Abstract
One of the key challenges of invasion biology in aquatic systems is determining the environmental conditions under which non-indigenous species establish populations in new habitats. It is widely believed that environmental degradation of streams and rivers may facilitate susceptibility to invasion; however, this has not yet been demonstrated consistently across a wide range of taxonomic groups. We analyzed macroinvertebrate data from 398 stream and river sites in Germany in order to test whether morphologically and physicochemically degraded stream and river habitats are more prone to invasion. Further, we identified the most important environmental variables facilitating invasion. The study confirmed that invaded sites were significantly more degraded than sites where only indigenous species were recorded. In both streams and rivers, invaded sites featured increased maximum temperatures, chloride and total organic carbon concentrations and a decreased morphological habitat quality. In streams, additionally the variables minimum temperature, oxygen, orthophosphate and ammonium contributed to greater degradation. In rivers also nitrate concentration was increased at invaded sites. Generalized linear models indicated that chloride was one of the most important variables that favored invasibility in both streams and rivers. In streams, the most indicative variables for invasion risk also included orthophosphate and maximum temperature. In rivers, in addition to chloride, morphological habitat quality was important. Our results confirm that the physicochemical and morphological intactness of riverine systems is a safeguard against invasion of aquatic non-indigenous macroinvertebrates. Based on this knowledge, management strategies can be developed to reduce invasion risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasions of non-indigenous species are one of the main threats to biodiversity (Lövei 1997; Vitousek et al. 1997; Dextrase and Mandrak 2006), leading to a homogenization of the regional sets of species, or in a more catchy term, to a “McDonaldisation” of the biosphere (Lövei 1997; Sax and Gaines 2003). Freshwater systems are especially affected, as invasion rates are higher compared to marine and terrestrial systems. Currently in Europe, approximately 200 non-indigenous benthic invertebrate species have been established, and the rate of invasion is further increasing (Strayer 2010).
These high freshwater invasion rates have been attributed to an increasing interconnectedness between habitats on a global scale. Canals directly connect previously separated systems, with ships acting as vectors for invasion. For example, the surface area of the catchments that are directly connected by inland water ways to the river Rhine (the busiest waterway of the world) has increased by a factor of 21.6 over the last two centuries (Leuven et al. 2009). Beside transport via shipping and dispersal by man-made waterways, ornamental trade and stocking are also important vectors for non-indigenous species spread (Gollasch and Nehring 2006).
While the pathways of species spread are well known, there is a lack of knowledge about the environmental conditions under which dispersing individuals establish populations in new habitats. Therefore, one of the key challenges of invasion biology is determining the environmental variables that control successful establishment of non-indigenous species. It has been shown that invaders are more tolerant to environmental stress (Karatayev et al. 2009) and based on this, it is believed that stressed habitats are consequently more prone to invasions. This assumption was derived from a range of case studies that mainly examined the effects of single morphological and physicochemical habitat variables on distribution patterns of non-indigenous species or their tolerance to certain habitat variables, e.g. the effects of oxygen depletion (Byers 2000b; MacNeil et al. 2000, 2001), temperature (Wijnhoven et al. 2003; Werner and Rothhaupt 2008; Weitere et al. 2009; Zukowski and Walker 2009), salinity (Wijnhoven et al. 2003; Grabowski et al. 2009; Zukowski and Walker 2009), ammonium (Prenter et al. 2004; Hong et al. 2007) and nitrate concentration (Alonso and Camargo 2003; Camargo et al. 2005) or structural degradations like dams and reservoirs (Havel et al. 2005; Johnson et al. 2008). Moreover, most of these studies focused on individual non-indigenous species or narrow taxonomical groups. Single species examples include Dikerogammarus villosus (Pöckl 2009; van der Velde et al. 2009), Corbicula fluminea (Werner and Rothhaupt 2008; Weitere et al. 2009) and various snail species (Byers 2000a, b; Schreiber et al. 2003; Alonso and Castro-Diez 2008; Zukowski and Walker 2009). The only taxonomic group above species level that has been well studied are the amphipods (MacNeil et al. 2000, 2001, 2009; Grabowski et al. 2007, 2009; Kestrup and Ricciardi 2009; Piscart et al. 2009). Only recently, a study addressed the relationships between a wide range of environmental variables and non-indigenous species from different taxonomic groups in slow-flowing artificial urban drainage systems (Vermonden et al. 2010). The relative impact of morphological habitat quality, pollutants and temperature on the occurrence of non-indigenous species in natural fast-flowing streams and rivers has not been assessed so far.
However, to draw general conclusions on exactly which variables facilitate invasibility of habitats, studies on a more complete range of non-indigenous species, as well as large sets of environmental variables within natural riverine systems are needed. Increased knowledge of these variables is necessary for developing management strategies for aquatic habitats to minimize the chances for non-indigenous species establishment. Such management strategies help to preserve a high degree of differentiation between the regional species pools in anthropocentrically-used freshwater systems and further prevent extensive establishment of non-indigenous species as a result of global change.
In this study, we addressed two questions: (1) Are morphologically and physicochemically degraded stream and river reaches more easily invaded by non-indigenous species than reaches that are less degraded? (2) Which are the most important environmental variables that facilitate invasions by aquatic non-indigenous species? To this end, we analyzed an extensive benthic macroinvertebrate data set from 398 sites in streams and rivers in the lower mountain ranges in Germany, comprising 20 non-indigenous species from eight different taxonomical groups. To answer the first question, differences in physicochemical and morphological loads between invaded and uninvaded sites were analyzed using a set of the nine environmental variables: morphological habitat quality, minimum temperature, maximum temperature, oxygen, orthophosphate, chloride, nitrate, ammonium and total organic carbon. To answer the second question, we employed generalized linear models to indicate the most important environmental variables favoring the establishment of aquatic non-indigenous species in streams and rivers. Streams and river habitats were analyzed separately, as their environmental conditions, as well as the set of indigenous species, differ (Vannote et al. 1980).
Materials and methods
Sampling sites
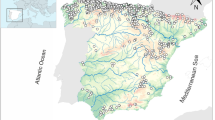
We analyzed benthic invertebrate data from 398 sampling sites located in streams and rivers in the lower mountain ranges in Germany (Fig. 1). Two hundred sixty-five data sets came from streams with a catchment area of 10–100 km2 and 133 sites from rivers with a catchment area of 100–10,000 km2.
Location of the sampling sites in streams (circles) and rivers (squares) in the lower mountain ranges in Germany. Filled symbols indicate sites that are invaded by non-indigenous species, empty symbols indicate uninvaded sites. For a better orientation the seven largest rivers in Germany are drawn in the map
The sites were divided into two groups with respect to the occurrence of aquatic non-indigenous species. The first group, NIS+, comprised sampling sites where non-indigenous species occurred (58 and 37 sites for streams and rivers, respectively). The second group, NIS−, comprised sampling sites where only indigenous species were found (207 and 96 sites for streams and rivers, respectively).
To ensure that the spatial structure of the sites does not confound the results of the analysis, we checked the distribution of NIS+ and NIS− sites across all sub-catchments that were covered by the dataset. The 398 sampling sites were located in 50 sub-catchments. The vast majority, of 359 sampling sites were located in sub-catchment were both NIS+ and NIS− sites were present. Only 39 sampling sites were located in sub-catchments with only one type, NIS+ or NIS− sites. However, this also included 9 cases where just one sampling site was available in a whole sub-catchment.
Benthic invertebrates
Benthic invertebrates were collected at all sampling sites following the official European Water Framework Directive (EU WFD) compliant sampling protocol applied in Germany (Haase et al. 2004). A multi-habitat sampling approach was used: at each site, 20 sub samples were taken, each notionally of 25 cm × 25 cm in dimensions, resulting in ca. 1.25 m2 of river bottom being sampled. The 20 subsamples were distributed on all microhabitat types present with ≥5 % cover, reflecting their relative occurrence. All invertebrate samples were taken between March and July from 2004 to 2008.
The samples were preserved in 70 % ethanol and transferred to the laboratory where they were sorted following Haase et al. (2004). Taxa were identified to the level proposed by Haase et al. (2006) ensuring that taxalists were inter-comparable with regard to their taxonomic resolution.
All species listed by DAISIE (2010) were considered as non-indigenous species according to the definition by (Genovesi and Shine 2004). The dataset included a total number of 20 aquatic non-indigenous species covering the taxonomic groups Amphipoda, Bivalvia, Decapoda, Gastropoda, Hirudinea, Isopoda Polychaeta and Turbellaria (Table 1).
Environmental variables
From each benthic invertebrate sampling site, measurements of water temperature as well as of oxygen (O2), orthophosphate (PO4), chloride (Cl), nitrate (NO3), ammonium (NH4) and total organic carbon (TOC) concentrations were available. All measurements were taken in the water column. The minimum resolution of the data was ten measurements per year. For chemical data, we calculated annual mean values. Additionally, the chemical quality class according to LAWA (1998) is given in figures, to facilitate comparisons between different variables. The chemical quality class is interval-scaled with four main classes: anthropogenically unloaded (I), moderate load (II), increased load (III), very high load (IV) and intermediate classes in between very low load (I–II), significant load (II–III), high load (III–IV). Values that fall into class I, I–II and II are considered to be still acceptable, while higher loads are considered critical and remedial measures should be taken. For temperature, only annual minimum (Tmin) and maximum (Tmax) value were reported. When physicochemical data directly measured at the sampling sites were lacking, representative measuring stations for the sites were selected according to the following criteria: (a) the time when the measurements were taken had to correspond to the benthic invertebrate sampling, (b) they had to be within a radius of 2 km around the invertebrate sampling site and (c) no other tributary or other water inlet was allowed between the measuring station and the sampling site.
In addition to physicochemical variables, habitat morphology was assessed using the morphological habitat quality (MHQ) index according to LAWA (2000). MHQ is assessed on a seven-step scale reaching from undisturbed (1) to totally disturbed (7). To this end, the river ecosystem is divided into three sectors: bed, bank and floodplain. Within these sectors the six main parameters of river morphology, plan form, longitudinal profile, bed structures, cross-section, bank structures and floodplain corridor are assessed. These main parameters are defined by 14 functional units, which themselves have 25 individual underpinning parameters. The final MHQ index is calculated by averaging the single assessment units (for details see Kamp et al. 2007).
Statistical analysis
Data for chemical variables (O2, PO4, Cl, NO3, NH4 and TOC) were log(x + 1)-transformed.
Differences in loads of all environmental variables of NIS+ and NIS− sites as well as between streams and rivers were analyzed using Mann–Whitney-U-tests.
Because some environmental variables were interrelated, we condensed the multivariate space using a detrended correspondence analysis (DCA). Because the length of environmental gradients within the invertebrate sampling sites was <3, a principle component analysis (PCA) was chosen for further analysis (Leyer and Wesche 2007). Eigenvectors were scaled to their standard deviations.
Statistical significance of the PCA-axes was tested using a randomization test (999 runs). For axes that were significant, Student’s t-tests were used to test for differences between the scores of the NIS+ and NIS− sites.
Lastly, the effects of environmental variables were integrated in one model predicting the occurrence of non-indigenous species. Streams and rivers were analyzed separately using generalized linear models (GLMs) with binomial errors. The analysis started with a baseline model containing all variables in which loads differed significantly between NIS+ and NIS− sites. Using backward selection, the complexity of the GLMs was reduced stepwise until a minimal Akaike Information Criterion (AIC) was achieved.
Univariate statistical analyses were performed with the software packages STATISTICA 8 (StatSoft, Inc., Tulsa, USA), and multivariate analyses were performed with PC-ORD 5 (MjM Software, Gleneden Beach, USA) and R software (R-Foundation, Vienna, Austria).
Results
Degradation of streams and rivers
Streams and rivers of the lower mountain ranges in Germany were morphologically rather degraded, indicated by an average MHQ (Fig. 2a) of “totally modified” (class 5). Substantial loads were also found for most of the physicochemical variables. In particular, PO4 concentration (Fig. 2e) in streams reached values categorized in “high load” (class III–IV), and Cl, NO3, NH4 and TOC concentrations (Fig. 2f, g, h, i) in “significant load” (class II–III).
Box-plots with all outliers of measurements of the nine variables at invaded (black bars) and uninvaded (gray bars) sites in streams and rivers. a MHQ = morphological habitat quality, b Tmin = minimum temperature, c Tmax = maximum temperature, d O2 = oxygen, e PO4 = orthophosphate, f Cl = chloride, g NO3 = nitrate, h NH4 = ammonium and i TOC = total organic carbon. Measurements of chemical variables (O2, PO4, Cl, NO3, NH4 and TOC) were log(x + 1)-transformed. Dotted horizontal lines give the threshold for “moderate” chemical water quality (class II) according to LAWA (1998). The asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001, NS not significant) between streams and rivers as well as between NIS+ and NIS− sites
Furthermore, for some environmental variables, average loads differed significantly between streams and rivers. In streams, Tmin (U = 15,303, p = 0.03) as well as the concentrations of PO4 (U = 11,551, p < 0.001), NO3 (U = 12,619, p < 0.001), NH4 (U = 12,879, p < 0.001) and TOC (U = 14,919, p = 0.01) were significantly higher than in rivers (Fig. 2b, e, g, h, i). Conversely, average Tmax was significantly lower in streams than in rivers (U = 11,954, p < 0.001; Fig. 2c).
Differences between NIS+ and NIS−
For streams, the first axis of the PCA was significant and inversely correlated with all variables indicating environmental stress (Table 2). The highest negative correlation coefficients were found for TOC, Cl, PO4 and Tmax. This axis was also positively correlated to O2, an indicator of good quality.
For rivers, the first two axes were found to extract a significant proportion of the variability in the data (Table 2). The highest negative correlation coefficients were found for TOC, PO4, Cl and NH4. O2 was not well represented on the first axis, but had the highest negative correlation in the second axis. In contrast, temperature parameters were positively correlated with the second axis.
Through the analysis of the scores of the significant PCA axes (streams: axis one; rivers: axis one and two), we found that for both streams and rivers, NIS+ sites had significantly more environmental stress than NIS− sites (streams axis 1: t = 6.84, p < 0.001; rivers axis 1: t = 3.57, p < 0.001, rivers axis 2: t = −2.12, p = 0.04; Fig. 3).
Box plots of the axis scores calculated with principle component analysis (PCA) for axis 1 and axis 2. The results comparing invaded (black bars) and uninvaded (gray bars) sites in a streams and b, c rivers are shown. Axis 2 in streams did not contribute significantly to the data variability and was therefore not considered. The asterisks indicate significantly different (*p < 0.05, **p < 0.01, ***p < 0.001) groups
In both streams and rivers, the MHQ (streams: 4,713, p = 0.01; rivers: U = 1,342, p = 0.03), Tmax (streams: U = 3,362, p < 0.001; rivers: U = 1,208, p = 0.004), Cl (streams: U = 2,834, p < 0.001; rivers: U = 987, p < 0.001) and TOC (streams: U = 4,364, p = 0.002; rivers: U = 1,274, p = 0.01) were significantly higher at NIS+ sites than at NIS− sites (Fig. 2a, c, f, i). In addition, Tmin (U = 4,223, p < 0.001), PO4 (U = 3,470, p < 0.001) and NH4 (U = 4,947, p = 0.04) in streams were significantly higher at NIS+ compared to NIS− sites, whereas O2 (U = 3,988, p < 0.001) was lower (Fig. 2b, d, e, h). In rivers, NO3 was significantly higher at NIS+ sites (streams: U = 5,330, p = 0.20; rivers: U = 1,360, p = 0.04; Fig. 2g).
Multiple regressions
Stepwise backward selection of the environmental variables in GLMs showed that in streams, PO4 followed by Cl and Tmax were the best predictors for non-indigenous occurrence (Table 3a). In rivers, increased Cl and degraded MHQ were the most predictive variables (Table 3b).
Discussion
Establishment of invasive species occurs more commonly in degraded habitats
Our study showed that stressed stream and river habitats are more prone to invasions of non-indigenous benthic macroinvertebrates than more pristine habitats. Although this has been demonstrated for single species and individual environmental variables, this has not been shown as a consistent pattern in a multi-species and multi-variable approach. The higher invasion success of non-indigenous species with increased loads of pollutants may reflect a selection process during anthropogenic invasion during which organisms often have to tolerate harsh environmental conditions, leading to non-indigenous species being a non-random, more tolerant subset of all benthic invertebrates (Karatayev et al. 2009). For example, when transported in ballast water of ships, a wide tolerance to various environmental stressors (salinity, oxygen depletion, high and low temperatures) is an important trait for survival and therefore for reaching new habitats. In this context, two hypotheses are proposed to explain why non-indigenous species manage to establish successfully, especially in stressed habitats. First, as non-indigenous species have a higher competitive fitness under environmental stress, they may outcompete indigenous competitors in stressed habitats. Alternatively, non-indigenous species may simply fill the gaps that have been left after more susceptible indigenous species have been exterminated from their habitats by various stressors (Didham et al. 2005; MacDougall and Turkington 2005). Both hypotheses emphasize that not only is human-aided dispersal of species a key to invasion, but so is the environmental condition of the habitat that is to receive non-indigenous species. We showed that the set of most important environmental variables varied between streams and rivers. In streams, we identified increased PO4 and Cl concentrations as well as increased Tmax as the most important variables for invasion, while in rivers, Cl and MHQ were most indicative for establishment of non-indigenous species. These differences may be attributed to the differences in land use around streams and rivers as well as to the differences in the physicochemistry and ecology of streams and rivers from the source to the mouth as described in the river continuum concept (Vannote et al. 1980). These principle differences between streams and rivers are also reflected in the different composition of indigenous species assemblages with different requirements in streams and rivers.
Environmental drivers for invasion success
Tmax was found to be one of the most important variables for invasion success in streams, where many indigenous species are adapted to low water temperatures. Therefore, they will be more affected by increasing temperature than river species which typically display wider ecological temperature amplitudes. At increased water temperatures, non-indigenous species, which are often tolerant to higher temperatures (Wijnhoven et al. 2003; Alonso and Castro-Diez 2008), may more effectively outcompete indigenous stream species than river species.
In addition to Tmax, we identified PO4 as an important variable for invasions in streams. The main source of PO4 in the study area is the diffuse input of agriculture (Schulz and Bischoff 2008). This is especially relevant in streams, where riparian buffers to agricultural land are often narrower than in rivers. In addition, the water mass of streams, in which PO4 is diluted, is smaller. Further, as many primary producers in freshwater are phosphorus-limited, uptake rates in rivers that are inhabited by phytoplankton are higher than in streams without phytoplankton. Consequently the average load of PO4 was significantly higher in streams than in rivers. In particular, PO4 loads at NIS+ sites were classified as “very high” (class IV). In contrast, most sampling sites in rivers were classified as “moderate” (class II).
With eutrophication induced by increased PO4, concentrations of other associated variables also change. Typically, productivity increases in the trail of increased PO4, followed by decreasing O2 concentration due to decomposition processes. The interplay of these processes may enable non-indigenous species to populate habitats which are highly contaminated by PO4 due to higher tolerance to O2 depletion. Also, increased availability of resources due to higher productivity may facilitate the invasion risk in PO4 enriched habitats, as these resources can be readily utilized by the new invaders (Davis et al. 2000; Zedler and Kercher 2004). These latter studies have focused on non-indigenous plants; however, in consumers, the direct relation to nutrient availability is less obvious than in primary producers. Nevertheless, Vermonden et al. (2010) showed that non-indigenous crustaceans profit from nutrient enrichment in urban waters.
In rivers, additionally to physicochemical variables, MHQ was correlated with the establishment of non-indigenous species. Structural habitat alterations like bank fixation, dams or reduction of run length are common anthropogenic impacts in riverine systems reducing habitat diversity. These anthropogenic changes favor euryoecious species with a high competitive fitness. Previously, Schreiber et al. (2003) showed a positive correlation between the presence of the non-indigenous mud snail Potamopyrgus antipodarum and anthropogenic development. Furthermore, our results extend prior findings that dams and reservoirs can promote invasions (Havel et al. 2005; Johnson et al. 2008), showing that morphological degradation may increase invasion risk in rivers in general.
In both streams and rivers, Cl was selected as important variable for invasion risk. Around 65 % of the invaded sites were classified as having “moderate” chemical quality (class II, 50–100 mgL−1 Cl) according to LAWA (1998). Therefore, even in concentrations below critical values, Cl was indicative for an increased invasion potential. These findings imply that the threshold for an acceptable “moderate load” of Cl might overestimate the tolerance of many native freshwater organisms to salinity. In particular, long-term effects leading to reduced competitive fitness may be hard to detect in eco-toxicological tests, which are often used to determine threshold levels.
Previously, for several non-indigenous amphipods, Grabowski et al. (2009) showed that they are more common and abundant at sides with raised salinity. Our study generalizes these previous finding showing that habitats which are contaminated by Cl are more vulnerable to invasion. As many non-indigenous species are transported in ballast water tanks of ships (Gollasch and Nehring 2006), salinity tolerance may reflect a selection process via anthropogenic introduction favoring euryhaline species (Alonso and Castro-Diez 2008).
Applications of our results
As invasions often do not progress with clear fronts, our results suggest that more loaded and degraded stretches, in particular, can be used as stepping stones for rapid invasions. Consequently, morphological and physicochemical intactness of water bodies are an important concern as intact water bodies are more resistant to invasion. Therefore, restoration measures increasing water and habitat quality can help to reduce invasion risk and thereby protect biodiversity.
Beside for the negative impact on biodiversity, invasions also accrue high economic costs (Pimentel et al. 2005 and references therein). Despite these facts, the introduction rate of non-indigenous species is still increasing. However, even if we stop the introduction of new non-indigenous species, those which have currently established will further advance until they reach the border of their ecological niches (Strayer 2010). Predicting the future spread of non-indigenous species and the corresponding consequences is difficult, as non-indigenous species may interact strongly with other environmental stressors, thereby modulating their effects. For example D. polymorpha was responsible for strong oxygen loss in the Seneca River due to high metabolic activity (Effler et al. 2004). This shows that the effects of non-indigenous species are bidirectional. Not only favors habitat degradation invasions, also non-indigenous species can degrade habitats, which in turn increases future invasion risk. Hence, non-indigenous species can fundamentally transform ecosystems into new, unknown so called “no-analogue ecosystems” (Williams and Jackson 2007; Strayer 2010). In such systems, experiences from the management strategies of known systems may not be a reliable guide (Strayer 2010), which makes sustainable management of these new systems more difficult or even impossible.
Since the management of invaded ecosystems is difficult and costly, the mitigation of invasions should have a high priority in environmental management. Beside the control of anthropogenic spread of non-indigenous species, the conservation (or restoration) of intact freshwater habitats is important, as this reduces the establishment success of non-indigenous species. Our results indicate that specific environmental variables can be used to assess invasion risk. The control of Cl, PO4, and MHQ may help to reduce the establishment success of non-indigenous species. Also, Tmax has been pinpointed as an important variable for invasions. Increased temperatures, as predicted by global climate change (IPCC 2007) will promote invasions (Rahel and Olden 2008), especially in streams, where indigenous cold water-adapted organisms may be more easily outcompeted by non-indigenous species with a wider temperature tolerance. Along with increased temperatures, some regions will also suffer from increased salinity due to summer desiccation.
Little is known about environmental patterns favoring invasions, and further research is needed to aggregate and conceptualize the many case studies that have been published in recent years. Doing so can lead to a clearer and more integrated picture of the relationship between climate change and other anthropogenic effects and invasion of our freshwater systems by non-indigenous species.
References
Alonso A, Camargo JA (2003) Short-term toxicity of ammonia, nitrite, and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bull Environ Contam Toxicol 70(5):1006–1012
Alonso A, Castro-Diez P (2008) What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia 614(1):107–116
Byers JE (2000a) Competition between two estuarine snails: Implications for invasions of exotic species. Ecology 81(5):1225–1239
Byers JE (2000b) Differential susceptibility to hypoxia aids estuarine invasion. Mar Ecol-Prog Ser 203:123–132
Camargo JA, Alonso A, Salamanca A (2005) Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58(9):1255–1267
DAISIE Delivering Alien Invasive Species Inventories for Europe (2010) European Invasive Alien Species Gateway. http://www.europe-aliens.org/. Accessed 1 Nov 2010
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88(3):528–534
Dextrase AJ, Mandrak NE (2006) Impacts of alien invasive species on freshwater fauna at risk in Canada. Biol Invasions 8(1):13–24
Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20(9):470–474
Effler SW, Matthews DA, Brooks-Matthews CM, Perkins MG, Siegfried CA, Hassett JM (2004) Water quality impacts and indicators of metabolic activity of the zebra mussel invasion of the Seneca River. J Am Water Resour Assoc 40(3):737–754
Genovesi P, Shine C (2004) European strategy on invasive alien species: Convention on the Conservation of European Wildlife and Natural Habitats. Council of Europe Publishing, Strasbourg Cedex
Gollasch S, Nehring S (2006) National checklist for aquatic alien species in Germany. Aquat Invasions 1(4):245–269
Grabowski M, Bacela K, Konopacka A (2007) How to be an invasive gammarid (Amphipoda: Gammaroidea)—comparison of life history traits. Hydrobiologia 590:75–84
Grabowski M, Bacela K, Konopacka A, Jazdzewski K (2009) Salinity-related distribution of alien amphipods in rivers provides refugia for native species. Biol Invasions 11(9):2107–2117
Haase P, Lohse S, Pauls S, Schindehütte K, Sundermann A, Rolauffs P, Hering D (2004) Assessing streams in Germany with benthic invertebrates: development of a practical standardised protocol for macro invertebrate sampling and sorting. Limnologica 34(4):349–365
Haase P, Sundermann A, Schindehütte K (2006) Informationstext zur Operationellen Taxaliste als Mindestanforderung an die Bestimmung von Makrozoobenthosproben aus Fließgewässern zur Umsetzung der EU-Wasserrahmenrichtlinie in Deutschland—Stand März 2006. http://www.fliessgewaesserbewertung.de/downloads/Informationstext_zur_Operationellen_Taxaliste_Stand_17Mrz06.pdf. Accessed 10 Dec 2010
Havel JE, Lee CE, Vander Zanden MJ (2005) Do reservoirs facilitate invasions into landscapes? Bioscience 55(6):518–525
Hong ML, Chen LQ, Sun XJ, Gu SZ, Zhang L, Chen Y (2007) Metabolic and immune responses in Chinese mitten-handed crab (Eriocheir sinensis) juveniles exposed to elevated ambient ammonia. Comp Biochem Physiol C-Toxicol Pharmacol 145(3):363–369
IPCC Intergovernmental Panel on Climate Change (2007) Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. http://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_synthesis_report.htm. Accessed 17 Nov 2010
Johnson PTJ, Olden JD, vander Zanden MJ (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6(7):359–365
Kamp U, Binder W, Hölzl K (2007) River habitat monitoring and assessment in Germany. Environ Monit Assess 127(1):209–226
Karatayev AY, Burlakova LE, Padilla DK, Mastitsky SE, Olenin S (2009) Invaders are not a random selection of species. Biol Invasions 11(9):2009–2019
Kestrup AM, Ricciardi A (2009) Environmental heterogeneity limits the local dominance of an invasive freshwater crustacean. Biol Invasions 11(9):2095–2105
LAWA Länderarbeitsgemeinschaft Wasser (1998) Beurteilung der Wasserbeschaffenheit von Fließgewässern in der Bundesrepublik Deutschland—Chemische Gewässergüteklassifikation-. Kulturbuchverlag, Berlin
LAWA Länderarbeitsgemeinschaft Wasser (2000) Gewässerstrukturgütekartierung in der Bundesrepublik Deutschland—Verfahren für kleine und mittelgroße Fließgewässer. Kulturbuchverlag, Berlin
Leuven R, van der Velde G, Baijens I, Snijders J, van der Zwart C, Lenders HJR, de Vaate AB (2009) The river Rhine: a global highway for dispersal of aquatic invasive species. Biol Invasions 11(9):1989–2008
Leyer I, Wesche K (2007) Multivariate Statistik in der Ökologie. Springer, Berlin
Lövei GL (1997) Biodiversity—global change through invasion. Nature 388(6643):627–628
MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86(1):42–55
MacNeil C, Dick JTA, Elwood RW (2000) Differential physico-chemical tolerances of amphipod species revealed by field transplantations. Oecologia 124(1):1–7
MacNeil C, Montgomery WI, Dick JTA, Elwood RW (2001) Factors influencing the distribution of native and introduced Gammarus spp. Irish river systems. Archiv Fur Hydrobiologie 151(3):353–368
MacNeil C, Dick JTA, Gell FR, Selman R, Lenartowicz P, Hynes HBN (2009) A long-term study (1949–2005) of experimental introductions to an island; freshwater amphipods (Crustacea) in the Isle of Man (British Isles). Divers Distrib 15(2):232–241
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52(3):273–288
Piscart C, Dick JTA, McCrisken D, MacNeil C (2009) Environmental mediation of intraguild predation between the freshwater invader Gammarus pulex and the native G. duebeni celticus. Biol Invasions 11(9):2141–2145
Pöckl M (2009) Success of the invasive Ponto-Caspian amphipod Dikerogammarus villosus by life history traits and reproductive capacity. Biol Invasions 11(9):2021–2041
Prenter J, MacNeil C, Dick JTA, Riddell GE, Dunn AM (2004) Lethal and sublethal toxicity of ammonia to native, invasive, and parasitised freshwater amphipods. Water Res 38(12):2847–2850
Rahel FJ, Olden JD (2008) Assessing the effects of climate change on aquatic invasive species. Conserv Biol 22(3):521–533
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends Ecol Evol 18(11):561–566
Schreiber ESG, Quinn GP, Lake PS (2003) Distribution of an alien aquatic snail in relation to flow variability, human activities and water quality. Freshw Biol 48(6):951–961
Schulz M, Bischoff M (2008) Variation in riverine phosphorus between 1994 and 2003 as affected by land-use and loading reductions in six medium-sized to large German rivers. Limnologica 38(2):126–138
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174
van der Velde G, Leuven R, Platvoet D, Bacela K, Huijbregts MAJ, Hendriks HWM, Kruijt D (2009) Environmental and morphological factors influencing predatory behaviour by invasive non-indigenous gammaridean species. Biol Invasions 11(9):2043–2054
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) River continuum concept. Can J Fish Aquat Sci 37(1):130–137
Vermonden K, Leuven R, van der Velde G (2010) Environmental factors determining invasibility of urban waters for exotic macroinvertebrates. Divers Distrib 16(6):1009–1021
Vitousek PM, Dantonio CM, Loope LL, Rejmanek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. N Z J Ecol 21(1):1–16
Weitere M, Vohmann A, Schulz N, Linn C, Dietrich D, Arndt H (2009) Linking environmental warming to the fitness of the invasive clam Corbicula fluminea. Global Change Biol 15(12):2838–2851
Werner S, Rothhaupt KO (2008) Mass mortality of the invasive bivalve Corbicula fluminea induced by a severe low-water event and associated low water temperatures. Hydrobiologia 613:143–150
Wijnhoven S, van Riel MC, van der Velde G (2003) Exotic and indigenous freshwater gammarid species: physiological tolerance to water temperature in relation to ionic content of the water. Aquat Ecol 37(2):151–158
Williams JW, Jackson ST (2007) Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ 5(9):475–482
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23(5):431–452
Zukowski S, Walker KF (2009) Freshwater snails in competition: alien Physa acuta (Physidae) and native Glyptophysa gibbosa (Planorbidae) in the River Murray, South Australia. Mar Freshw Res 60(10):999–1005
Acknowledgments
We are grateful to the Umweltbundesamt and the Hessisches Landesamt für Umwelt und Geologie (HLUG) for kindly providing the species and physicochemical and morphological data. Further, we wish to thank Sami Domisch, Heike Kappes and two anonymous referees for constructive comments. The present study was funded by the research funding program “LOEWE—Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of Hesse’s Ministry of Higher Education, Research, and the Arts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Früh, D., Stoll, S. & Haase, P. Physicochemical and morphological degradation of stream and river habitats increases invasion risk. Biol Invasions 14, 2243–2253 (2012). https://doi.org/10.1007/s10530-012-0226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0226-9