Abstract
Responding differentially to kin and non-kin is known to be adaptive in many species. One example is the inclusive fitness benefits of reducing aggression toward closer relatives. Little is known, however, about the ability of animals to assess differential degrees of genetic relatedness and to respond accordingly with differential levels of aggression. In the present study, we tested whether aggressiveness between body mass-matched pairs of fire salamander (Salamandra infraimmaculata) larvae covaried with the genetic similarity between them. We quantified aggressiveness at three levels of genetic similarity by selecting pairs within and across pools from recently genotyped populations. We also assessed aggression between pairs of siblings. Aggression and associated injuries decreased as genetic similarity increased across the groups. These findings suggest that cannibalistic salamanders can assess their degree of genetic relatedness to conspecifics and vary their behavioral responses depending on the degree of similarity between them along a genetic relatedness continuum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differential behavioral responses to conspecifics based on genetic relatedness are thought to be adaptive in many social interactions. The benefits associated with these responses include optimizing mate choices to promote heterozygosity and genetic variability and to avoid the detrimental fitness consequences of inbreeding and outbreeding depression (Bateson 1983; Templeton 1986; Thornhill 1993; Tregenza and Wedell 2000) and reducing hostility and violence (including cannibalism) toward related individuals (Walls and Roudebush 1991; Pfennig et al. 1993), thereby enhancing inclusive fitness (Hamilton 1964).
The consensus on the benefits of making behavioral distinctions between individuals based on genetic relatedness does not extend to the nature of the mechanisms that enable such distinctions, however. One aspect of this lack of consensus arose because most studies were not designed to distinguish between categorical distinctions (of kin from non-kin) and graded discriminations along a genetic relatedness continuum (Todrank and Heth 2003). Recently, it became clear that when mice respond differentially to individual odors of conspecifics of differing degrees of genetic relatedness, these distinctions and the associated odor preferences depend on the degree (not simply the category) of genetic similarity between the subject and the odor donor (Heth et al. 2003; Todrank et al. 2005). These graded differential responses are probably possible because the perceptual similarity of individual odors covaries with the genetic similarity between the odor donors (“odor-genes covariance”; Heth and Todrank 2000). Many studies of rodents’ responses to conspecific odors are consistent with a mechanism that enables graded responses by using odor similarities as a proxy for genetic similarities (Todrank and Heth 2003), but to our knowledge, differential responses along a continuum of genetic relatedness had not been explored explicitly in non-mammalian vertebrates.
Recent studies on kin recognition in amphibians, for example, do show that some anuran and salamander species can discriminate (presumably also based on chemical cues) between kin and non-kin (e.g., Pfennig et al. 1994; Pfennig 1997; Gibbons et al. 2003). Temporary nares occlusion eliminated kin discrimination (Pfennig et al. 1994), further substantiating the importance of olfaction in genetic relatedness assessment processes in salamanders. Masters and Forester (1995) reported that female mountain dusky salamanders (Desmognathus ochrophaeus) spend more time brooding genetically similar eggs collected close to their own nests as opposed to dissimilar eggs collected from more distant sites, suggesting a relationship between genetic relatedness and maternal care in this amphibian species. Until now, no studies of amphibian species have addressed differential behavioral interactions based on differential relatedness along a genetic similarity continuum, which is important for understanding genetic relatedness assessment mechanisms (Todrank and Heth 2003).
A study of genetic diversity among fire salamander (Salamandra infraimmaculata) populations in Israel (within and across two mountain ranges separated by at least 20 km of low-elevation plains that are inhospitable to salamanders) indicated greater genetic similarity among individuals with increasing proximity between certain pools where they were collected (Peleg 2009). Also, fire salamander larvae are known to be aggressive toward one another in nature, and cannibalism is common, making these salamanders an ideal species for studying the relationship between differential aggression and differential genetic similarity. As fire salamander larvae disperse as they are larviposited into pools that typically contain larvae from other females, new larvae can encounter other larvae of variable genetic similarity to themselves from the time of their larviposition. Thus, any mechanism enabling genetic relatedness assessment in this species would have to be effective from the moment they are larviposited and would be less reliable if it depended on learning post-larviposition from other larvae already in the pool.
The fire salamander is found in the mountains of northern Israel, including on Mount Carmel and in the Lower Galilee (Degani 1996), the areas selected for this study. During misty or rainy nights over the fall and winter, female fire salamanders larviposit larvae in standing pools, often frequenting the same breeding sites (Warburg 1994). The larvae prey on crustaceans, insects, and amphibians (Blaustein et al. 1996), including each other (Degani 1993). The timing of larviposition is important because the early cohort faces a risk of desiccation when the rains are inconsistent; whereas, the later cohort faces a greater risk of competition with and predation by other, especially older, larvae in the pool (Reques and Tejedo 1996; Eitam et al. 2005). Thus, larvae from these cohorts suffer differential levels of intraspecific hostility and violence, which varies from non-injurious bites to cannibalism (Reques and Tejedo 1996). Bites and efforts to avoid others’ aggression may affect growth and foraging efficiency as well as increase susceptibility to pathogens, and, therefore, they may reduce the fitness of the attacked individual (Walls and Jaeger 1987). In such circumstances, a mechanism for assessing different degrees of genetic similarity and reducing aggression toward more genetically similar individuals would provide an adaptive advantage.
In the present study, we specifically addressed the question of whether there is a predictable relationship between differential genetic relatedness among fire salamander larvae and their level of aggression toward each other when housed in pairs. If these larvae can assess their degree of genetic similarity to other larvae in their pool, we would predict lower aggression between more genetically similar pairs and increasing aggression as the genetic similarity between pairs declines. These within-pool distinctions may be subtle and difficult to quantify in the laboratory but should still be evident in assessments of greater genetic dissimilarity than typically found within pools. Here, we assessed differential aggressiveness along a broad within-species genetic relatedness continuum: at three levels of genetic similarity within and across pools and populations where the mothers were collected (same pool, close populations, and distant populations) and between siblings. Finding differential aggressiveness between these groups would not only provide additional evidence of genetic relatedness assessment processes, extending them to include amphibian species, but also demonstrate an experimental methodology in which this assessment mechanism is manifest adaptively in differential responses to conspecifics.
Materials and methods
Collection and maintenance of animals
On rainy nights during the end of November 2007, we collected two gravid females from a Mount Carmel breeding site (Ein el Balad), seven gravid females from one Lower Galilee site (Kaukab Springs), and two gravid females from a second Lower Galilee site (Manof). These specific sites were chosen because it was already known from a recent genotyping study (Peleg 2009) that these sites represent distinct populations, but there is greater genetic similarity between individuals from the Manof and Kaukab Springs pools compared with those from the Ein el Balad pools. Manof and Kaukab Springs were selected as the close populations in the present study. The Ein el Balad population, which is 21 km from Kaukab Springs and 25 km from Manof, was selected as the distant population. Females were maintained in a climate-controlled room (12L:12D, 22°C) individually in separate aquariums (20 × 35 × 20 cm) filled with 4 L of aged tap water and stones to allow resting above the water. The water was shallow enough to prevent stratification of larvae after they had been larviposited. To avoid disturbing the females while they were larvipositing and to ensure that there were sufficient larvae from all the females to complete the experiment, between 16 (the minimum number of larvae per female to complete the experimental groups described below) and 25 larvae were taken from each female 1 week after the initiation of larviposition. Thereafter, the females and the rest of their larvae were returned to their original natural pool.
To control for any environmental effects, all the salamander larvae were fed only mosquito larvae (at a rate consistent with their consumption in nature), and they were housed in identical individual tubs for 3 weeks before photographing and measurement of body mass, snout–vent length, snout–tail length, head width, injuries, and missing parts of gills, legs, and tail in preparation for the experiment. We photographed each larva just before the experiment to allow individual identification by its unique tail fin spots (following Eitam and Blaustein 2002) and at the end of the experiment to quantify any injuries that occurred during the study. During the experiment, each pair of larvae was placed in a shallow tub filled with 0.5 L aged tap water and provided a stone as shelter. Females have been observed to larviposit at such densities in nature, and such densities can be exceeded if the pool is drying (Blaustein, unpublished data). At the end of the experiment, all salamander larvae were returned to the pool where their mother had been collected.
Experimental design
Our goal in the experimental design was to have as many unique combinations of paired larvae as possible, taking into account both the mother and the population where she was collected in each of four experimental groups and to be consistent in our selection of pairs across the groups. The groups included pairs of (1) siblings (larvae from the same female), (2) larvae from the same population (different females collected in the same pool), (3) larvae from close populations (different females collected from different Lower Galilee breeding sites), and (4) larvae from distant populations (one larva from a female in a Mount Carmel pool and another from a Lower Galilee pool). For siblings, there were 11 possible unique combinations, one pair from each of the 11 mothers. For pairs from the same population to be consistent across groups, we selected 11 of the possible 23 combinations of mothers, being sure that all the mothers from each pool were represented. For pairs from the two close populations, we selected 11 of the possible 14 combinations of mothers. For pairs from the distant populations, we selected 11 of the possible 18 combinations of mothers, using both mothers from the Manof (Lower Galilee) population with both mothers from the Mount Carmel population for four of the pairs; the other seven pairs came from combining different mothers from the Kaukab Springs (Lower Galilee) population with both mothers from the Mount Carmel population. There were sufficient larvae available to test two sets of pairs from each combination of mothers for a total of 22 pairs in each of the groups. To complete this matrix, we randomly selected pairs of larvae from the chosen mothers that were matched in snout–tail length to enhance the chances of comparable aggressive strength between the paired individuals. We used these measures to match the pairs because there are no measures available to determine differential health or vigor between larvae apart from encounters between them and that would have introduced a prior experience confound. There were no significant differences in the average larval size in the two sets of pairs from each combination of mothers as expressed in terms of snout–tail length (one-way ANOVA: F 3,40 = 1.0, p = 0.40, all Fisher’s least significant difference (LSD) pairwise comparison tests between the groups showed p values >0.05) nor in the initial number of tail injuries (one-way ANOVA: F 3,40 = 1.44, p = 0.243, all Fisher’s LSD pairwise comparison tests between the groups showed p values >0.05) across the groups at the beginning of the experiment. Thus, all larvae in the experiment had the same experience and treatment prior to the experiment to ensure that the only difference among the pairs was the relative level of genetic similarity across the experimental groups. The pairs of larvae remained together in their tubs for a month prior to testing. For a week before the observations, we fed each salamander larva three mosquito larvae daily, as fire salamander larvae are reported to be more aggressive when they have been fed recently (Cohen et al. 2005).
To minimize disturbance to the larvae, we carefully moved one tub at a time to a separate testing room, allowed 1 min for acclimation and then observed the pair of larvae for 10 min. During the observation period, we recorded the number of times each individual approached and bit the other, using the spot patterns and brightness to distinguish between the two larvae. Very few of these “bites” resulted in noticeable physical injury.
We repeated this observational procedure after an additional month. The order of conducting the trials across the four groups within the experimental days was random. At the end of the experiment, we recorded the same body measurements again to quantify body size changes during the experimental period. Injuries were found almost exclusively on the tails and not on other body parts, such as the legs and gills; thus, we focused on tail injuries in the analysis. All procedures were carried out under criteria set by the Israeli animal rights and welfare council and were approved by the animal care committee at the University of Haifa.
Statistical analysis
In this experimental design, the validity of any claim that differential aggressiveness covaries with genetic similarity depends not only on the measures of aggression but also on the confidence that the range of genetic similarity within each group does not overlap with the comparison groups. In addition to the genotyping evidence from the sampled populations (Peleg 2009), the distances as well as the terrain between Mount Carmel and the Lower Galilee populations preclude the possibility of any recent gene flow between the “distant” collection sites. The maximum observed dispersal distance during a single season in the fire salamander in Israel was 1.2 km (Bar-David et al. 2007), thus, even though some unobserved salamanders may have dispersed over longer distances, it is unlikely that there are paternal half-siblings in the “close” populations, which are 4 km apart. Although it is possible that some pairs of larvae were cousins or more distant relatives due to dispersal over more than one season, it is improbable that the instances of such relatedness were sufficient to result in an overlap in the ranges of genetic similarity of pairs in the same and the close population groups. The same cannot be said for the same population and the siblings groups, however, because of the possible confound of unknown paternity, females within a population may be fertilized by multiple males, and males may fertilize multiple females, even in separate pools in a given area (Steinfartz et al. 2006). Although, in all likelihood, the genetic similarity is higher overall in the “siblings” than in the “same pool” group, some of the “siblings” may have been maternal half-siblings (diminishing the genetic similarity within the group), and some of the pairs from the same pool could have been paternal half-siblings (inflating the genetic similarity within the group), resulting in some uncertainty as to the degree of overlap in the genetic similarity ranges for these groups. As we did not have information on the individual genotypes of the larvae used in these groups (i.e., “siblings” and “same pool” larvae) to determine the precise genetic similarity between the pairs, we treated these groups as possibly overlapping. In addition, because females often stop larvipositing if they are disturbed, we did not separate the larvae from the aquarium with their mother and siblings as they were larviposited, creating a potential familiarity confound for the siblings group. Thus, we did not include them in the analysis but rather present those data for visual comparison. The statistical analysis compared the three groups (i.e., same pool, close populations, and distant populations) that did not have overlapping genetic similarity ranges. As an additional check for consistent genetic variability across pools, we conducted a preliminary analysis for the siblings and same pool groups comparing the results for larvae from the pools where only two mothers had been collected (and thus, the genetic variability may have been lower) with the results for larvae from the pool where seven mothers had been collected (and the genetic variability could have been higher) to ensure that there were no statistically significant differences in larval aggression levels between pools depending on the number of gravid females found there (siblings group: F 1,9 = 0.011, p = 0.917; same pool group: F 1,9 = 0.010, p = 0.920).
For the analysis, to avoid pseudoreplication, we averaged the number of bites for the two sets of pairs from each unique combination of mothers and averaged across the two behavioral trials to produce one datum point for each of the two sets of pairs. Thus, although there were 44 larvae in each experimental group, the statistics were calculated based on n = 11 data points (i.e., each point was the average per two pairs of larvae from a unique combination of mothers) per group. The number of tail injuries is an unambiguous measure of aggressive behavior across the 2 months during which the pairs shared a tub. Therefore, we calculated how many tail injuries occurred during the experiment for each individual in the set of four larvae from each unique mother–mother combination and averaged them to produce one datum point for each set. We used one-way ANOVAs to test the effect of genetic similarity on the number of bites and injuries across the groups. We used Fisher’s LSD pairwise comparison post hoc tests to identify significant differences in bites and additional injuries across the groups. As an additional analysis to look at overall relationships, we used a Spearman correlation to test for the correlation between genetic similarity and aggression (both bites and tail injuries).
We analyzed changes in head width, snout–vent length, snout–tail length, and body mass from the beginning of the experiment to its end to assess whether there were statistically significant changes in these parameters that may have affected the other results and to test whether there were morphological growth costs associated with our design. We used Systat (Systat Software Inc., San Jose, CA, USA) version 10 for all our statistical tests.
Results
Behavioral observations
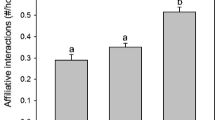
The ANOVA demonstrated that genetic similarity had a significant effect on the average number of bites observed across the “same pool”, “close populations”, and “distant populations” groups (F 2,30 = 13.09, p = 0.0001, Fig. 1). Fisher’s LSD pairwise comparison post hoc tests demonstrated that pairs of larvae from distant populations were significantly more aggressive than pairs of larvae from close populations (p = 0.004) and pairs of larvae from the same pool (p < 0.001). Pairs of larvae from the same pool were less aggressive than pairs from close populations (p = 0.043). There were fewer bites among larvae in the “siblings” group than in the other groups (Fig. 1). A Spearman rank correlation also showed a strong negative relationship between aggression and genetic similarity across the groups (r s = 0.68, n = 33, p < 0.0001).
The mean (+SE) number of average bites per set of four larvae from each unique combination of mothers that occurred during two 10-min trials in fire salamander in each experimental group: larvae from “distant populations” (black bar), larvae from “close populations” (dark gray bar), larvae from the “same pool” (gray bar), and “siblings” (open bar). Different letters denote significant differences (p < 0.05) between the three non-overlapping genetic similarity groups: distant populations, close populations, and same pool
Accumulated injuries and body size changes
The ANOVA indicated that the degree of genetic similarity between the pairs had a significant effect on the number of tail injuries accumulated during the study period (F 2,30 = 21.24, p < 0.0001, Fig. 2). Fisher’s LSD pairwise comparison post hoc tests demonstrated that pairs of larvae from distant populations had more tail injuries than pairs from close populations (p < 0.001) and pairs from the same pool (p < 0.001). Despite the clear gradient (see Fig. 2), the differences between the close populations and the same pool groups were not significant (p = 0.273). The overall relationship between aggression and genetic relatedness was also strongly supported by a Spearman rank correlation with a significant increase in the number of injuries as genetic similarity decreased (r s = 0.66, n = 33, p < 0.0001). The siblings group had the lowest average number of tail injuries (Fig. 2). There were no significant changes between the beginning and the end of the experiment in head width, snout–vent length, and body mass between the groups (all p values >0.05).
The mean (+SE) increase in the average number of tail injuries per set of four larvae from each unique combination of mothers from the beginning to the end of the experimental period in fire salamander from each experimental group: larvae from “distant populations” (black bar), larvae from “close populations” (dark gray bar), larvae from the “same pool” (gray bar), and “siblings” (open bar). Different letters denote significant differences (p < 0.05) between the three non-overlapping genetic similarity groups: distant populations, close populations, and same pool
Discussion
In this study, assessing covariance between aggression and genetic similarity in pairs of fire salamander larvae, aggression was significantly less intense (as expressed by both bites during the observation period and tail injuries accumulated during the 2-month study period) in more genetically similar pairs. Although the mechanism enabling the assessment of differential genetic relatedness cannot be determined using this methodology, the degree of genetic similarity probably modulates the levels of aggression because environmental factors that might alter larval odors and mask chemical cues to genetic similarity were carefully controlled. Observed bites and accumulated injuries, which are both acts of or consequences of aggression, occurred most frequently between larvae from distant populations, and there was a graded decrease as the genetic similarity between the individuals in the pair increased from close populations to the same pool. The siblings showed the lowest average number of bites and injuries. In fact, the number of bites and accumulated injuries in the siblings group was so low that it is unlikely that it would be possible to detect differences in aggression between full-siblings and maternal half-siblings using the current paradigm even if the genotypes of the larvae were known. This may also be true for distinctions between paternal half-siblings and non-siblings in the same pool group.
It is noteworthy that significant differences in the number of bites between groups could be detected even in the two short (10 min) observation periods during the 2 months of sharing the same tub. As most observed “bites” did not result in physical injury, it was important to supplement these results with the analysis of the injuries that accumulated over the 2 months as consequences of aggression when the pairs were not being observed. Although the differences in the number of additional injuries between the same and close populations were not statistically significant, the gradient of increased injuries with increasing genetic dissimilarity is clear.
As most studies of differential responses to conspecifics based on genetic similarity focus on distinctions between kin and non-kin, we wanted to include a siblings group in our study as well as the three groups representing decreasing degrees of genetic similarity from pairs of larvae whose mothers were collected from the same pool or from close populations to those whose mothers came from distant populations. We could be confident that the latter three represented distinct genetic similarity groups along the continuum given the genetic analysis of these populations conducted by Peleg (2009). Because another study on a closely related congeneric species (Salamandra salamandra) indicated multiple fertilizations within and across females by males of this genus, even in separate nearby pools (Steinfartz et al. 2006), we recognized that there was some possibility of overlap in the ranges of genetic similarity for the siblings and same pool groups, which may have included some half-siblings, and treated them as such in the analysis.
Because the larvae in this study spent up to a week with their siblings and mother prior to separation, we cannot rule out a contribution of postnatal learning in genetic similarity assessment processes in this species. The biology of the species, however, militates against a mechanism underlying discriminative behavior on the basis of genetic similarity that depended on learning after larvipositing for several reasons. First, because females may carry larvae from multiple males, larvae can be larviposited not only with full-siblings but with maternal half-siblings as well, making it difficult to distinguish degrees of kinship if larvae learn a kinship template, even from larvae in their immediate vicinity shortly after being larviposited. Second, because males can fertilize multiple females, and thus larvae can encounter paternal half-siblings larviposited by other females in the same pool, it would be biologically adaptive to discriminate these unfamiliar relatives from less related cousins and non-siblings in the pool. Furthermore, because larvae are larviposited into pools where a mix of variously related and unrelated larvae often is already present, larvae could mistakenly treat unrelated larvae as kin if their mechanism for distinguishing degrees of genetic relatedness was developed from the mix of chemosignals already existing in the pool at the time they were larviposited.
Suggested benefits of kin recognition abilities in cannibalistic salamanders include the reduction of aggression toward and cannibalism of kin (Walls and Roudebush 1991; Pfennig and Collins 1993; Pfennig et al. 1994), and therefore, a reduction in the likelihood of being injured by kin and being exposed to pathogens (Walls and Jaeger 1987), and a reduction in missed opportunity costs associated with lost time that could have been used to perform alternative activities such as foraging. Broadening these discriminative abilities along a continuum of genetic similarity would extend the ability to minimize aggression between more genetically similar non-kin that have been larviposited in the same pool. This type of discrimination is consistent with the data presented here even though half of the larvae in this study encountered larvae from other pools, which would not occur in nature. The ability to assess genetic relatedness along a continuum and respond adaptively could also be beneficial for adult salamanders in other contexts besides aggression, such as in mate choice within and between populations and species (e.g., Dawley 1984, 1986; Verrell 1999, 2003). In newborn and adult rodents, it was possible to show much more finely tuned odor discriminations and preferences along a genetic relatedness continuum from siblings to across species (Heth et al. 2003; Todrank and Heth 2003; Busquet and Baudoin 2005; Todrank et al. 2005) than can be assessed through studies of differential aggression, but this does not preclude the possibility that salamander larvae are able to make such finely tuned discriminations as well (see description of discrimination between full-siblings and first cousins by cannibalistic salamander larvae in Pfennig and Collins (1993) and Pfennig et al. (1994)). Interestingly, Kaib et al. (2004) showed correlations between genetic similarity, odors (cuticular hydrocarbon composition), aggression, and geographic distance in termite colonies, suggesting that the phenomenon of genetic relatedness assessment modulating responses to conspecifics could also be expressed in invertebrates.
The primary purpose of this investigation was to extend the demonstration of genetic relatedness assessment along a continuum of genetic similarity to include amphibian species, but the findings in this study may have important conservation implications for this, and presumably other, species where aggression may vary with genetic relatedness. In an effort to enhance survival rates, conservation strategies call for moving animals (including fire salamander larvae) from one population to another sparser population (Hughes et al. 2003). Because the levels of aggression increase as genetic dissimilarity increases, as it often does with geographic distance between populations, the results presented here suggest that this practice may actually reduce future reproduction and survival of both populations when larvae are transferred between distant populations. A better compromise may be to introduce transferred larvae to a pool close to their native pool, thereby ensuring enough genetic variability among the mixed population and, at the same time, avoiding high aggression levels.
References
Bar-David S, Segev O, Peleg N, Hill N, Templeton AR, Schultz CB, Blaustein L (2007) Long distance movements by fire salamanders (Salamandra infraimmaculata) and implications for habitat fragmentation. Isr J Ecol Evol 53:143–159
Bateson P (1983) Optimal outbreeding. In: Bateson PPG (ed) Mate choice. Cambridge University Press, Cambridge, UK, pp 257–277
Blaustein L, Friedman J, Fahima T (1996) Larval Salamandra drive temporary pool community dynamics: evidence from an artificial pool experiment. Oikos 76:392–402
Busquet N, Baudoin C (2005) Odour similarities as a basis for discriminating degrees of kinship in rodents: evidence from Mus spicilegus. Anim Behav 70:997–1002
Cohen M, Flam R, Sharon R, Ifrach H, Yeheskely-Hayon D, Warburg MR (2005) The evolutionary significance of intra-cohort cannibalism in larvae of a xeric-inhabiting salamander: an inter-cohort comparison. Curr Herpetol 24:55–66
Dawley EM (1984) Recognition of individual, sex and species odors by salamanders Plethodon glutinosus and Plethodon jordani complex. Anim Behav 32:353–361
Dawley EM (1986) The evolution of chemical signals as a premating isolating mechanism in a complex of terrestrial salamanders. In: Duvall D, Muller-Schwarze D, Silverstein RM (eds) Chemical signals in vertebrates 4. Plenum, New York, pp 241–224
Degani G (1993) Cannibalism among Salamandra salamandra larvae. Isr J Zool 39:125–129
Degani G (1996) Salamandra salamandra at the southern limit of its distribution. Laser Pages Publishing, Jerusalem, Israel
Eitam A, Blaustein L (2002) Noninvasive individual identification of larval Salamandra using tailfin spot patterns. Amphib-reptil 23:215–219
Eitam A, Blaustein L, Mangel M (2005) Density and intercohort priority effects on larval Salamandra salamandra in temporary pools. Oecologia 146:36–42
Gibbons ME, Ferguson AM, Lee DR, Jaeger RG (2003) Mother-offspring discrimination in the red-backed salamander may be context dependent. Herpetologica 59:322–333
Hamilton WD (1964) The genetical evolution of social behaviour. I and II. J Theor Biol 7:1–52
Heth G, Todrank J (2000) Individual odour similarities across species parallel phylogenetic relationships in the S. ehrenbergi superspecies of mole rats. Anim Behav 60:789–795
Heth G, Todrank J, Busquet N, Baudoin C (2003) Genetic relatedness assessment through individual odour similarities (G-ratios) in mice. Biol J Linn Soc Lond 78:595–603
Hughes J, Goudkamp K, Hurwood D, Hancock M, Bunn S (2003) Translocation causes extinction of a local population of the freshwater shrimp Paratya australiensis. Conserv Biol 17:1007–1012
Kaib M, Jmhasly P, Wilfert L, Durka W, Franke S, Francke W, Leuthold RH, Brandl R (2004) Cuticular hydrocarbons and aggression in the termite Macrotermes subhyalinus. J Chem Ecol 30:365–385
Masters BS, Forester DC (1995) Kin recognition in a brooding salamander. Proc R Soc Lond B Biol Sci 261:43–48
Peleg N (2009) Studies on the conservation of the fire salamander, Salamandra infraimmaculata, in Israel. PhD dissertation. University of Haifa, Israel
Pfennig DW (1997) Kinship and cannibalism. Bioscience 47:667–675
Pfennig DW, Collins JP (1993) Kinship affects morphogenesis in cannibalistic salamanders. Nature 362:836–838
Pfennig DW, Reeve HK, Sherman PW (1993) Kin recognition and cannibalism in spadefoot toad tadpoles. Anim Behav 46:87–94
Pfennig DW, Sherman PW, Collins JP (1994) Kin recognition and cannibalism in polymorphic salamanders. Behav Ecol 5:225–232
Reques R, Tejedo M (1996) Intraspecific aggressive behaviour in fire salamander larvae (Salamandra salamandra): the effects of density and body size. J Herpetol 6:15–19
Steinfartz S, Stemshorn K, Kuesters D, Tautz D (2006) Patterns of multiple paternity within and between annual reproduction cycles of the fire salamander (Salamandra salamandra) under natural conditions. J Zool 268:1–8
Templeton AR (1986) Coadaptation and outbreeding depression. In: Soule M (ed) Conservation biology: science of scarcity and diversity. Sinauer, Sunderland, MA, pp 105–116
Thornhill NW (ed) (1993) The natural history of inbreeding and outbreeding. Chicago Press, Chicago
Todrank J, Heth G (2003) Odor-genes covariance and genetic relatedness assessment: rethinking odor-based “recognition” mechanisms in rodents. Adv Study Behav 32:77–130
Todrank J, Busquet N, Baudoin C, Heth G (2005) Preferences of newborn mice for odours indicating closer genetic relatedness: is experience necessary? Proc R Soc Lond B Biol Sci 272:2083–2088
Tregenza T, Wedell N (2000) Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol 9:1013–1027
Verrell PA (1999) Geographic variation in sexual behaviour: sex, signals, and speciation. In: Foster SA, Endler JA (eds) Geographic variation in behaviour: perspectives on evolutionary mechanisms. Oxford University Press, New York, pp 262–282
Verrell PA (2003) Population and species divergence of chemical cues that influence mate recognition of females in desmognathine salamanders. Ethology 109:577–586
Walls SC, Jaeger RG (1987) Aggression and exploitation as mechanisms of competition in larval salamanders. Can J Zool 65:2938–2944
Walls SC, Roudebush RE (1991) Reduced aggression toward siblings as evidence of kin recognition in cannibalistic salamanders. Am Nat 138:1027–1038
Warburg MR (1994) Population ecology, breeding activity, longevity, and reproductive strategies of Salamandra salamandra during an 18-year long study of an isolated population on Mt. Carmel, Israel. Mertensiella 4:399–421
Acknowledgements
We thank Yoav Shulman for help in collecting and maintaining the adult females. We also thank Alan Templeton, Marc Mangel, Yoav Shulman, Asaf Sadeh, Alon Silberbush, Ori Segev, Nir Peleg, and Shirli Bar-David for fruitful discussions and R. W. Elwood and two anonymous reviewers for constructive comments on an earlier version of the manuscript. Permission to use the Salamandra was granted by the Israel Nature and Parks Authority. This study was funded by US–Israel Binational Science Foundation grant 2002-365 awarded to L. B. and Marc Mangel. The experiment complies with the current animal protection laws of Israel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Christensen-Dalsgaard
Rights and permissions
About this article
Cite this article
Markman, S., Hill, N., Todrank, J. et al. Differential aggressiveness between fire salamander (Salamandra infraimmaculata) larvae covaries with their genetic similarity. Behav Ecol Sociobiol 63, 1149–1155 (2009). https://doi.org/10.1007/s00265-009-0765-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0765-y