasive floating plant state to an invasive submerged plant state are likely, particularly when the submerged plants have high RGRs and the ability to auto-fragment, such as E. densa. The evidence presented has the potential to better inform management of South Africa’s freshwater systems and promote a more holistic approach to invasive plant management and ecosystem restoration.
References
Andersen, T., J. Carstensen, E. Hernandez-Garcia & C. M. Duarte, 2009. Ecological thresholds and regime shifts: approaches to identification. Trends in Ecology & Evolution 24: 49–57.
Barko, J. W., R. M. Smart, D. G. McFarland & R. L. Chen, 1988. Interrelationships between the growth of Hydrilla verticillata (Lf.) Royle and sediment nutrient availability. Aquatic Botany 32: 205–216.
Basson, M. S., P. H. Van Niekerk & J. A. Van Rooyen, 1997. Overview of Water Resources Availability and Utilization in South Africa. Department of Water Affairs and Forestry, Pretoria.
Beisner, B. E., D. T. Haydon & K. Cuddington, 2003. Alternative stable states in ecology. Frontiers in Ecology and the Environment 1: 376–382.
Benton, T. G., M. Solan, J. M. Travis & S. M. Sait, 2007. Microcosm experiments can inform global ecological problems. Trends in Ecology & Evolution 22: 516–521.
Bickel, T. O. & G. P. Closs, 2008. Fish distribution and diet in relation to the invasive macrophyte Lagarosiphon major in the littoral zone of Lake Dunstan, New Zealand. Ecology of Freshwater Fish 17: 10–19.
Biggs, R., S. R. Carpenter & W. A. Brock, 2009. Turning back from the brink: detecting an impending regime shift in time to avert it. PNAS 106: 826–831.
Bini, L. M., S. M. Thomaz, K. J. Murphy & A. F. M. Camargo, 1999. Aquatic macrophyte distribution in relation to water and sediment conditions in the Itaipu Reservoir, Brazil. Hydrobiologia 415: 147–154.
Brix, H. & H. H. Shierup, 1989. The use of aquatic macrophytes in water pollution control. Ambio 18: 100–107.
Cabrera-Walsh, G., Y. Magalí Dalto, F. M. Mattioli, R. I. Carruthers & L. W. Anderson, 2013. Biology and ecology of Brazilian elodea (Egeria densa) and its specific herbivore, Hydrellia sp., in Argentina. BioControl 58: 1–15.
Cao, T., P. Xie, Z. Li, L. Ni, M. Zhang & J. Xu, 2009. Physiological stress of high NH4 + concentration in water column on the submersed macrophyte Vallisneria natans L. Bulletin of Environmental Contamination and Toxicology 82: 296–299.
Caraco, N., J. Cole, S. Findlay & C. Wigand, 2006. Vascular plants as engineers of oxygen in aquatic systems. BioScience 56: 219–225.
Carrillo, Y., A. Guarín & G. Guillot, 2006. Biomass distribution, growth and decay of Egeria densa in a tropical high mountain reservoir (NEUSA, Colombia). Aquatic Botany 85: 7–15.
Carvalheiro, L. G., Y. M. Buckley, R. Ventim, S. V. Fowler & J. Memmott, 2008. Apparent competition can compromise the safety of highly specific biocontrol agents. Ecology Letters 11: 690–700.
Center, T. D., 1994. Biological control of weeds: water hyacinth [Eichhornia crassipes] and water lettuce [Pistia stratiotes]. Food and Agriculture Organization of the United Nations. http://agris.fao.org/agris-search/search.do?recordID=GB9602237 [accessed July 12 2016].
Champion, P. D., & R. D. Wells, 2014. Proactive management of aquatic weeds to protect the nationally important Northland dune lakes, New Zealand. Proceedings from: 19th Australasian Weeds Conference, 1–4 September 2014, Tasmania, Australia: 139–142.
Chen, R. L. & J. W. Barko, 1988. Effects of freshwater macrophytes on sediment chemistry. Journal of Freshwater Ecology 4: 279–289.
Chimney, M. J. & K. C. Pietro, 2006. Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecological Engineering 27: 301–321.
Cilliers, C. J., 1991. Biological control of water lettuce, Pistia stratiotes (Araceae), in South Africa. Agriculture, Ecosystems & Environment 37: 225–229.
Coetzee, J. A., M. P. Hill, M. J. Byrne & A. Bownes, 2011. A review of the biological control programmes on Eichhornia crassipes (C.Mart.) Solms (Pontederiaceae), Salvinia molesta D.S.Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoides Lam. (Azollaceae) in South Africa. African Entomology 19: 451–468.
Conversi, A., V. Dakos, A. Gårdmark, S. Ling, C. Folke, P. J. Mumby, C. Greene, M. Edwards, T. Blenckner, M. Casini & A. Pershing, 2015. A holistic view of marine regime shifts. Philosophical Transactions of the Royal Society B: Biological Science 370(1659): 20130279.
Cook, C. D. & K. Urmi-König, 1984. A revision of the genus Egeria (Hydrocharitaceae). Aquatic Botany 19: 73–96.
Daehler, C. C., 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Review of Ecology Evolution and Systematics 34: 183–211.
Davis, M. A., J. P. Grime & K. Thompson, 2000. Fluctuating resources in plant communities: a general theory of invasibility. Journal of Ecology 88: 528–534.
Dawson, W., M. Fischer & M. Van Kleunen, 2011. The maximum relative growth rate of common UK plant species is positively associated with their global invasiveness. Global Ecology and Biogeography 20: 299–306.
De Wit, C. T. & J. P. Van den Bergh, 1965. Competition between herbage plants. Netherlands Journal of Agricultural Science 13: 212–221.
DeAngelis, D. L. & J. C. Waterhouse, 1987. Equilibrium and nonequilibrium concepts in ecological models. Ecological Monographs 57: 1–21.
Diop, O., J. A. Coetzee & M. P. Hill, 2010. Impact of different densities of Neohydronomus affinis (Coleoptera: Curculionidae) on Pistia stratiotes (Araceae) under laboratory conditions. African Journal of Aquatic Science 35: 267–271.
Evans, G. C., 1972. Relative growth rate. In Anderson, D. J., P. Greig-Smith & F. A. Pitelka (eds), Studies in Ecology, Vol. 1., The quantitative analysis of plant growth University of California Press. Berkeley, California: 295–314.
Folke, C., S. Carpenter, B. Walker, M. Scheffer, T. Elmqvist, L. Gunderson & C. S. Holling, 2004. Regime shifts, resilience and biodiversity in ecological management. Annual Review of Ecology Evolution and Systematics 35: 557–581.
Fordham, D. A., 2015. Mesocosms reveal ecological surprises from climate change. PLoS Biology 13: e1002323.
FrÜh, D., S. Stoll & P. Haase, 2012. Physicochemical and morphological degradation of stream and river habitats increases invasion risk. Biological Invasions 14: 2243–2253.
Gaertner, M., R. Biggs, M. Te Beest, C. Hui, J. Molofsky & D. M. Richardson, 2014. Invasive plants as drivers of regime shifts: identifying high-priority invaders that alter feedback relationships. Diversity and Distributions 20: 733–744.
Grotkopp, E., M. Rejmanek & T. L. Rost, 2002. Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. The American Naturalist 159: 396–419.
Henry-Silva, G. G., A. F. M. Camargo & M. M. Pezzato, 2008. Growth of free-floating aquatic macrophytes in different concentrations of nutrients. Hydrobiologia 610: 153–160.
Hill, B. H., 1979. Uptake and release of nutrients by aquatic macrophytes. Aquatic Botany 7: 87–93.
Hill, M. P., 2003. The impact and control of alien aquatic vegetation in South African aquatic ecosystems. African Journal of Aquatic Science 28: 19–24.
Hill, M. P. & J. Coetzee, 2017. The biological control of aquatic weeds in South Africa: current status and future challenges. Bothalia-African Biodiversity & Conservation 47: 1–12.
Hoagland, D. R. & D. I. Arnon, 1938. Growing Plants Without Soil by the Water Culture Method. California Agricultural Experimental Station, University of California, College of Agriculture, Berkeley: 29–32.
Hood, G. H. & R. J. Naiman, 2000. Vulnerability of riparian zones to invasion by exotic vascular plants. Plant Ecology 148: 105–114.
Janes, R. A., J. W. Eaton & K. Hardwick, 1996. The effects of floating mats of Azolla filiculoides Lam. and Lemna minuta Kunth. on the growth of submerged macrophytes. Hydrobiologia 340: 23–26.
Janse, J. H. & P. J. T. M. Van Puijenbroek, 1998. Effects of eutrophication in drainage ditches. Environmental Pollution 102: 547–552.
Jewell, W. J., 1971. Aquatic weed decay: dissolved oxygen utilization and nitrogen and phosphorus regeneration. Water Pollution Control Federation 43: 1457–1467.
Jha, H. K., B. S. Singh & A. K. Varshney, 2015. Local survey of Hydrilla verticillata (L.F.) Royle: an invasive and valuable aquatic weed. The Journal of the Indian Botanical Society 94: 149–152.
Kelly, R., C. Harrod, C. A. Maggs & N. Reid, 2015. Effects of Elodea nuttallii on temperate freshwater plants, microalgae and invertebrates: small differences between invaded and uninvaded areas. Biological Invasions 17: 2123–2138.
Kinzig, A., P. Ryan, M. Etienne, H. Allison, T. Elmqvist & B. Walker, 2006. Resilience and regime shifts: assessing cascading effects. Ecology and Society 11: 20.
Kolar, C. S. & D. M. Lodge, 2001. Progress in invasion biology. Trends in Ecology & Evolution 16: 199–204.
Longhi, D., M. Bartoli & P. Viaroli, 2008. Decomposition of four macrophytes in wetland sediments: organic matter and nutrient decay and associated benthic processes. Aquatic Botany 89: 303–310.
López-Núñez, F. A., R. H. Heleno, S. Ribeiro, H. Marchante & E. Marchante, 2017. Four-trophic level food webs reveal the cascading impacts of an invasive plant targeted for biocontrol. Ecology 98: 782–793.
Madeira, P. T., J. A. Coetzee, T. D. Center, E. E. White & P. W. Tipping, 2007. The origin of Hydrilla verticillata recently discovered at a South African dam. Aquatic Botany 87: 176–180.
Martin, G. D. & J. A. Coetzee, 2011. Pet stores, aquarists and the internet trade as modes of introduction and spread of invasive macrophytes in South Africa. Water SA 37: 371–380.
Matthews, J., K. R. Koopman, R. Beringen, B. Ode, R. Pot, G. Velde, J. L. C. H. Van Valkenburg & R. S. E. W. Leuven, 2014. Knowledge Document for Risk Analysis of the Non-native Brazilian Waterweed (Egeria densa) in the Netherlands. Department of Environmental Science, Radboud University, Nijmegen.
McConnachie, A. J., M. P. Hill & M. J. Byrne, 2004. Field assessment of a frond-feeding weevil, a successful biological control agent of red water fern, Azolla filiculoides, in southern Africa. Biological Control 29: 326–331.
Midgley, J. M., M. P. Hill & M. H. Villet, 2006. The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. African Journal of Aquatic Science 31: 25–30.
Mitchell, D. S., 1985. Surface-floating aquatic macrophytes. In Denny, P. (ed.), the ecology and Management of African Wetland Vegetation, Vol. 6., Geobotany Springer, Netherlands: 109–124.
Morris, K., K. A. Harrison, P. C. E. Bailey & P. I. Boon, 2004. Domain shifts in the aquatic vegetation of shallow urban lakes: the relative roles of low light and anoxia in the catastrophic loss of the submerged angiosperm Vallisneria americana. Marine & Freshwater Research 55: 749–758.
Morrison, G., O. S. Fatoki, E. Zinn & D. Jacobsson, 2001. Sustainable development indicators for urban water systems: a case study evaluation of King William’s Town, South Africa, and the applied indicators. Water SA 27: 219–232.
Netten, J. J. C., G. H. P. Arts, R. Gylstra, E. H. Van Nes, M. Scheffer & R. M. M. Roijackers, 2010. Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii in mesocosms. Fundamental and Applied Limnology 177: 125–132.
Nofemela, R. S., 2013. The effect of obligate hyperparasitoids on biological control: differential vulnerability of primary parasitoids to hyperparasitism can mitigate trophic cascades. Biological Control 65: 218–224.
Oberholster, P. J. & P. J. Ashton, 2008. State of the Nation Report: An Overview of the Current Status of Water Quality and Eutrophication in South African Rivers and Reservoirs. Parliamentary Grant Deliverable, Council for Scientific and Industrial Research (CSIR), Pretoria.
Odume, O. N., C. G. Palmer, F. O. Arimoro & P. K. Mensah, 2016. Chironomid assemblage structure and morphological response to pollution in an effluent-impacted river, Eastern Cape, South Africa. Ecological Indicators 67: 391–402.
Pistori, R. E. T., A. F. M. Camargo & G. G. Henry-Silva, 2004. Relative growth rate and doubling time of the submerged aquatic macrophyte Egeria densa Planch. Acta Limnologica Brasiliensia 16: 77–84.
Pokorný, J. & E. Rejmánková, 1983. Oxygen regime in a fishpond with duckweeds (Lemnaceae) and Ceratophyllum. Aquatic Botany 17: 125–137.
Polis, G. A., A. L. Sears, G. R. Huxel, D. R. Strong & J. Maron, 2000. When is a trophic cascade a trophic cascade? Trends in Ecology & Evolution 15: 473–475.
R Development Core Team, 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Rietkerk, M. & J. Van de Koppel, 1997. Alternate stable states and threshold effects in semi-arid grazing systems. Oikos 79: 69–76.
Scheffer, M., 2009. Alternative stable states and regime shifts in ecosystems. In Levin, S. A., R. S. Carpenter, H. C. J. Godfray, A. P. Kinzig, M. Loreau, J. B. Losos, B. Walker & B. S. Wilcove (eds), The Princeton Guide to Ecology. Princeton University Press, Princeton: 359–406.
Scheffer, M., S. Szabó, A. Gragnani, E. H. van Nes, S. Rinaldi, N. Kautsky, J. Norberg, R. M. M. Roijackers & R. J. M. Franken, 2003. Floating plant dominance as a stable state. Proceedings of the National Academy of Sciences 100: 4040–4045.
Schefffer, M. & S. R. Carpenter, 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends in Ecology & Evolution 18: 648–656.
Schoener, T. W., 1973. Population growth regulated by intraspecific competition for energy or time: some simple representations. Theoretical Population Biology 4: 56–84.
Schroder, A., L. Persson & A. M. De Roos, 2005. Direct experimental evidence for alternative stable states: a review. Oikos 110: 3–19.
Sharip, Z., S. Schooler, M. R. Hipsey & R. Hobbs, 2012. Eutrophication, agriculture and water level control shift aquatic plant communities from floating-leaved to submerged macrophytes in Lake Chini, Malaysia. Biological Invasions 14: 1029–1044.
Shilla, D., T. Asaeda, T. Fujino & B. Sanderson, 2006. Decomposition of dominant submerged macrophytes: implications for nutrient release in Myall Lake, NSW Australia. Wetlands Ecology and Management 14: 427–433.
Simberloff, D., 2014. Biological invasions: what’s worth fighting and what can be won? Ecological Engineering 65: 112–121.
Smart, R. M., J. W. Barko, & D. W. McFarland, 1994. Competition between Hydrilla. verticillata and Vallisneria americana under different environment conditions. Technical Report A-94-1. NTIS No. AD A279 172. US Army Engineer Waterways Experiment Station, Vicksburg.
Spivak, A. C., M. J. Vanni & E. M. Mette, 2011. Moving on up: can results from simple aquatic mesocosm experiments be applied across broad spatial scales? Freshwater Biology 56: 279–291.
Stewart, R. I., M. Dossena, D. A. Bohan, E. Jeppesen, R. L. Kordas, M. E. Ledger, M. Meerhoff, B. Moss, C. Mulder, J. B. Shurin & B. Suttle, 2013. Mesocosm experiments as a tool for ecological climate-change research. In Woodward, G. & E. J. O’Gorman (eds), Global Change in Multispecies Systems: Advances in Ecological Research. Elsevier, London: 71–182.
Stiers, I., N. Crohain, G. Josens & L. Triest, 2011. Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biological Invasions 13: 2715–2726.
Suding, K. N., K. L. Gross & G. R. Houseman, 2004. Alternative states and positive feedbacks in restoration ecology. Trends in Ecology & Evolution 19: 46–53.
Téllez, T. R., E. M. D. R. López, G. L. Granado, E. A. Pérez, R. M. López & J. M. S. Guzmán, 2008. The water hyacinth, Eichhornia crassipes: an invasive plant in the Guadiana River Basin (Spain). Aquatic Invasions 3: 42–53.
Tyler, A. C., J. G. Lambrinos & E. D. Grosholz, 2007. Nitrogen inputs promote the spread of an invasive marsh grass. Ecological Applications 17: 1886–1898.
Uddin, M. N. & R. W. Robinson, 2017. Can Nutrient Enrichment Influence the Invasion of Phragmites australis?. In Press, Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2017.06.131.
Van Ginkel, C. E., 2011. Eutrophication: present reality and future challenges for South Africa. Water SA 37: 693–701.
Vermaat, J. E., L. Santamaria & P. J. Roos, 2000. Water flow across and sediment trapping in submerged macrophyte beds of contrasting growth form. Archiv für Hydrobiologie 148: 549–562.
Viaroli, P., M. Bartoli, G. Giordani, M. Naldi, S. Orfanidis & J. M. Zaldivar, 2008. Community shifts, alternative stable states, biogeochemical controls and feedbacks in eutrophic coastal lagoons: a brief overview. Aquatic Conservation: Marine and Freshwater Ecosystems 18: 105–117.
Walker, B. & J. A. Meyers, 2004. Thresholds in ecological and social–ecological systems: a developing database. Ecology and Society 9: 3.
Wang, C., S. H. Zhang, P. F. Wang, J. Hou, W. Li & W. J. Zhang, 2008. Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte Vallisneria natans (Lour.) Hara. Aquatic Toxicology 87: 88–98.
Weyl, P. S. & J. A. Coetzee, 2014. The invasion status of Myriophyllum spicatum L. in southern Africa. Management of Biological Invasions 5: 31–37.
Yarrow, M., V. H. Marín, M. Finlayson, A. Tironi, L. E. Delgado & F. Fischer, 2009. The ecology of Egeria densa planchon (Liliopsida: Alismatales): A wetland ecosystem engineer. Revista Chilena de Historia Natural 82: 299–313.
Acknowledgements
This research was funded through the Department of Environmental Affairs, Natural Resource Management Programme’s Working for Water programme. Further funding for this work was provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa. Thanks are extended to the staff of the Centre for Biological Control (South Africa) for their help in the building and maintenance of the experiments, particularly Rosie Mangan and Rosalie Smith for plant collection and care. The authors also thank Jordie Netten for her valuable and constructive comments on earlier drafts of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: John E. Havel, Sidinei M. Thomaz, Lee B. Kats, Katya E. Kovalenko & Luciano N. Santos / Aquatic Invasive Species II
Rights and permissions
About this article
Cite this article
Strange, E.F., Hill, J.M. & Coetzee, J.A. Evidence for a new regime shift between floating and submerged invasive plant dominance in South Africa. Hydrobiologia 817, 349–362 (2018). https://doi.org/10.1007/s10750-018-3506-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3506-2