Abstract
Many amphibian populations are in decline worldwide. Surprisingly, few studies have examined how such declines may benefit mosquitoes. Amphibian larvae may compete with and prey upon mosquito larvae, and may alter oviposition habitat selection (OHS) of mosquito adults. However, often overlooked, observed among-pool egg distributions attributed to OHS may additionally or alternatively be explained by egg predation. Temporary pools of mountainous areas of the Mediterranean serve as larval habitat for both the mosquito, Culiseta longiareolata, and the salamander, Salamandra infraimmaculata. We found Culiseta larvae and egg rafts to be highly vulnerable to predation by pre-metamorphosing Salamandra larvae, but not to metamorphosing ones. In outdoor mesocosm experiments, oviposition avoidance by Culiseta females in response to caged Salamandra was not demonstrated regardless of salamander developmental stage. Egg raft abundance was significantly reduced in free-roaming, pre-metamorphosing Salamandra but not by metamorphosing ones. Thus, Salamandra larvae may have little deterrence on Culiseta oviposition. Instead, fewer egg rafts are attributed largely to egg predation. This study highlights the importance of egg raft predation in addition to OHS when interpreting the influence of predators on prey egg distributions. It also highlights that a cost of declining amphibian populations is their reduced impacts on mosquito populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many species of amphibians worldwide, more than other taxa, are experiencing population declines, local extinctions, and even global extinctions (Alford & Richards, 1999). One taxonomic group that may benefit from these amphibian population declines and extinctions are mosquitoes. Surprisingly few studies have considered the roles of amphibians in affecting mosquitoes. Yet, these limited studies demonstrate that anuran larvae may be strong competitors (Blaustein & Margalit, 1994, 1996; Mokany & Shine, 2003; Stav et al., 2010) and sometimes predators (Blaustein & Margalit, 1996; Blum et al., 1997) of mosquito larvae, while urodele larvae, known to drastically structure communities via predation (Morin, 1980; Blaustein et al., 1996; Benoy, 2008), may be important predators of mosquito larvae as well (Brodman & Dorton, 2006; DuRant & Hopkins, 2008; Rubbo et al., 2011; Reinhardt et al., 2013).
Amphibians may also influence oviposition habitat selection (OHS) by mosquitoes. Natural selection should favor the ability of gravid females to assess habitat quality for their progeny. In temporary pools, habitat quality can be defined in significant part by predation pressure (e.g., Morin et al., 1983; Wellborn et al., 1996). Future risk of predation to progeny in temporary pool habitats, if detectable, can be fairly predictable to a gravid female searching for an oviposition site because most predatory stages of aquatic predators are confined to the pool into which they were deposited by their mother (Blaustein, 1999). Indeed, there is a growing body of literature that is demonstrating that many pond organisms with complex life cycles including mosquitoes (reviewed in Vonesh & Blaustein, 2010), other aquatic insects (e.g., Resetarits, 2001), and amphibians (e.g., Binckley & Resetarits, 2008) are able to assess water bodies for risk of predation to their progeny, and choose oviposition sites accordingly. OHS by mosquitoes in response to risk of predation by urodeles has very rarely been assessed (Rubbo et al., 2011).
Experiments that demonstrate that there are fewer prey eggs observed in pools containing predators have not necessarily demonstrated that the mechanism for this observed distribution is OHS in response to predation risk; when the predator is not caged, a plausible explanation for such a pattern is that the prey females are ovipositing randomly with regards to among-pool predator distributions, but that the predators are preying upon the eggs, leaving fewer eggs for the researcher to observe and count. Few studies have considered this possibility, yet egg predation may be prevalent across prey species and predator species in temporary pools (Vonesh, 2005). Ecologists may try to rule out this possibility by including a caged-predator treatment, where presumably, the predator risk cues, in particular, predator-released kairomones, are readily detectable by prey females outside the cage in the pool but the predators cannot eat the eggs (e.g., Blaustein et al., 2004). Alternatively, experimental designs may employ predator-conditioned water but without the predator itself (Munga et al., 2006; Silberbush et al., 2010). However, an experimental design demonstrating no reduction in a caged-predator or predator-conditioned water treatment but a reduction in a free-roaming predator treatment does not necessarily indicate by default, egg predation: prey females may still detect cues of predation risk in free-roaming predator treatments and not in caged-predator or predator-conditioned water treatments by visual or tactile cues (Devereaux & Mokany, 2006). Mosquito egg raft predation or egg raft disruption has rarely been tested, yet it has been demonstrated for backswimmers (Chesson, 1984) and odonates (Stav et al., 1999).
Of interest in this paper are the potential effects of the larval fire salamander, Salamandra infraimmaculata Martens, on egg raft distributions of the mosquito, Culiseta longiareolata Macquart. This ubiquitous and abundant mosquito is likely found along the entire range of S. infraimmaculata and over much of the range of other Salamandra species (Margalit et al., 1988; Roiz et al., 2007; Becker & Hoffmann, 2011). S. infraimmaculata is considered locally endangered in Israel (Dolev & Perevolotsky, 2004). Their larvae are keystone, size-selective predators in temporary and permanent pools (Blaustein et al., 1996; Eitam et al., 2005), drastically reducing amphibian and large macroinvertebrate densities, including those of C. longiareolata (Segev & Blaustein, 2007; Blaustein, unpublished data). C. longiareolata oviposit egg rafts on the water surface between dusk and dawn. C. longiareolata larvae, relative to other mosquito species, are highly vulnerable to a wide variety of predators (Blaustein, 1998; Roberts, 2012). The females of this species have been shown to be able to chemically detect and avoid various backswimmer (Notonectidae) species (Eitam et al., 2002; Silberbush et al., 2010) when ovipositing, and to avoid Anax imperator (Aeshnidae: Anisoptera) larvae when ovipositing, apparently by means other than chemical cues (Stav et al., 1999, 2000). C. longiareolata egg rafts have also been shown to be vulnerable to predation by A. imperator (Stav et al., 1999).
The risk of predation by amphibians may depend on the developmental stage of the amphibian present in the pool. Many, but not all, amphibian species cease to feed when metamorphosing (Wells, 2007). This has never been explicitly tested for larval Salamandra. If Salamandra larvae, which are voracious predators for 2 months or more prior to metamorphosis (Degani, 1996; Eitam et al., 2005), cease to predate at some point in the metamorphic process, they would still be present in the water but nonlethal to prey for between several days and several weeks while completing metamorphosis. For some aquatic prey species, detecting predation risk comes in the form of detecting cues from consumed or injured prey (e.g., Laurila et al., 1997; Mogali et al., 2011). If C. longiareolata are capable of detecting risk of predation from chemical cues resulting from ingested prey, then these cues may not exist for metamorphosing larvae. Thus, if C. longiareolata avoids ovipositing in response to cues left from consumed larvae, they will not avoid the non-feeding metamorphosing larvae when ovipositing.
Here, we first conducted experiments to compare predation rates on C. longiareolata larvae and egg rafts by metamorphosing S. infraimmaculata larvae and similarly sized pre-metamorphosing larvae. We then conducted experiments to determine whether among-pool egg raft distributions were affected by either caged or free-roaming Salamandra larvae and if so, whether this distribution was affected by different developmental states—i.e., pre-metamorphosing versus metamorphosing larvae. These experiments, all combined, allowed us to assess whether any among-treatment differences in distribution in egg rafts could be attributed to egg raft predation and/or to OHS.
Methods
Study animals
Salamandra infraimmaculata larvae used in these experiments were collected from breeding sites in the Galilee Mountains, Israel. Both pre-metamorphosing and metamorphosing Salamandra used were the same size: 3.4–3.6 cm snout-vent length. While anurans have very specific, described developmental stages from egg to complete metamorphosis (Wells, 2007), no such general description exists for Salamandra. Pre-metamorphosing Salamandra larvae are characterized by full tail fins and long gills compared to shrunken, narrow tail fins and shrunken, short gills of metamorphosing larvae (e.g., Degani, 1996). For metamorphosing individuals, we chose individuals that had roughly one-half the width in tail fin and one-half the length of gills found in pre-metamorphosing larvae. Larvae of some, but not all, urodele species cease to feed during the process of metamorphosis (Wells, 2007), though it was not documented, prior to this study if metamorphosing Salamandra cease to feed.
The experiments were conducted with permission of the Israel Nature and Parks Authority permit 2009/36605 and the Haifa University Animal Experimentation Ethics Committee permit 190/10.
Experiment 1. Predation by metamorphosing and pre-metamorphosing Salamandra infraimmaculata larvae on Culiseta longiareolata larvae
To compare predation rates by metamorphosing and pre-metamorphosing salamander larvae on C. longiareolata larvae, we conducted laboratory predation trials between March and April 2010. Rectangular laboratory tubs (length × width × height: 29 × 18 × 13 cm) with 3 l of aged tap water served as arenas to measure predation. Treatments consisted of (1) control (no Salamandra larva); (2) one pre-metamorphosing Salamandra larva; (3) one metamorphosing Salamandra larva. Salamandra larvae were not fed for 24 h prior to a predation trial. Twenty-third-instar C. longiareolata larvae, collected from outdoor, water-filled plastic tubs, were placed into each tub. The number surviving was counted after 1 h. Temperatures ranged from 22 to 24° C. Each treatment was replicated eight times.
Experiment 2. Egg raft predation
To determine whether metamorphosing and pre-metamorphosing Salamandra larvae prey upon C. longiareolata egg rafts, we conducted an outdoor experiment in plastic containers (54 × 41 × 20 cm) filled to 20 l of aged tap water. Treatments consisted of (1) control (no Salamandra larvae); (2) three pre-metamorphosing Salamandra larvae; (3) three metamorphosing Salamandra larvae. This density is well within the range of natural densities (Blaustein, unpublished data). The salamander larvae were allowed to acclimate to the tubs for 48 h prior to adding egg rafts. Trials, seven per treatment, were conducted using Culiseta egg rafts between mid-April and mid-May 2010. Just prior to sunset, to each tub, we added three C. longiareolata egg rafts that had been oviposited in other tubs the previous night. We covered the top with mosquito window screening to prevent additional mosquito oviposition and influences by most other animals, but not necessarily small insects. Fire ants have been shown to prey on single mosquito eggs on moist soil above the water surface (Lee et al., 1994). The next morning, we counted remaining egg rafts.
In addition, to further determine if missing egg rafts in the outdoor mesocosm experiment were due to Salamandra egg raft predation, we also conducted observations in the laboratory. Into rectangular plastic tubs (length × width × height: 29 × 18 × 13 cm) containing 2 l of water, we added a single C. longiareolata egg raft to 7 tubs without a Salamandra larvae and to 14 tubs containing a single Salamandra larvae of similar size to other experiments and starved for 48 h. In none of the replicates were the Salamandra initially attracted to any movement caused by the placement of the egg raft into the water. We observed whether the egg raft was still present after 2 h. We additionally videoed several tubs with a Salamandra larva and egg raft (Nikon D3100 camera with 105 macro lens) to capture potential egg raft predation.
Experiment 3. Effects of pre-metamorphosing Salamandra on among-pool egg raft distribution
We conducted an outdoor, artificial pool experiment to determine if pre-metamorphosing Salamandra larvae (caged or free) could influence the observed among-pool distribution of C. longiareolata egg rafts. The experiment was conducted in the rural village of Koranit, Lower Galilee, Israel (460 m asl) in a yard partially shaded by Punica granatum trees. To serve as artificial pools, we used plastic tubs identical to those used in the egg raft predation experiment (length × width × height: 54 × 41 × 20 cm) with ~20 l of a mixture of rainwater and aged tap water. We added 1 g of dry cat food (Friskies Indoor Delight) to serve as a nutrient base. We randomly assigned artificial pools to one of three treatments: (1) control (no Salamandra larvae); (2) caged pre-metamorphosing Salamandra larvae (three Salamandra were caged and thus were unable to prey on deposited egg rafts or interfere with alighting mosquito females); (3) free pre-metamorphosing Salamandra (three Salamandra outside the cage and thus could potentially prey upon egg rafts and attack or interfere with alighting mosquito females).
On 5 March, we added three pre-metamorphosing Salamandra larvae to all free and caged Salamandra tubs. Individuals that initiated metamorphosis were removed and replaced. Each treatment was replicated with six tubs (total = 18 tubs). Inter-tub distances were ~0.5–0.7 m. Previous studies showed C. longiareolata adults to be able to discriminate between non-predator and predator (backswimmer) tubs at these inter-pool distances (e.g., Eitam et al., 2002; Blaustein et al., 2004). To all 18 tubs, regardless of treatment, we added a cage [2 l plastic bottle with a grid of holes (3 mm diameter every 2 cm)]. The holes allowed chemicals released by a caged Salamandra to diffuse from the cage into the tub. We added cages to all pools (not just the caged Salamandra pools) so that any potential effects of Salamandra on mosquito behavior observed in the caged treatment would not be confounded with effects of the cage itself. To further ensure that the tub water itself outside the cage was conditioned with Salamandra-released chemicals, each morning, we lifted the cage above the tub momentarily to allow the entire water content inside the cage to drain into the tub, and then immediately returned the cage to the tub. Because caged Salamandra likely fed less than free-roaming Salamandra, and because we were limited by our permit in the number of larvae that we could use, every 2 days, we removed all Salamandra larvae, and those inside cages were transferred to a randomly chosen free-Salamandra tub and those that were outside the cages (free treatment) were transferred into cages in randomly chosen caged treatment tubs. C. longiareolata egg rafts were counted and collected from 7–15 March 2010 (=9 nights).
Experiment 4. Effects of metamorphosing Salamandra larvae on among-pool egg raft distributions
This experiment was identical to the previous one, except that we used metamorphosing larvae instead of pre-metamorphosing ones, nearly completely metamorphosed individuals were replaced with partially metamorphosed as described earlier, and egg rafts were counted and collected from 29 March to 7 April 2010 (=9 nights).
Statistical analysis
For response variables C. longiareolata larval survival (experiment 1), egg raft survival (experiment 2), and total number of egg rafts per pool (experiments 3 and 4), we first subjected the data to a Levene’s test for homogeneity of variance. In each case, we failed to detect a deviation in homogeneity of variance and thus used the non-transformed numbers in conducting analyses of variance, choosing an alpha level of 0.05 (two tailed). In the cases of experiments 3 and 4, there were no indications of time × treatment interactions, and particularly because of the low number of egg rafts on any given day, we used the sum across all days for each pool as the response variable. If the ANOVA revealed a statistically significant difference, we then tested for treatment differences using a Tukey’s HSD test.
Results
Experiment 1. Predation by pre-metamorphosing and metamorphosing Salamandra infraimmaculata larvae on Culiseta longiareolata larvae
In the laboratory experiment, Culiseta larvae were vulnerable to predation by pre-metamorphosing salamander larvae only. There was virtually no larval Culiseta mortality in the control replicates (98.1% survival) or in the metamorphosing Salamandra treatment (93.2% survival) but mortality was strongly reduced in the presence of pre-metamorphosing salamander larvae (43.1% survival), a statistically significant reduction (F 2,23 = 67.84; P < 0.0001; Fig. 1a).
Larval survival (a) and egg raft survival (b) of C. longiareolata in the absence of S. infraimmaculata, and in the presence of metamorphosing or pre-metamorphosing Salamandra larvae. Error bars are one standard error. Different letters above error bars indicate statistically significant differences based on Tukey’s HSD test. The larval survival experiment was conducted in the laboratory and the egg raft survival experiment was conducted in outdoor-screened mesocosms
Experiment 2. Predation by pre-metamorphosing and metamorphosing Salamandra infraimmaculata larvae on Culiseta longiareolata egg rafts
Consistent with the results of Experiment 1, in the outdoor tub egg raft predation experiment, Culiseta egg rafts were vulnerable only to pre-metamorphosing salamander larvae. Egg raft survival was high in both the control and metamorphosing Salamandra tubs, but was reduced by 50% in the pre-metamorphosing salamander larvae tubs (F 2,18 = 7.00, P = 0.0056; Fig. 1b). In the laboratory observations, we found that 7 of 7 egg rafts were still present on the water surface after 48 h in the absence of a Salamandra larva while only 5 of 14 were still present in the presence of a pre-metamorphosing Salamandra larva. An online video demonstrates the ‘capture’ and swallowing of egg rafts (Online Resource 1).
Experiment 3. Effects of pre-metamorphosing Salamandra on among-pool egg raft distribution
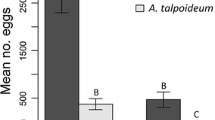
Egg rafts were most abundant in control pools. There were 24% fewer in the caged Salamandra treatment though this difference was not statistically significant. Compared to the control tubs, there was a statistically significant 68% reduction of egg rafts in the free-roaming Salamandra tubs (F 2,15 = 4.01; P = 0.040; Fig. 2a).
Culiseta longiareolata egg raft distribution among outdoor mesocosms with no, caged, or free-roaming S. infraimmaculata. Graph a represents the experiment done with pre-metamorphosing Salamandra larvae. Graph b represents the experiment done with metamorphosing Salamandra larvae. Error bars are one standard error. Different letters above error bars indicate statistically significant differences based on Tukey’s HSD test
Experiment 4. Effects of metamorphosing Salamandra on among-pool egg raft distribution
Metamorphosing Salamandra, caged or free-roaming, did not cause any statistically significant reduction in egg rafts (F 2,15 = 0.12, P = 0.889; Fig. 2b).
Discussion
Many urodele species worldwide are of conservation concern, and their population declines and extinctions may be beneficial for mosquito populations. Our study adds to a surprisingly small number of studies that tests and demonstrates the strong predatory effects that larval urodeles can have on mosquito larvae (Brodman & Dorton, 2006; DuRant & Hopkins, 2008; Rubbo et al., 2011). Our study also demonstrates for the first time that a urodele preys upon mosquito egg rafts.
We found that C. longiareolata larvae are highly vulnerable to predation by pre-metamorphosing S. infraimmaculata larvae but not to metamorphosing ones. Vulnerability of this larval mosquito to pre-metamorphosing salamander larvae is not surprising. S. infraimmaculata is a generalist predator (e.g., Blaustein et al., 1996; Eitam et al., 2005) and C. longiareolata larvae, even in comparison with larvae of other mosquito species, are highly vulnerable to a wide range of predators (Blaustein & Whitman, 2009; Roberts, 2012).
We found that pre-metamorphosing, but not metamorphosing S. infraimmaculata prey on egg rafts. Very few studies have examined and shown that predators of any taxonomic group (backswimmers: Chesson, 1984; odonate larvae: Stav et al., 1999) prey upon, or disrupt and break up, mosquito egg rafts. Fire ants can prey upon single mosquito eggs laid on moist soil (Lee et al., 1994). Some terrestrial arthropods may prey upon egg rafts on the water surface but to our knowledge, this has not been studied. We know of no previous studies demonstrating that urodele larvae are predators of mosquito eggs. Although Salamandra larvae generally are strongly attracted to prey movement, they also may use olfactory cues to detect prey. For example, larvae this species have been fed in the laboratory with minced beef liver (Cohen et al., 2006). They apparently use chemical cues to determine genetic similarity of conspecific larvae and modulate their aggression or cannibalism accordingly (Markman et al., 2009). Egg rafts can also become a moving prey item and thus provide a visual-movement cue; they are commonly wind-blown across the water surface or may be moved by water turbulence when a Salamandra larva or other organisms swim near the egg raft.
That metamorphosing S. infraimmaculata did not prey on C. longiareolata is not a trivial finding as many urodele species continue to be predaceous while they metamorphose, though becoming less efficient as they transition from an aquatic suction feeder to a terrestrial predator (Lauder & Shaffer, 1986). Consistent with a reduction to cessation of feeding as they metamorphose, S. infraimmaculata loses considerable weight while metamorphosing (Blaustein, unpublished data). Thus, if prey can accurately track risk of predation, in the case of S. infraimmaculata, it should be able to distinguish between pre-metamorphosing and metamorphosing larvae. One way this could be tracked is if the cue comes from excretions of consumed prey (Laurila et al., 1997).
Traditionally, experiments in real aquatic habitats or outdoor mesocosms have measured the putative effect of mosquito antagonists (predators or competitors) by comparing mosquito larval densities or mosquito emergence in control versus antagonist plots. To assess this, experimenters were implicitly assuming random oviposition with respect to the antagonist. We now know that in many cases, this assumption is not true—that many mosquitoes detect many predators of their offspring and avoid ovipositing in pools of high antagonist risk (Vonesh & Blaustein, 2010). Thus, assuming random oviposition likely overestimates the effect of the antagonists on mosquito populations (Spencer et al., 2002; Blaustein et al., 2010), it is important to understand OHS of mosquitoes and also to properly interpret a reduction of eggs in predator pools. Although we failed to find a reduction of egg rafts in the caged pre-metamorphosing Salamandra treatment (which would indicate OHS), we cannot necessarily conclude that the reduction of egg rafts in the free-roaming pre-metamorphosing Salamandra treatment can be attributed completely to egg raft predation and that OHS does not contribute at all to this reduction. Future studies can determine whether the small but statistically insignificant reduction of egg rafts that we found in the caged treatment is real or additionally, whether the cage itself masks other predator risk cues such as visual or tactile cues that the ovipositing mosquito would normally use to detect and avoid Salamandra including whether mosquito females that have already alighted onto the water surface are frightened off by attacking Salamandra. C. longiareolata has shown strong oviposition avoidance of other insect predators (e.g., Stav et al., 1999; Blaustein et al., 2004). Additionally, crustacean eggs have shown a hatching inhibition in the presence of S. infraimmaculata larvae, presumably an evolutionary response to risk of predation (Blaustein, 1997; Spencer & Blaustein, 2001).
Culiseta longiareolata may not have evolved oviposition avoidance to this particular predator due to low temporal and spatial overlap despite a substantial predatory impact of young salamander larvae on its eggs and larval stages. Salamandra breed largely during the winter and metamorphose by end of April in temporary pools in this region while C. longiareolata breed largely between late March and early June. In addition, our work was conducted near the southern-most limit of S. infraimmaculata (Warburg, 1994; Degani, 1996). C. longiareolata is found both South of Salamandra’s southern border and also at lower elevations within the same region (Margalit et al., 1988; Blank & Blaustein, 2012).
In summary, we have demonstrated that S. infraimmaculata larvae, prior to initiating metamorphosis, prey heavily on the mosquito C. longiareolata—both on its larvae and its eggs. We were unable to demonstrate that this mosquito can also avoid pools containing Salamandra larvae. Understanding the mechanisms explaining fewer egg rafts in the presence of pre-metamorphosing Salamandra larvae is very important when it comes to understanding its effects on prey populations. When a prey mosquito species can avoid predator pools, the effect of the predator on the adult population is reduced (Spencer et al., 2002; Kershenbaum et al., 2012). Thus, predators that do not elicit an oviposition response or a weak oviposition response in mosquitoes may be more important natural or biocontrol agents than predators that do elicit oviposition avoidance.
References
Alford, R. A. & S. J. Richards, 1999. Global amphibian declines: a problem in applied ecology. Annual Review of Ecology and Systematics 30: 133–165.
Becker, N. & D. Hoffmann, 2011. First record of Culiseta longiareolata (Macquart) for Germany. European Mosquito Bulletin 29: 143–150.
Benoy, G. A., 2008. Tiger salamanders in prairie potholes: a fish in amphibian’s garments? Wetlands 28: 464–472.
Binckley, C. A. & W. J. Resetarits Jr, 2008. Oviposition behaviour partitions aquatic landscapes along predation and nutrient gradients. Behavioral Ecology 19: 552–557.
Blank, L. & L. Blaustein, 2012. Using ecological niche modeling to predict the distributions of two endangered amphibian species in aquatic breeding sites. Hydrobiologia 693: 157–167.
Blaustein, L., 1997. Nonconsumptive effects of larval Salamandra on its crustacean prey: can eggs detect predators? Oecologia 110: 212–217.
Blaustein, L., 1998. Influence of the predatory backswimmer, Notonecta maculata, on pool community structure. Ecological Entomology 23: 246–252.
Blaustein, L., 1999. Oviposition habitat selection in response to risk of predation: consequences for populations and community structure. In Wasser, S. P. (ed.), Evolutionary Processes and Theory: Modern Perspectives. Kluwer Academic Publishers, Amsterdam: 441–456.
Blaustein, L. & J. Margalit, 1994. Mosquito larvae (Culiseta longiareolata) compete with and prey upon toad (Bufo viridis) immatures. Journal of Animal Ecology 63: 841–850.
Blaustein, L. & J. Margalit, 1996. Priority effects in temporary pools: nature and outcome of mosquito larva-toad tadpole interactions depend on order of entrance. Journal of Animal Ecology 65: 77–84.
Blaustein, L. & D. W. Whitman, 2009. Behavioral plasticity in response to risk of predation: oviposition habitat selection by a mosquito. In Ananthakrishnan, T. N. & D. Whitman (eds), Phenotypic Plasticity in Insects: Mechanisms and Consequences. Science Pub Inc., Plymouth: 263–280.
Blaustein, L., J. Friedman & T. Fahima, 1996. Larval Salamandra drive temporary pool community dynamics: evidence from an artificial pool experiment. Oikos 76: 392–402.
Blaustein, L., M. Kiflawi, A. Eitam, M. Mangel & J. E. Cohen, 2004. Oviposition habitat selection in response to risk of predation: mode of detection consistency across experimental venue. Oecologia 138: 300–305.
Blaustein, L., R. S. Ostfeld & R. D. Holt, 2010. A community-ecology framework for understanding vector and vector-borne disease dynamics. Israel Journal of Ecology & Evolution 56: 251–262.
Blum, S., T. Basedow & N. Becker, 1997. Culicidae (Diptera) in the diet of predatory stages of anurans (Amphibia) in humid biotopes of the Rhine valley in Germany. Journal of Vector Ecology 22: 23–29.
Brodman, R. & R. Dorton, 2006. The effectiveness of pond-breeding salamanders as agents of larval mosquito control. Journal of Freshwater Ecology 21: 467–474.
Chesson, J., 1984. Effect of Notonectids (Hemiptera: Notonectidae) on mosquitoes (Diptera: Culicidae): predation or selective oviposition? Environmental Entomology 13: 531–538.
Cohen, M., D. Yeheskely-Hayon, M. R. Warburg, D. Davidson, G. Halevi & R. Sharon, 2006. Differential growth identified in salamander larvae half-sib cohorts: survival strategy? Development, Growth & Differentiation 48: 537–548.
Degani, G., 1996. Salamandra salamandra at the Southern Limit of Its Distribution. Laser Pages Publication, Kazrin.
Devereaux, J. S. L. & A. Mokany, 2006. Visual and chemical cues from aquatic snails reduce chironomid oviposition. Australian Journal of Zoology 54: 79–86.
Dolev, A. & A. Perevolotsky (eds), 2004. The Red Book of Vertebrates in Israel. Israel Nature and National Parks Protection Authority and Society for Protection of Nature in Israel press, Jerusalem.
DuRant, S. E. & W. A. Hopkins, 2008. Amphibian predation on larval mosquitoes. Canadian Journal of Zoology 86: 1159–1164.
Eitam, A., L. Blaustein & M. Mangel, 2002. Effects of Anisops sardea (Hemiptera: Notonectidae) on oviposition habitat selection by mosquitoes and other dipterans and community structure in artificial pools. Hydrobiologia 485: 183–189.
Eitam, A., L. Blaustein & M. Mangel, 2005. Density and intercohort priority effects on larval Salamandra salamandra in temporary pools. Oecologia 146: 36–42.
Kershenbaum, A., M. Spencer, L. Blaustein & J. E. Cohen, 2012. Modelling evolutionarily stable strategies in oviposition site selection, with varying risks of predation and intraspecific competition. Evolutionary Ecology 26: 955–974.
Lauder, G. V. & H. B. Shaffer, 1986. Functional design of the feeding mechanism in lower vertebrates: unidirectional and bidirectional flow systems in the tiger salamander. Zoological Journal of the Linnean Society 88: 277–290.
Laurila, A., J. Kujasalo & E. Ranta, 1997. Different antipredator behaviour in two anuran tadpoles: effects of predator diet. Behavioral Ecology and Sociobiology 40: 329–336.
Lee, D. K., A. P. Bhatkar, S. B. Vinson & J. K. Olson, 1994. Impact of foraging red imported fire ants (Solenopsis invicta) (Hymenoptera: Formicidae) on Psorophora columbiae eggs. Journal of the American Mosquito Control Association 10: 163–173.
Margalit, Y., C. Dimentman & A. S. Tahori, 1988. Geographical, seasonal and ecological distribution of mosquito larvae (Diptera–Culicidae) in Southern Israel. Archiv Fur Hydrobiologie 112: 233–249.
Markman, S., N. Hill, J. Todrank, G. Heth & L. Blaustein, 2009. Differential aggressiveness covaries with genetic similarity in fire salamander larvae. Behavioral Ecology and Sociobiology 63: 1149–1155.
Mogali, S. M., S. K. Saidpur & B. A. Bhagyashri, 2011. Levels of predation modulate antipredator defense behavior and metamorphic traits in the toad Bufo melanostictus. Journal of Herpetology 45: 428–431.
Mokany, A. & R. Shine, 2003. Competition between tadpoles and mosquito larvae. Oecologia 135: 615–620.
Morin, P. J., 1980. Salamander predation and the structure of temporary pond communities. American Zoologist 20: 810.
Morin, P. J., H. M. Wilbur & R. N. Harris, 1983. Salamander predation and the structure of experimental communities – responses of Notophthalmus and microcrustacea. Ecology 64: 1430–1436.
Munga, S., N. Minakawa, G. Zhou, O. O. J. Barrack, A. K. Githeko & G. Yan, 2006. Effects of larval competitors and predators on oviposition site selection of Anopheles gambiae sensu stricto. Journal of Medical Entomology 43: 221–224.
Reinhardt T., S. Steinfartz, A. Paetzold & M. Weitere, 2013. Linking the evolution of habitat choice to ecosystem functioning: direct and indirect effects of pond reproducing fire salamanders on aquatic–terrestrial subsidies. Oecologia (in press). doi:10.1007/s00442-013-2592-0.
Resetarits, W. J., 2001. Colonization under threat of predation: avoidance of fish by an aquatic beetle, Tropisternus lateralis (Coleoptera: Hydrophilidae). Oecologia 129: 155–160.
Roberts, D., 2012. Responses of three species of mosquito larvae to the presence of predatory dragonfly and damselfly larvae. Entomologia Experimentalis et Applicata 145: 23–29.
Roiz, D., R. Eritja, R. Escosa, J. Lucientes, E. Marques, R. Melero-Alcibar, S. Ruiz & R. Molina, 2007. A survey of mosquitoes breeding in used tires in Spain for the detection of imported potential vector species. Journal of Vector Ecology 32: 10–15.
Rubbo, M. J., J. L. Lanterman, R. C. Falco & T. J. Daniels, 2011. The influence of amphibians on mosquitoes in seasonal pools: can wetlands protection help minimize disease risk? Wetlands 31: 799–804.
Segev, O. & L. Blaustein, 2007. Priority effects of the early breeding fire salamander on the late breeding banded newt. Hydrobiologia 583: 28–275.
Silberbush, A., S. Markman, E. Lewinsohn, E. Bar, J. E. Cohen & L. Blaustein, 2010. Mosquitoes use hydrocarbons to detect larval predators when selecting an oviposition site. Ecology Letters 13: 1129–1138.
Spencer, M. & L. Blaustein, 2001. Hatching responses of temporary pool invertebrates in response to environmental signals. Israel Journal of Zoology 47: 397–418.
Spencer, M., L. Blaustein & J. E. Cohen, 2002. Oviposition habitat selection by mosquitoes (Culiseta longiareolata) and consequences for population size. Ecology 83: 669–679.
Stav, G., L. Blaustein & J. Margalith, 1999. Experimental evidence for predation sensitive oviposition by a mosquito, Culiseta longiareolata. Ecological Entomology 24: 202–207.
Stav, G., L. Blaustein & Y. Margalit, 2000. Influence of nymphal Anax imperator (Odonata: Aeshnidae) on oviposition by the mosquito Culiseta longiareolata (Diptera: Culicidae). Journal of Vector Ecology 25: 190–202.
Stav, G., B. P. Kotler & L. Blaustein, 2010. Foraging response to risks of predation and competition in temporary pools. Israel Journal of Ecology & Evolution 56: 9–20.
Vonesh, J. R., 2005. Egg predation and predator-induced hatching plasticity in the African reed frog, Hyperolius spinigularis. Oikos 110: 241–252.
Vonesh, J. R. & L. Blaustein, 2010. Implications of predator-induced shifts in mosquito oviposition site selection for vector control: a meta-analysis. Israel Journal of Ecology & Evolution 56: 263–279.
Warburg, M. R., 1994. Population ecology, breeding activity, longevity, and reproductive strategies of Salamandra salamandra during an 18-year long study of an isolated population on Mt. Carmel, Israel. Mertensiella 4: 399–421.
Wellborn, G. A., D. K. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27: 337–363.
Wells, K. D., 2007. The Ecology & Behavior of Amphibians. University of Chicago Press, Chicago.
Acknowledgments
We are grateful to the many participants of the European Pond Conservation Network Conference held in Luxembourg, 2012, to Ori Segev, Amots Dafni, and two anonymous reviewers for providing interesting and useful feedback and to Anna Gershberg and Eden Orion for technical help with the video of egg raft predation. This study was funded by Israel Science Foundation Grant 961-2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: R. Céréghino, D. Boix, H.-M. Cauchie, K. Martens & B. Oertli / Understanding the role of ponds in a changing world
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1. Video of two predation events by Salamandra infraimmaculata larva on two Culiseta longiareolata egg rafts.Supplementary material 1 (MPG 2564 kb)
Rights and permissions
About this article
Cite this article
Blaustein, J., Sadeh, A. & Blaustein, L. Influence of fire salamander larvae on among-pool distribution of mosquito egg rafts: oviposition habitat selection or egg raft predation?. Hydrobiologia 723, 157–165 (2014). https://doi.org/10.1007/s10750-013-1554-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1554-1