Abstract
The invasive aquatic plant species Elodea nuttallii could pose a considerable risk to European freshwater ecosystems based on its current distribution, rate of spread and potential for high biomass. However, little research has been conducted on the impacts of this species on native biota. This study takes an ecosystem-wide approach and examines the impact of E. nuttallii on selected physicochemical parameters (dissolved oxygen and pH), algae, invertebrate and macrophyte communities. Elodea nuttallii had small but significant impacts on plant, invertebrate and algal species. The richness of algal periphyton was lower on E. nuttallii than on native macrophytes. The taxonomic composition of invertebrate communities associated with E. nuttallii differed from that associated with similar native plant species, but did not differ in terms of total biomass or species richness. Macrophyte species richness and total cover were positively correlated with percentage cover of E. nuttallii. Not all macrophyte species responded in the same way to E. nuttallii invasion; cover of the low-growing species, Elodea canadensis and charophytes were negatively correlated with E. nuttallii cover, whilst floating-rooted plants were positively correlated with E. nuttallii cover. All observed differences in the macrophyte community were small relative to other factors such as nutrient levels, inter-annual variation and differences between sites. Despite this, the observed negative association between E. nuttallii and charophytes is a key concern due to the rarity and endangered status of many charophyte species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater systems have been shown to be at particularly high risk from biological invasions (Sala et al. 2000) and invasive aquatic plants are widely considered to be a major threat to both species diversity and ecosystem functioning (Strayer 2010). The assessment of potential impacts of invasive species on ecosystems is essential for the effective prioritisation of resources (Leung et al. 2012), and traits associated with successful naturalisation cannot be reliably used to infer potential impact (Hulme 2012). Despite this, in Europe there is a lack of studies directly assessing the impacts of aquatic species on natural ecosystems across trophic levels (Caffrey et al. 2014).
Invasive macrophytes can be ‘ecosystem engineers’, fundamentally altering ecosystems through alterations to habitat structure and water chemistry (Strayer 2010). The impacts of invasive macrophytes on native macrophytes are more frequently studied than their impacts on algae or invertebrates (Evangelista et al. 2014). Invasive macrophytes are frequently observed to be dominant in plant assemblages. They may reduce overall macrophyte richness (Carniatto et al. 2013; Michelan et al. 2010; Stiers et al. 2011) and native seed banks (deWinton and Clayton 1996), and alter plant community composition (Mjelde et al. 2012; O’Hare et al. 2012). However, invasive macrophytes may also benefit native plant species by altering the physical environment (e.g. stabilisation of sediment, reduction of turbidity or altering water clarity, Rybicki and Landwehr 2007; Thomaz et al. 2012). Previous laboratory experiments conducted with Elodea nuttallii have shown that it can out-compete other submerged species (Barrat-Segretain 2005) and floating species when nutrient concentrations are not limiting (Szabo et al. 2010). However, floating species are likely to out-compete E. nuttallii in high nutrient conditions due to their superior ability to compete for light (Netten et al. 2010; Szabo et al. 2010).
Algal periphyton is a key link between macrophytes and aquatic invertebrate species (Hamilton et al. 1992). Algal periphyton communities differ between plant hosts (Toporowska et al. 2008) both as a result of plant architecture (Declerck et al. 2007; Warfe and Barmuta 2006) and chemical exudates (Erhard and Gross 2006). Suppression of algal taxa by macrophyte exudates has been observed for several submersed species, including E. nuttallii and its congener Elodea canadensis (van Donk and van de Bund 2002; Wu et al. 2009). As competition with periphyton and phytoplankton is a major limiting factor for aquatic macrophytes, such allelopathy could constitute a substantial competitive advantage for these species.
Allelopathic exudates may also affect zooplankton and macroinvertebrates, e.g. negative effects of Elodea spp. on growth and development of Daphnia spp. (Burks et al. 2000) and lepidopteran larvae in the family Pyralidae (Erhard et al. 2007). Many macrophyte species contain chemicals that deter grazing, and invertebrates and fish may preferentially select native macrophyte species as food (Burks and Lodge 2002; Schultz and Dibble 2012). Furthermore, the physical structure of different macrophytes provides varying amounts of predator-free refuge space (Kovalenko and Dibble 2014; Valinoti et al. 2011). In some cases, the increase in plant biomass associated with invasive macrophytes may increase the overall productivity of the invaded system, resulting in an increase in biomass and diversity of invertebrate species and changes in invertebrate community composition (Schultz and Dibble 2012).
Elodea nuttallii is a submerged freshwater plant species which occurs in lakes and slow moving rivers, and which could pose a significant risk to European waterbodies based on its rapid spread and high abundance (Champion et al. 2010) and the observed impacts of E. canadensis. Whilst spread rates and suitability of European waterbodies for the establishment of E. nuttallii have been studied (Hussner 2012; Kelly et al. 2014a, b), little research has been conducted on the impacts of this species in invaded waterbodies.
Elodea nuttallii was first introduced to Europe in 1939 and has spread rapidly, replacing the ecologically similar E. canadensis in many locations (Thiébaut et al. 2008). E. canadensis is considered to be one of the ‘100 worst’ invasive species in Europe (DAISIE 2015) and has impacts on macrophyte communities and aquatic food webs (e.g. deWinton and Clayton 1996; Kelly and Hawes 2005; Kornijow et al. 2005). E. nuttallii and E. canadensis are so similar that they may be ecologically and functionally redundant (Herault et al. 2008), in which case their distribution and impacts could be expected to be similar. Both E. canadensis and E. nuttallii have high photosynthetic rates, show strong effects on pH, dissolved oxygen and CO2 levels within plant stands (James et al. 1999) and may play an important role in phosphorus cycling in eutrophic systems (Angelstein and Schubert 2008). Field evidence suggests that E. nuttallii is replacing E. canadensis (Barrat-Segretain 2001; Barrat-Segretain et al. 2002) and laboratory experiments have shown that E. nuttallii is more competitive than E. candensis (Barrat-Segretain 2005). Hence, the impacts of E. nuttallii could be more severe than those of E. canadensis.
According to the “invasional meltdown” hypothesis (Simberloff 2006) invasive species may facilitate the establishment or growth of other invasive species leading to accelerating rates of invasion; however, there are few empirical examples of this (Montgomery et al. 2012). Recent research on invasive macrophytes found evidence of facilitation of Egeria densa by Ludwigia grandiflora, but mutual inhibition between L. grandiflora and Myriophyllum aquaticum (Thouvenot et al. 2013), suggesting that such interactions may be species- and/or context-specific. Therefore, it is important to examine the potential interactions between E. canadensis and E. nuttallii where they co-occur in order to ascertain whether impacts on native biota are amplified by the interaction of these species.
Here, we describe two correlational studies which provide insights into the potential impacts of Elodea. Firstly, we used historical data on the macrophyte communities in two large lakes over the course of an invasion to examine the impact of E. nuttallii on other macrophyte species, and to examine interactions between E. nuttallii and E. canadensis. Secondly, we used a paired survey design to examine differences in micro-algae and invertebrates associated with native macrophytes and invasive E. nuttallii within six waterbodies. We used a combination of standard community metrics (e.g. biomass and species richness) and multivariate analyses of communities, both in terms of taxonomic groups and broader functional or structural groups, to examine impacts at different trophic levels.
Methods
Macrophyte study sites
Lough Erne in County Fermanagh, Northern Ireland, comprises Upper Lough Erne (ca. 29 km2) and Lower Lough Erne (ca. 104 km2). Lough Erne is a naturally eutrophic lake system with high alkalinity due to the underlying geology of the area. Upper Lough Erne is the shallower of the two lakes with a mean depth of 2.9 m; Lower Lough Erne has a mean depth of 11.9 m. Over the period of this study pH in these lakes ranged from 6.2 to 9.3, total phosphorus from 10 to 780 μg l−1 and nitrates from 20 to 1,080 μg l−1 [data provided by Northern Ireland Environment Agency (NIEA), based on monthly measurements at ten monitoring points from 2006 to 2010]. Lough Erne is notable for its conservation value, being designated as a Special Area of Conservation (SAC) and Ramsar site and containing many Irish Red Data List species, including the pointed stonewort (Nitella mucronata) and aquatic invertebrates such as the pond skater (Limnoporus rufoscutellatus), water beetles (Donacia aquatica, D. bicolora, Gyrinus distinctus, G. natator and Hydroporus glabriusculus) and white-clawed crayfish (Austropotabius pallipes). E. nuttallii was first recorded in Lough Erne in 2006.
Macrophyte field and laboratory methods
Data on macrophyte community composition were obtained for both Upper and Lower Lough Erne from the Water Management Unit of the NIEA. These data represent a total of 15 transects in Upper Lough Erne during 2007 and 2010 and 18 transects in Lower Lough Erne during 2006 and 2009. Surveys were carried out by wading and by boat depending on water depth. Macrophyte species and percentage cover were recorded within 5 m2 quadrats positioned every 5 m along each transect perpendicular to the shoreline until the edge of the macrophyte zone was reached. Nitrogen and phosphorus (NO3N, NO2N, NH4N, total organic nitrogen, soluble P, and total P) were measured in surface waters in late July or August for each survey year at a central point in Upper Lough Erne and two points in Lower Lough Erne (Fig. 1). These chemistry data are included to account for differences between lakes and over time, rather than smaller scale differences between transects. Unfortunately, it was not possible to obtain more detailed information on water chemistry due to the historical nature of the dataset. We have also accounted for this issue by using a paired statistical design which means that we are not comparing between quadrats from different parts of the lakes. Only quadrats which were surveyed in both years were used in the analysis (n = 728 quadrats).
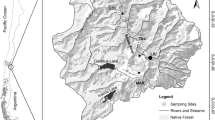
a Field sites for study of impacts of E. nuttallii on dissolved oxygen, chorophyll a, pH, algae and invertebrates. Samples were paired within sites such that samples were taken from a stand of E. nuttallii and a stand of native plants within each site, b inset map of Ireland showing field site locations
In order to determine whether the presence of E. nuttallii affected the structure of macrophyte beds, each macrophyte species was allocated to one of eight groups based on its structural characteristics: emergent, free-floating, floating rooted, submerged (canopy forming), submerged (low growing), bryophytes, filamentous algae and charophytes.
Dissolved oxygen, pH, algae and invertebrate study sites
A paired survey design of six sites in Northern Ireland was used to examine the associations between E. nuttallii, dissolved oxygen, pH, and algal and invertebrate communities, between July and September 2010 (Fig. 2). At each site a native macrophyte stand and a stand of the invader were chosen within the same water body (distance between macrophyte stands <500 m). Native species differed between sites, but all had a predominantly submerged habit. Native species and sites were as follows: Potamogeton pectinatus (Lagan), Potamogeton perfoliatus/Myriophyllum spicatum (Ballyronan), Potamogeton natans (Lough Cashel), Ceratophyllum demersum (Loughbrickland and Upper Bann), Sagittaria sagittifolia (Lower Bann). Waterbodies were selected to represent the most common site conditions in which E. nuttallii was found and included three lake sites and three slow-flowing river sites. All samples were taken in shallow water between 0.45 m and 1.05 m in depth. There was no consistent pattern as to whether E. nuttallii or native plants occurred in deeper water (the mean difference in depth between E. nuttallii and native plants within sites was 14 cm). Sites covered a range of nutrient levels from mesotrophic to hypereutrophic (measured total phosphorus ranging from 18 to 1168 μg l−1 and total dissolved nitrogen between 4.61 and 530 μg l−1).
Dissolved oxygen, pH, algae and invertebrate field and laboratory methods
Water chemistry, environmental data and algal sampling took place monthly for 3 months from July to September 2010. The pH and dissolved oxygen were recorded at each site using a Hanna pHep 4 pH meter and a portable dissolved oxygen meter (VWR DO200). Two litres of water was collected within each macrophyte bed for chlorophyll a analysis, filtered using a 0.45 μm Metricel® membrane filter and stored at −20 °C. Chlorophyll a analysis was conducted using methanol-based pigment extraction and spectrophotometry readings (Hamilton 2010). A further two litres of water was collected for nutrient analyses: soluble reactive phosphorus (SRP), total phosphorus (TP), total soluble phosphorus (TSP), total organic nitrogen (TON), ammonium (NH4), nitrogen dioxide (NO2), nitrates (NO3) and total dissolved nitrogen (TDN). Nutrient analyses were conducted by the Agri-Food and Biosciences Institute, Newforge Lane, Belfast, Northern Ireland.
Algal periphyton was collected by taking approximately 10 cm length of plant material from both the tip and the base of the macrophyte with approximately 15 ml of water immediately surrounding the macrophyte leaves. Care was taken to carry out this procedure slowly and carefully in situ to minimise loss of periphyton. Water samples were filtered through a 250 μm mesh within 10 min of sampling to remove zooplankton and preserved using Lugol’s Iodine solution (5 g iodine (I2), 10 g potassium iodide (KI), 85 ml distilled H2O). One algal sample was taken in each invaded and each uninvaded macrophyte bed in each of July, August and September. Algal samples were kept in the dark at 5–7 °C before processing.
Algal periphyton was separated from plant samples by vigorous shaking for 60 s. The algal sample was then transferred into a sterile 20 ml tube. Plant material was dried at 60 °C for 72 h and the dry mass was recorded. The algal sample was placed in a Lund chamber. Five horizontal transects of the chamber were carried out at 100× magnification and larger species were identified and counted. A further 20 random fields of view (450 μm2) were examined at 400× magnification and all species were identified and counted. Taxa were identified to genus level where possible, or to the lowest practical taxonomic level (Bellinger and Sigee 2010; Cox 1996; John et al. 2002). It was not possible to accurately identify all cells under 10 μm; those which could not be identified were measured for biovolume and recorded as “unidentified genera” (1.9 % of total algal biovolume). For unicellular and colonial algae, the first 10 cells or colonies of each genus or species were measured. For filamentous algae, the first 30 filaments were measured as there was greater variation observed in filament length than in cell or colony size. Mean cell biovolumes were calculated using the ‘WISER phytoplankton counter spreadsheet’ (Carvalho et al. 2007) and biovolume formulae were added for new taxa as defined in Hillebrand et al. (1999).
Algal species were categorised into seven functional groups based on Kruk et al. (2010) plus an eighth group of ‘uncategorised genera’ (Supplementary Material, Table S1). These groups have been proposed to be useful predictors of algal responses to environmental variables as they are closely linked with functional characteristics such as prey avoidance, K and r strategies and sinking rates (Kruk et al. 2010).
Invertebrates were sampled during July and late September/early October using two methods at each sampling date. Firstly, at each site, four replicate core samples of sediment were taken from each macrophyte bed using a KC Denmark Kayak core sampler 45 mm in diameter (hereafter, referred to as ‘sediment invertebrate samples’). Secondly, invertebrates present in macrophyte material were collected using a bespoke bucket and mesh trap of 379 cm2 surface area and 300 μm mesh size (hereafter, referred to as ‘macrophyte invertebrate samples’).
Invertebrates were separated from samples using a 250 μm sieve and stored in 70 % ethanol. Plant material was dried at 60° C for 72 h and its dry mass recorded for calculation of macrophyte stand density. All invertebrates were identified to the lowest possible taxonomic level (Edington and Hildrew 1995; Elliott and Mann 1998; Fitter and Manuel 1986; Friday 1998; Gledhill et al. 1993; Savage 1989; Wallace et al. 1990). For sediment invertebrate samples, specimen length, width and dry mass were measured (n = 523). Linear regressions based on the length or width and biomass (transformed by Log10 or a natural logarithm depending on best fit described by the adjusted R2 value) were conducted using SigmaPlot 10 to describe the relationship between individual length/width and biomass for each common invertebrate family or genus (Supplementary Material, Table S2). In taxa that exhibited a significant relationship between length/width and body mass these regression formulae were used to calculate the biomass of individuals of that taxa in the macrophyte invertebrate samples. For all other species dry mass was measured directly. Invertebrate species were further categorised into six functional feeding guilds: collector filterers, collector gatherers, herbivore piercers, predators, scraper grazers and shredders following (Chaloner et al. 2009; Compin and Cereghino 2007; Cummins and Klug 1979; Heino 2008) (Supplementary Material, Table S3).
Statistical analyses
Macrophytes
In Lough Erne, the impact of Elodea spp. on total macrophyte cover, non-Elodea macrophyte cover and species richness (i.e. of native plants) was examined using a Generalized Linear Mixed Model (GLMM) approach. Explanatory variables in the models were year (fitted as a factor with four levels: 2006, 2007, 2009 or 2010), water depth, nutrient concentration, the percentage cover of E. nuttallii, the percentage cover of E. canadensis, and the interaction of E. nuttallii and E. canadensis. Nutrient concentration was expressed as the first axis of a PCA analysis of nitrogen and phosphorus values, which explained 62.7 % of the variance with a positive relationship with nitrogen variables (r = 0.95) and a negative relationship with phosphorus variables (r = −0.67). Quadrat nested within lake was included as a random factor.
All GLMMs were first fitted with a Gaussian distribution and identity link function. Model residuals were tested for normality using a Shapiro–Wilk test. Models for which residuals were not normally distributed were refitted using alternative distributions more suited to the response data. Specifically, gamma distributions with a log-link function were used for continuous response data and a Poisson distribution with a log link function was used for count data (i.e. species richness). In each GLMM, all possible subsets of explanatory variables were ranked using the Akaike Information Criterion adjusted for small sample sizes (AICc), and the most optimal model was taken as that with the lowest AICc value.
Multivariate responses in macrophyte communities were assessed using partial Canonical Correspondence Analysis (pCCA). Two pCCAs were conducted, the first with a response matrix of percentage cover of macrophyte structural groups and a second with percentage cover of macrophyte genera. The associated environmental matrix included the percentage cover of E. nuttallii, E. canadensis, Year (as a factor), water depth and nutrient content. Quadrat was fitted as a random factor. The optimal model was obtained following stepwise forward selection followed by backward stepwise elimination. Explanatory variables were sequentially added to a null model (with site fitted as a random factor) where these variables significantly improved model AICc values based on a permutation test (P < 0.05 for inclusion), and then successively dropped from the model based on the same inclusion criteria. As E. canadensis was not included in the final pCCA model, it was then added to the response matrices (i.e. plant genera and structural datasets).
In order to assess whether species communities where E. nuttallii was present were more similar to each other than those without E. nuttallii, an analysis was carried out on multivariate homogeneity of group dispersion using the function “betadisper” in R. This was conducted based on a Jaccard dissimilarity distance between species communities (i.e. the proportion of species which differed between quadrats where E. nuttallii was present vs. the proportion of species which differed between quadrats where E. nuttallii was not present).
Dissolved oxygen, pH, algae and invertebrates
GLMMs were used to examine all univariate dependent variables in relation to the presence of E. nuttallii. Water chemistry response variables (dissolved O2 saturation, pH and chlorophyll a) were tested for correlation prior to GLMM analysis using Spearman’s rank correlation test. There was no significant correlation between these variables (dissolved O2–chlorophyll a (rho = 0.168, P = 0.327), dissolved O2–pH (rho = 0.286, P = 0.091) and chlorophyll a and pH (rho = 0.086, P = 0.617). Explanatory variables for these physiochemical variables were the presence or absence of E. nuttallii and month (July, August or September), waterbody type (i.e. two level factor “Lake” or “River”) and the interaction between E. nuttallii presence and waterbody type. Site was fitted as a random factor.
Explanatory variables for GLMMs of algal biovolume, algal species richness and macrophyte bed density were the presence and absence of E. nuttallii, month, waterbody type (i.e. a two level factor “Lake” or “River”) and the interaction between E. nuttallii presence and waterbody type, nutrient concentration and the interaction of E. nuttallii and nutrient concentration. Nutrient concentration was expressed as the first axis of a PCA analysis of nitrogen and phosphorus values which explained 64.1 % of the total variance and had a positive relationship with both nitrogen (r = 0.83) and phosphorus variables (r = 0.73). Site was fitted as a random factor.
Invertebrate richness and biomass in both macrophyte samples and sediment core samples were examined as above for algae. However, macrophyte bed density was added as an explanatory variable to each model. Model selection was as above for previous GLMMs.
Multivariate community responses were assessed using pCCA. Response matrices for algae were biovolume of each algal functional group and biovolume of each algal taxon (per unit of plant dry mass). Response matrices for invertebrate species were the biomass of invertebrate feeding guilds and biomass of invertebrate taxa. The associated explanatory environmental matrix included the same factors and covariates as those used in univariate analyses i.e. the presence/absence of E. nuttallii, month and nutrient concentrations, waterbody type and the interaction between E. nuttallii presence and waterbody type, with the addition of plant density in invertebrate models only. Site was fitted as a random factor. Model optimisation was conducted as previously described for pCCAs of macrophyte communities.
In order to assess whether algal and invertebrate communities on E. nuttallii were more similar to each other than those on native plants were to each other we conducted an analysis of multivariate homogeneity of group dispersion using the function “betadisper” in R (as per macrophyte community data).
Unless otherwise stated all analyses were performed using R 3.0.2 (R Core Development Team 2012) and the packages glmmADMB (Fournier et al. 2012), MuMIn (Barton 2013) and vegan (Oksanen et al. 2013).
Results
Macrophytes
Elodea nuttallii was present in 2 % of the 728 quadrats in the initial survey in 2006–2007 and increased to presence in 70 % of quadrats in 2009–2010. Over the same period, the percentage cover of E. nuttallii within each quadrat increased from a mean of 0.03 (0–4 %) to 21.3 (0–100 %) on resurvey in 2009–2010. E. canadensis declined in presence from 33 to 9 % of quadrats and in mean cover per quadrat from 1.1 (0–70 %) to 0.5 (0–30 %) over the same period. A total of 71 other macrophyte species were recorded. E. canadensis and E. nuttallii were the only invasive species recorded in these surveys.
Total macrophyte cover within quadrats was positively associated with cover of both E. nuttallii (β = 0.013 ± 0.003, χ2 = 20.24, P < 0.001) and E. canadensis (β = 0.029 ± 0.012, χ2 = 5.53, P = 0.019). Excluding both Elodea species from the total macrophyte cover, the cover of remaining species was not significantly associated with the cover of either E. nuttallii or E. canadensis, but declined with water depth and differed between years. Both total macrophyte cover and the cover of non-Elodea species were negatively associated with water depth, the PCA axis of nutrient concentration and differed between years (see Supplementary Material, Table S5).
Species richness of macrophytes other than E. nuttallii and E. canadensis (i.e. native species) was positively associated with percentage cover of both E. nuttallii (β = 0.002 ± 0.001, χ2 = 3.85, P = 0.050) and E. canadensis (β = 0.013 ± 0.004, χ2 = 11.58, P < 0.001) and with the PCA axis of nutrient concentrations and negatively associated with water depth and differed between years (see Supplementary Material, Table S5). There was no evidence of an interaction between E. canadensis and E. nuttallii in any model.
The pCCA of macrophyte structural groups showed that year and percentage cover of E. nuttallii influenced structural composition and explained 4.6 % of the variation in plant structure after variation between quadrats (69 %) was accounted for (P = 0.005; Fig. 3). The pCCA of macrophyte genera showed that water depth, year and percentage cover of E. nuttallii influenced composition of genera significantly and explained 3.9 % of the variation after between-quadrat variation (53.9 %) was accounted for (P = 0.005). The percentage cover of E. nuttallii alone (with the other factors accounted for by pCCA) explained only 0.6 and 0.5 % of the variation in structural groups and genera respectively (P = 0.033 and P = 0.005, respectively; Supplementary Material, Table S6). The cover of submersed low-growing species and charophytes was negatively associated with the cover of E. nuttallii, whilst the surface-growing plants (both free-floating and rooted) were positively associated with E. nuttallii (Table 1). At a taxonomic level, the most negatively affected species was E. canadensis whilst Nuphar lutea and Stratiotes aloides were the most positively associated (Table 2). However, variance in plant community explained by E. nuttallii was very low relative to variance between quadrats and between years (Tables 1, 2).
Plot of partial Canonical Correspondence Analysis showing relationships between E. nuttallii and plant functional groups, when year is also fitted an explanatory factor and quadrat ID is accounted for as a random factor. Species scores are unscaled. Axis labels show % of total variation in macrophyte communities explained by each CCA axis. Grey ellipse shows 95 % confidence interval around sites where E. nuttallii is present, dashed grey ellipse shows 95 % confidence interval around sites where E. nuttallii is not present
Analysis of multivariate homogeneity of group dispersion showed that quadrats containing E. nuttallii were more homogeneous (mean Jaccard dissimilarity = 0.43, SE <0.01) than those that did not contain E. nuttallii (mean Jaccard dissimilarity = 0.49, SE <0.01) (F = 24.34, P < 0.01.
Plot of partial Canonical Correspondence Analysis showing relationships between E. nuttallii and invertebrate taxa in lakes, when site is accounted for as a random factor. Species scores are unscaled. Taxonomic groups which were present in more than one sample and for which > 0.5 % of variation is explained by E. nuttallii are shown. Axis labels show % of total variation in macrophyte communities explained by each CCA axis. Grey ellipse shows 95 % confidence interval around sites where E. nuttallii is present, dashed grey ellipse shows 95 % confidence interval around sites where E. nuttallii is absent
Plot of partial Canonical Correspondence Analysis showing relationships between E. nuttallii and invertebrate taxa in rivers, when site is accounted for as a random factor. Species scores are unscaled. Taxonomic groups which were present in more than one sample and for which > 0.5 % of variation is explained by E. nuttallii are shown. Axis labels show % of total variation in macrophyte communities explained by each CCA axis. Grey ellipse shows 95 % confidence interval around sites where E. nuttallii is present, dashed grey ellipse shows 95 % confidence interval around sites where E. nuttallii is absent
Dissolved oxygen, pH, algae and invertebrates
Dissolved O2 saturation differed between lakes and rivers being higher in lakes than in rivers. The presence of E. nuttallii was included in the best model of dissolved O2 saturation (χ2 = 3.21, P = 0.073), being higher in E. nuttallii stands (mean ± SE = 93.97 % ± 5.46) than in native plant stands (85.13 ± 3.86 %). Chlorophyll a showed no significant association with rivers or lakes, months or the presence of E. nuttallii. The pH varied significantly between months, but was not significantly associated with the presence of E. nuttallii (Supplementary Material, Table S7).
Macrophyte bed density did not differ between E. nuttallii and native macrophyte beds and was not associated with any of the other variables tested. The optimal model for algal species richness contained E. nuttallii with marginal significance (χ2 = 3.67, P = 0.055) and month, but not nutrient concentration. Algal biovolume per gram of plant dry mass varied significantly between months. Algal biovolume was not affected by either the presence of E. nuttallii or nutrient concentration (Supplementary Material, Table S8).
The pCCA of algal community data showed no significant effect of E. nuttallii on algal community composition in terms of either functional groups or taxa. The community composition in terms of algal functional groups was not significantly associated with any of the explanatory variables tested. However, nutrient concentration and month significantly affected community composition in terms of algal taxa (P = 0.015). Analysis of multivariate homogeneity of group dispersion did not show any significant difference in the variance between algal communities on E. nuttallii and those on native plants (F = 0.42, P = 0.521).
None of the community metrics of invertebrate species on macrophytes or in the sediment differed between E. nuttallii and native macrophyte samples. Invertebrate species richness (derived from macrophyte samples) varied significantly between months. Invertebrate biomass in macrophyte samples also varied significantly between months and was positively correlated with plant density and nutrient concentration. Invertebrate species richness in sediment cores was not significantly associated with any of the environmental parameters. Invertebrate biomass in the sediment cores was positively associated with nutrient, but not with any of the other environmental parameters (Supplementary Material, Table S9).
The pCCAs of invertebrate taxonomic communities sampled from macrophytes showed a significant effect of the interaction of waterbody type and the presence of E. nuttallii, suggesting that the impact of E. nuttallii on invertebrate communities differed between lakes and rivers. This interaction explained 10 % of the variation in invertebrate communities (P = 0.043) after variation between sites (45 %) was accounted for (P = 0.005). When rivers and lakes were examined separately, E. nuttallii was found to explain 9 % of variation in invertebrate communities in lakes and 13 % of the variation in rivers, after accounting for variation between sites (41 and 33 % respectively; Tables 3, 4; Figs. 4, 5). The pCCAs of invertebrate functional groups from the macrophyte invertebrate samples and the pCCAs of invertebrate community in sediment core samples showed no association with any of the tested variables after accounting for variation between sites (Supplementary Material, Table S10). In addition, analysis of multivariate homogeneity of group dispersion did not show any significant difference in the variance between invertebrate communities associated with E. nuttallii stands and those associated with native plant stands in either macrophyte (F = 0.15, P = 0.702) or sediment samples (F = 1.92, P = 0.179).
Discussion
Freshwater communities associated with E. nuttallii differed in small but significant ways from uninvaded communities. Specifically, we observed differences in oxygen saturation, plant and algal richness, and invertebrate and macrophyte species composition. However, observed differences were small relative to other factors such as nutrient levels, inter-annual variation and differences between sites. Furthermore, there was no evidence of any effect of E. nuttallii on the biovolume of periphytic algae, biomass of invertebrate species or the cover of native macrophyte species. In addition, whilst plant communities in quadrats containing E. nuttallii were more similar to each other than quadrats in which E. nuttallii was not present, no similar effect was observed on algal or invertebrate communities.
The effects of E. nuttallii on species communities could be seen as both positive and negative, for example, the increased species richness of macrophyte species may be contrasted with the lower richness of algal taxa. Increases in floating plants associated with E. nuttallii can be contrasted with declines in submerged species. The association between floating plant species and E. nuttallii may arise as a result of structural complexity where E. nuttallii reaches the water surface, which reduces surface turbidity and provides anchorage for floating species. In addition, floating species are most likely to out-compete E. nuttallii for light and have been shown to out-compete E. nuttallii in high nutrient conditions (Netten et al. 2010; Szabo et al. 2010). Submerged species which are negatively associated include low-growing species which are likely to be shaded by E. nuttallii (such as Eleocharis acicularis, Isoetes spp., Littorella uniflora), canopy-forming submerged species occupying a similar niche space to E. nuttallii (including E. canadensis) and charophyte species.
Although the observed negative association between E. nuttallii and charophytes is small, this is of concern due to the rarity and conservation status of charophyte species. Charophytes are usually low-growing (<0.5 m in height) and are likely to be out-competed for light by E. nuttallii. While this negative association could arise in this study from charophytes reducing the likelihood of establishment of E. nuttallii, this seems unlikely as charophytes have been previously shown to be out-competed by structurally similar invaders from the same plant family (e.g. Lagarosiphon major (Barrs et al. 2008) and E. canadensis (Mjelde et al. 2012)).
The observed negative association between the cover of E. nuttallii and E. canadensis suggests a competitive interaction between these two closely related invasive species. We did not find any indication that E. nuttallii or E. canadensis interact to increase impacts on native macrophyte cover or richness. Therefore, our findings do not support the invasional meltdown hypothesis in the case of E. nuttallii and E. canadensis. In addition, the observed rapid increase range and abundance of E. nuttallii in Lough Erne (such that it is much now much more frequently observed than E. canadensis), supports the suggestion that E. nuttallii may be replacing E. canadensis in parts of its invaded range (Barrat-Segretain 2001; Barrat-Segretain et al. 2002).
It is perhaps surprising that species richness of native macrophytes was positively associated with the presence of E. nuttallii and E. canadensis in Lough Erne, after differences in nutrient levels and between years had been accounted for. Mechanisms for facilitation of native plant species could include alteration of flow rate and turbidity, or increases in primary productivity over time through the release of nutrients from the sediment. However, these alterations could also make conditions suitable for further establishment of E. nuttallii, which can absorb nutrients directly from the water column and is adapted to low-light conditions (Angelstein and Schubert 2008, 2009). An alternative explanation for the positive correlation between E. nuttallii and species richness of native macrophytes is that some other environmental factor, unaccounted for here, facilitates both an increase in E. nuttallii cover (or its establishment) and macrophyte species richness. Previous studies have suggested that while species richness increases resistance to invasion at small spatial scales (Kennedy et al. 2002), such effects may be overwhelmed by environmental factors which co-vary with species richness, such as propagule pressure, resulting in an apparent positive relationship between invasive species and native species richness (Levine 2000; Lonsdale 1999). Furthermore, a recent large-scale study of invasive species in macrophyte communities found no clear relationship between native species richness and exotic species richness (Capers et al. 2007).
In common with previous authors we found that plant density was significantly correlated with the biomass of invertebrate species living on macrophytes (Schultz and Dibble 2012). However, in our study plant density and invertebrate biomass did not differ between E. nuttallii and native plants, reflecting an explicit decision to examine differences between similar native and invasive plant beds. Whilst E. nuttallii may not alter the biomass of invertebrate species relative to similar-sized plants, results from our macrophyte dataset suggest that E. nuttallii may be replacing low-growing species and increasing overall macrophyte cover. Hence, by altering the relative regional abundance of different plant functional groups, E. nuttallii may produce corresponding changes in invertebrate biomass at larger spatial scales.
Differences in invertebrate assemblages associated with macrophytes have also been shown previously for similar submerged invasive species (Hogsden et al. 2007; Kelly and Hawes 2005; Stiers et al. 2011). The reasons for the observed differences in invertebrate species composition may be varied and complex, and are likely to relate to differences in plant architecture, plant palatability, chemical exudates, water chemistry and water flow rates. Oxygen saturation is an important factor in determining invertebrate communities in freshwater environments. Higher oxygen saturation levels associated with E. nuttallii may have influenced species composition here: there was a lower abundance of some species groups associated with low oxygen saturation levels such as true fly larvae in the family Chironomidae, Alderflies (Sialis lutaria), leeches in the genera Erpobdella and Theromyzon, and Asellus isopods, and a higher abundance of some species associated with higher oxygen saturation such as caddisflies in the family Linephiidae. However, several species behaved contrary to expectation based on oxygen saturation alone, suggesting that other factors influence their distributions, for example damselflies in the family Coengriidae were negatively associated with E. nuttallii, leeches in the family Glossiphonidae were positively associated with E. nuttallii, and freshwater snails in the genera Hippeautis, Lymnea, Valvata, Physa and Bithynia, which have similar oxygen requirements, show a range of different responses. Allelopathy may explain observed negative association between E. nuttalii and lepidopteran larvae in the family Pyralidae, as E. nuttalii has been previously shown to retard the growth and reduce the survival of the Pyralidae species Acentria ephemerella under laboratory conditions (Erhard et al. 2007). Where Pyralidae larvae exist in large numbers they may substantially reduce cover of other macrophyte species providing an indirect advantage to Elodea spp. (Gross et al. 2001).
One weakness of the pairing of native and invasive plant beds in this study was that it was not possible to use sites where only E. nuttallii was present (i.e. highly invaded sites). Therefore, if native species are required at particular points in invertebrate life cycles (e.g. reproduction), population declines associated with their absence may not have been detected as invertebrate species could move between plant beds if necessary. Additionally, many Northern Irish water bodies, such as those sampled here, have been subject to considerable pressure from eutrophication, pollution and human disturbance, especially in lowland areas (Heegaard et al. 2001) prior to the introduction of invasive species, such as E. nuttallii. The algal and invertebrate communities present in these waterbodies differ from those in more pristine sites, especially in the relative lack of rare species. Impacts of invasive macrophytes may also differ depending on trophic status of waterbodies (Strayer 2010) and in some cases the same invasive macrophyte species has opposite effects on invertebrates in different study systems (Schultz and Dibble 2012). Therefore, it is possible that the impact of E. nuttallii on invertebrate and algal communities would have been different in oligotrophic sites or more pristine sites which had not been previously impacted by anthropogenic pressures.
Together these field studies provide insights into the potential impacts of the widespread invader E. nuttallii on a range of taxa in temperate waterbodies. Due to the correlational nature of these studies it is not possible to determine cause-and-effect, or to reveal the exact drivers of change in biological communities. Here, where possible, we have used closely paired sites within waterbodies to minimise potentially confounding differences between sites. We suggest that the results of this research may be used to direct further research including both field and laboratory experiments focused on the interaction of E. nuttallii with particular species of concern (e.g. the observed negative association of E. nuttallii and charophytes).
In conclusion, our findings suggest that whilst E. nuttallii significantly altered freshwater communities, observed differences were small relative to other factors such as nutrient levels, inter-annual variation and differences between sites. In addition, we add to a growing body of literature that suggests that the impacts of aquatic invasive plant species are not consistently negative and they may, for example, increase the richness of native plant species or the abundance of invertebrate species if total plant biomass increases as a result of invasion (Schultz and Dibble 2012; Strayer 2010; Thomaz et al. 2012).
References
Angelstein S, Schubert H (2008) Elodea nuttallii: uptake, translocation and release of phosphorus. Aquat Biol 3:209–216
Angelstein S, Schubert H (2009) Light acclimatisation of Elodea nuttallii grown under ambient DIC conditions. Plant Ecol 202:91–101
Barrat-Segretain M (2001) Invasive species in the Rhone River floodplain (France): replacement of Elodea canadensis Michaux by E. nuttallii St. John in two former river channels. Archiv Fur Hydrobiologie 152:237–251
Barrat-Segretain M (2005) Competition between invasive and indigenous species: impact of spatial pattern and developmental stage. Plant Ecol 180:153–160
Barrat-Segretain M, Elger A, Sagnes P, Puijalon S (2002) Comparison of three life-history traits of invasive Elodea canadensis Michx. and Elodea nuttallii (Planch.) H. St. John. Aquat Bot 74:299–313
Barrs JR, Keenan EA, O’Callaghan P, Caffrey J (2008) Research and control programme for Lagarosiphon major (Hydrocharitaceae). Central Fisheries Board, Ireland
Barton K (2013) MuMIn: multi-model inference. R package version 1(9):11
Bellinger EG, Sigee DC (2010) Freshwater algae: identification and use as bioindicators. Wiley-Blackwell, UK
Burks R, Lodge D (2002) Cued in: advances and opportunities in freshwater chemical ecology. J Chem Ecol 28:1901–1917
Burks R, Jeppesen E, Lodge D (2000) Macrophyte and fish chemicals suppress Daphnia growth and alter life-history traits. Oikos 88:139–147
Caffrey JM, Baars J-R, Barbour JH, Boets P, Boon P, Davenport K, Dick J, Early J, Edsman L, Gallagher C, Gross J, Heinimaa P, Horrill C, Hudin S, Hulme PE, Hynes S, MacIsaac HJ, McLoone P, Millane M, Moen TL, Moore N, Newman J, O’Conchuir R, O’Farrell M, Colette O’Flynn, Oidtmann B, Renals T, Ricciardi A, Roy H, Shaw R, van Valkenburg JLCH, Olaf Weyl, Williams F, Lucy FE (2014) Tackling invasive alien species in Europe: the top 20 issues. Manag Biol Invasion 5:1–20
Capers RS, Selsky R, Bugbee GJ, White JC (2007) Aquatic plant community invasibility and scale-dependent patterns in native and invasive species richness. Ecology 88:3135–3143
Carniatto N, Thomaz SM, Cunha ER, Fugi R, Ota RR (2013) Effects of an invasive alien Poaceae on aquatic macrophytes and fish communities in a neotropical reservoir. Biotropica 45:747–754
Carvalho L, Dudley B, Dodkins I, Clarke R, Jones I, Thackeray S, Maberly S (2007) Final report project WFD80 Phytoplankton classification tool (Phase 2). SNIFFER, Edinburgh
Chaloner DT, Hershey AE, Lamberti GA (2009) Benthic invertebrate fauna. Elsevier, New York
Champion PD, Clayton JS, Hofstra DE (2010) Nipping aquatic plant invasions in the bud: weed risk assessment and the trade. Hydrobiologia 656:167–172
Compin A, Cereghino R (2007) Spatial patterns of macroinvertebrate functional feeding groups in streams in relation to physical variables and land-cover in Southwestern France. Landsc Ecol 22:1215–1225
Cox EJ (1996) Identification of freshwater diatoms from live material. Chapman and Hall, London
Cummins KW, Klug MJ (1979) Feeding ecology of stream invertebrates. Annu Rev Ecol Syst 10:147–172
DAISIE (2015) 100 of the worst. http://www.europe-aliens.org/speciesTheWorst.do. Accessed 16 Jan 2015
Declerck S, Vanderstukken M, Pals A, Muylaert K, de Meester L (2007) Plankton biodiversity along a gradient of productivity and its mediation by macrophytes. Ecology 88:2199–2210
deWinton MD, Clayton JS (1996) The impact of invasive submerged weed species on seed banks in lake sediments. Aquat Bot 53:31–45
Edington JM, Hildrew AG (1995) Sp. 53 caseless cadis larvae (Trichoptera): a revised key to the caseless cadis larvae of the British Isles, with notes on their ecology. Freshwater Biological Association, Ambleside
Elliott JM, Mann KH (1998) Sp. 40 Leeches. A key to the British Freshwater Leeches, with notes on their life cycles and ecology. Freshwater Biological Association, Ambleside
Erhard D, Gross EM (2006) Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton. Aquat Bot 85:203–211
Erhard D, Pohnert G, Gross EM (2007) Chemical defense in Elodea nuttallii reduces feeding and growth of aquatic herbivorous Lepidoptera. J Chem Ecol 33:1646–1661
Evangelista HBA, Thomaz SM, Umetsu CA (2014) An analysis of publications on invasive macrophytes in aquatic ecosystems. Aquat Invasions 9:521–528
Fitter R, Manuel R (1986) Field guide to lakes, rivers, streams and ponds of north-west Europe. Harper Collins, Hong Kong
Fournier D, Skaug H, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, Sibert J (2012) AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw 27:233–249
Friday LE (1998) A key to the adults of British water beetles. J Field Stud Counc 7:1–152
Gledhill T, Sutcliffe DW, Williams WD (1993) British freshwater Crustacea Malacostraca: a key with ecological notes. Freshwater Biological Association, Cumbria
Gross EM, Johnson RL, Hairston NG (2001) Experimental evidence for changes in submersed macrophyte species composition caused by the herbivore Acentria ephemerella (Lepidoptera). Oecologia 127:105–114
Hamilton G (2010) The determination of Chlorophyll a in freshwater. Agri-food and biosciences standard operating procedure. Agri-food and Biosciences Institute, Belfast
Hamilton SK, Lewis WM, Sippel SJ (1992) Energy-sources for aquatic animals in the Orinoco River floodplain—evidence from stable isotopes. Oecologia 89:324–330
Heegaard E, Birks H, Gibson C, Smith SJ, Wolfe-Murphy S (2001) Species–environmental relationships of aquatic macrophytes in Northern Ireland. Aquat Bot 70:175–223
Heino J (2008) Patterns of functional biodiversity and function-environment relationships in lake littoral macroinvertebrates. Limnol Oceanogr 53:1446–1455
Herault B, Bornet A, Tremolieres M (2008) Redundancy and niche differentiation among the European invasive Elodea species. Biol Invasions 10:1099–1110
Hillebrand H, Durselen C, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Hogsden K, Sager E, Hutchinson T (2007) The impacts of the non-native macrophyte Cabomba caroliniana on littoral biota of Kasshabog Lake, Ontario. J Great Lakes Res 33:497–504
Hulme PE (2012) Weed risk assessment: a way forward or a waste of time? J Appl Ecol 49:10–19
Hussner A (2012) Alien aquatic plant species in European countries. Weed Res 52:297–306
James C, Eaton J, Hardwick K (1999) Competition between three submerged macrophytes, Elodea canadensis Michx, Elodea nuttallii (Planch.) St John and Lagarosiphon major (Ridl.) moss. Hydrobiologia 415:35–40
John DM, Whitton BA, Brook AJ (2002) The freshwater algal flora of the British Isles: An identification guide to freshwater and terrestrial algae. Cambridge University Press, Cambridge
Kelly D, Hawes I (2005) Effects of invasive macrophytes on littoral-zone productivity and foodweb dynamics in a New Zealand high-country lake. J N Am Benthol Soc 24:300–320
Kelly R, Leach K, Cameron A, Maggs CA, Reid N (2014a) Combining global climate and regional landscape models to improve prediction of invasion risk. Divers Distrib 20:884–894
Kelly R, Lundy MG, Mineur F, Harrod C, Maggs CA, Humphries NE, Sims DW, Reid N (2014b) Historical data reveal power-law dispersal patterns of invasive aquatic species. Ecography 37:581–590
Kennedy T, Naeem S, Howe K, Knops JMH, Tilman D, Reich P (2002) Biodiversity as a barrier to ecological invasion. Nature 417:636–638
Kornijow R, Vakkilainen K, Horppila J, Luokkanen E, Kairesalo T (2005) Impacts of a submerged plant (Elodea canadensis) on interactions between roach (Rutilus rutilus) and its invertebrate prey communities in a lake littoral zone. Freshw Biol 50:262–276
Kovalenko KE, Dibble ED (2014) Invasive macrophyte effects on littoral trophic structure and carbon sources. Hydrobiologia 721:23–34
Kruk C, Huszar V, Peeters E, Bonilla S, Costa L, Lurling M, Reynolds CS, Scheffer M (2010) A morphological classification capturing functional variation in phytoplankton. Freshw Biol 55:614–627
Leung B, Roura-Pascual N, Bacher S, Heikkila J, Brotons L, Burgman MA, Dehnen-Schmutz K, Essl F, Hulme PE, Richardson DM, Sol D, Vila M (2012) TEASIng apart alien species risk assessments: a framework for best practices. Ecol Lett 15:1475–1493
Levine J (2000) Species diversity and biological invasions: Relating local process to community pattern. Science 288:852–854
Lonsdale W (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80:1522–1536
Michelan T, Thomaz S, Mormul R, Carvalho P (2010) Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshwater Biol 55:1315–1326
Mjelde M, Lombardo P, Berge D, Johansen SW (2012) Mass invasion of non-native Elodea canadensis Michx. in a large, clear-water, species-rich Norwegian lake—impact on macrophyte biodiversity. Int J Limnol 48:225–240
Montgomery WI, Lundy MG, Reid N (2012) ‘Invasional meltdown’: evidence for unexpected consequences and cumulative impacts of multispecies invasions. Biol Invasions 14:1111–1125
Netten J, Arts G, Gylstra R, Roijackers RMM (2010) Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii in mesocosms. Fundam Appl Limnol 177:125–132
O’Hare M, Gunn I, Chapman D, Dudley BJ, Purse BV (2012) Impacts of space, local environment and habitat connectivity on macrophyte communities in conservation lakes. Divers Distrib 18:603–614
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) vegan: Community Ecology Package. R Package Version 2.0-9
R Core Development Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rybicki N, Landwehr J (2007) Long-term changes in abundance and diversity of macrophyte and waterfowl populations in an estuary with exotic macrophytes and improving water quality. Limnol Oceanogr 52:1195–1207
Sala OE, Chapin FS III, Armesto JJ, Eric Berlow, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Savage AA (1989) Adults of the British Aquatic Hemiptera Heteroptera: a key with ecological notes. Freshwater Biological Association, Ambleside
Schultz R, Dibble E (2012) Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: the role of invasive plant traits. Hydrobiologia 684:1–14
Simberloff D (2006) Invasional meltdown 6 years later: important phenomenon, unfortunate metaphor, or both? Ecol Lett 9:912–919
Stiers I, Crohain N, Josens G, Triest L (2011) Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biol Invasions 13:2715–2726
Strayer D (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174
Szabo S, Scheffer M, Roijackers R, Waluto B, Braun M, Nagy PT, Borics G, Zambrano L (2010) Strong growth limitation of a floating plant (Lemna gibba) by the submerged macrophyte (Elodea nuttallii) under laboratory conditions. Freshw Biol 55:681–690
Thiébaut G, Di Nino F, Peltre M-C, Wagner P (2008) Management of aquatic exotic plants: the case of Elodea species. In: Sengupta M, Dalwani R (eds) The 12th world lake conference. Taal, pp 1058–1066
Thomaz SM, Silveira MJ, Michelan TS (2012) The colonization success of an exotic Poaceae is related to native macrophyte richness, wind disturbance and riparian vegetation. Aquat Sci 74:809–815
Thouvenot L, Puech C, Martinez L, Haury J, Thiebaut G (2013) Strategies of the invasive macrophyte Ludwigia grandiflora in its introduced range: Competition, facilitation or coexistence with native and exotic species? Aquat Bot 107:8–16
Toporowska M, Pawlik-Skowronska B, Wojtal AZ (2008) Epiphytic algae on Stratiotes aloides L., Potamogeton lucens L., Ceratophyllum demersum L. and Chara spp. in a macrophyte-dominated lake. Oceanol Hydrobiol St 37:51–63
Valinoti C, Ho C, Armitage A (2011) Native and exotic submerged aquatic vegetation provide different nutritional and refuge values for macroinvertebrates. J Exp Mar Biol Ecol 409:42–47
van Donk E, van de Bund W (2002) Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquat Bot 72:261–274
Wallace ID, Wallace B, Philipson GN (1990) A key to the case-bearing caddis larvae of Britain and Ireland. Freshwater Biological Association, Ambleside
Warfe DM, Barmuta LA (2006) Habitat structural complexity mediates food web dynamics in a freshwater macrophyte community. Oecologia 150:141–154
Wu Z, Gao Y, Wang J, Liu BY, Zhou QH, Zhang YY (2009) Allelopathic effects of phenolic compounds present in submerged macrophytes on Microcystis aeruginosa. Allelopathy J 23:403–410
Acknowledgments
This research was funded by the Natural Heritage Research Partnership (NHRP) between the Northern Ireland Environment Agency (NIEA) and Quercus, Queen’s University Belfast (QUB) under a PhD studentship (QU08-05). Water chemistry analyses for the field study were conducted by the Agri-Food and Biosciences Institute, Newforge Lane, Belfast. Data from Lough Erne surveys were kindly supplied by Brenda Walker of the NIEA. Thanks to Irena Tománková for her assistance with invertebrate identification. We also thank our NIEA client officers, John Early and Tony Waterman, for their support. Thanks also to two anonymous reviewers whose advice substantially improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kelly, R., Harrod, C., Maggs, C.A. et al. Effects of Elodea nuttallii on temperate freshwater plants, microalgae and invertebrates: small differences between invaded and uninvaded areas. Biol Invasions 17, 2123–2138 (2015). https://doi.org/10.1007/s10530-015-0865-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0865-8