Abstract
Habitat permanence and threat of predation are primary drivers of community assembly and composition in lentic freshwater systems. Pond-breeding amphibians select oviposition sites to maximize fitness and minimize risks of predation and desiccation of their offspring, typically facing a trade-off between the two as predation risk often increases as desiccation risk decreases. To experimentally determine if Hyla chrysoscelis partition oviposition along gradients of relative desiccation risk and predation risk, we tested oviposition site preference in a natural population of treefrogs colonizing experimental ponds that varied in water depth and contained predatory larvae of two Ambystoma salamander species. Hyla chrysoscelis selected habitats with both lower predation risk, avoiding A. talpoideum over A. maculatum, and lower desiccation risk, selecting ponds with three times greater depth. We demonstrate that adult oviposition site choices simultaneously minimize relative predation risk and desiccation risk and that closely related salamander species produce functionally different responses among colonizing animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies of multiple risk factors in ecology have tended to focus on multiple predators, in contrast to risks derived from the interaction of biotic and abiotic factors (Sih et al. 1998; Schmitz and Sokol-Hessner 2002; Grabowski et al. 2008; Touchon and Warkentin 2009). Predators play critical roles in structuring communities, but the conditions that allow for the persistence of predators can be driven by the abiotic environment (Paine 1966; Carpenter et al. 1985; Schneider and Frost 1996; Skelly 1996). Lethal and sublethal effects of the abiotic environment, such as those of desiccation, fire, or pollutants, can equally affect both predators and organisms at lower trophic levels (Menge and Sutherland 1987; Scott and Sloman 2004). Although different species of predators may be equally affected by the abiotic environment, they often produce functionally diverse effects on communities (DeWitt et al. 2000; Resetarits and Chalcraft 2007; Schmitz 2009; Resetarits and Pintar 2016). This generates landscapes of habitat patches that vary in risk due to the interaction between abiotic and biotic factors (Menge and Sutherland 1987; Wellborn et al. 1996; Jackson et al. 2001), and colonizing organisms must assess this risk when selecting habitat patches. How various sources of risk are perceived is a window on the strength of selection exerted by multiple factors.
Pond permanence and predator presence are primary drivers of freshwater aquatic community composition. Ponds exist on a hydroperiod gradient, and as hydroperiod increases, ponds are typically inhabited by more dominant predators (Wellborn et al. 1996). Differences in aquatic communities were traditionally ascribed to varying strengths of lethal processes (predation and desiccation), but habitat selection can play an equal or greater role in determining community composition (Resetarits and Wilbur 1989; Skelly 1996; Resetarits and Binckley 2009; Kraus and Vonesh 2010). Predatory fish occupy most permanent ponds and play extremely important roles at both colonization and post-colonization stages, both repelling colonizers and shaping community structure through direct predation (Petranka et al. 1987; Resetarits and Wilbur 1989). Thus, many other aquatic taxa have evolved to live in, and are more often found in, temporary ponds. Even among temporary ponds, those with longer hydroperiods typically have more and larger predators (Woodward 1983; Wellborn et al. 1996).
During oviposition, females select habitats for their offspring based on a variety of abiotic and biotic site characteristics, such as predator or competitor presence, canopy cover, or sediment depth (Resetarits and Wilbur 1989; Thompson and Pellmyr 1991; Rudolf and Rödel 2005; McGuffin et al. 2006; Silberbush and Blaustein 2011). Oviposition site selection is a strong determinant of reproductive success, as larvae cannot leave their habitat patch before metamorphosis. Thus, it is imperative that adults make decisions that minimize risk and maximize reward. However, selecting breeding sites based on their perceived permanence (volume/depth), or other characteristics that often covary with permanence such as conductivity, temperature, and dissolved oxygen necessitates the ability to simultaneously assess the relative predation risk (Saward-Arav et al. 2016). Because predation risk is typically inversely correlated with desiccation risk, site selection based on one of these two factors may place colonizing individuals at high risk due to variation in the second factor. Predation risk varies greatly across aquatic taxa, from often very strong effects of fish, to more moderate effects of larval salamanders, to the often low relative risk of some insects (Morin et al. 1988; Wilbur 1997).
Hyla chrysoscelis (Cope’s gray treefrog) selectively oviposits in ponds without predators as well as newly filled ponds; the latter reduces desiccation risk, and offspring of early arrivers gain advantages over later breeders (Wilbur and Alford 1985; Resetarits and Wilbur 1989; Pintar and Resetarits 2017a). With metamorphosis often occurring in less than one month, H. chrysoscelis is capable of surviving in short-lived temporary ponds. However, this can come at a cost of smaller sizes at metamorphosis and shifting of growth between the aquatic and terrestrial stages (Wilbur and Collins 1973). In the southeastern United States, H. chrysoscelis typically breeds in fishless lentic habitats where the top predators are often larval salamanders, particularly Ambystoma talpoideum (mole salamander) and/or A. maculatum (spotted salamander) (Petranka 1998). Ambystoma talpoideum pose a greater predation risk to larval treefrogs than do A. maculatum as they grow to larger sizes and persist as larvae or paedomorphs in ponds for a year or more, whereas A. maculatum metamorphose by mid-summer. To assess oviposition responses to perceived risk in different oviposition sites, we experimentally examined the effects of pond water depth and presence of larvae of these two predatory Ambystoma salamanders on oviposition site preferences of a natural population of H. chrysoscelis.
Materials and methods
On 17-Jan-2015, we set up an array of experimental mesocosms (plastic cattle tanks: diameter = 1.83 m, maximum depth = 0.61 m; N = 63) in a field at the University of Mississippi Field Station near Oxford, Mississippi, USA, 25 m north and west of nearby fish-containing ponds. This experiment was designed to assess the effects of flooding and competitor addition on larval Ambystoma development. Thus, our primary goal in experimental design was to achieve an optimal setup for development, and hence, our mesocosms were separated by 0.5 m edge-to-edge and arranged in a quasi-rectangular array due to highly uneven ground. A 5 × 8 set of mesocosms formed the core of the array, with the remaining mesocosms arranged around the periphery, while maintaining equal spacing. This resulted in six spatial blocks (distance south to north from a treeline), which we included in our initial analyses, but ultimately had no effect on oviposition. The spatial design of our experiment was within the range of areas occupied by similar experiments, which allows females to assess chemical cues from multiple mesocosms prior to and during oviposition (Resetarits and Wilbur 1991; Binckley and Resetarits 2008).
Using pond water pumped through 1.3 mm mesh, we filled the mesocosms to a depth of 16 cm (~400 L); mesocosms were fitted with PVC standpipes to adjust water levels. On 19-Jan, we randomly assigned and added 3 kg of dry hardwood leaf litter (primarily Platanus occidentalis) to each mesocosm. Originally, using a 2 × 2 × 2 factorial design, we randomly assigned treatments of salamander species (Ambystoma maculatum, A. talpoideum) crossed with water depth (16 cm = low, 50 cm = full) and the addition of competitors midway through the larval stage (5 A. maculatum, no salamanders). On 28-Jan, we added 15 larvae of either A. maculatum or A. talpoideum to each mesocosm and allowed larvae to develop until 21-Apr when mesocosms in the full treatment were filled to a depth of 50 cm (~1200 L) with pond water. The five additional A. maculatum competitors were added on 22-Apr; however, these competitors had no effect, so we eliminated this factor from additional analyses, resulting in a 2 × 2 factorial design. The A. talpoideum/low treatment (TL) had 12 replicates, and all other treatments had 17 replicates: A. maculatum/full (MF), A. maculatum/low (ML), and A. talpoideum/full (TF). We checked all mesocosms daily, and once oviposition by H. chrysoscelis began in mid-May, we collected eggs daily (early morning), counted them with photographs, and placed all eggs into nearby fishless ponds.
Although predators were not caged, Ambystoma were unable to consume eggs prior to counting, and we have not observed consumption of H. chrysoscelis eggs by Ambystoma larvae. Ambystoma maculatum may be incapable of feeding on Ranidae eggs, whereas A. talpoideum has minimal success (Anderson et al. 2013; Tumlison and Serviss 2013). The small body size and low abundance of our larvae would make them incapable of consuming a small number of the eggs in each tank, as the gelatinous H. chrysoscelis egg masses occupy volumes that would greatly exceed the size of our larvae (Petranka 1998). Thus, while predation by Ambystoma on Hyla eggs was possible, the likelihood of any meaningful consumption of H. chrysoscelis eggs by Ambystoma was minimal.
The total number of eggs laid per mesocosm during the course of our experiment was our response variable, and in our initial 2 × 2 × 2 analysis, we conducted ANOVA on square root transformed mean total eggs using type III SS on factors of salamander species, addition of competitors, water level, and block. In our reduced 2 × 2 analysis (without added competitors), we examined the effects of salamander species, water level, and block in a similar ANOVA to the initial analysis, along with effect size and post hoc Tukey. To account for potential effects of spillover from density-dependent oviposition if preferred breeding sites could not accommodate all breeders on a given night, we used simple linear regression to compare the proportion of eggs found in the preferred patch type to the total number of eggs laid on a night. On 12-May, the closest date to the oviposition period for which we have data, we measured the conductivity, oxygen, pH, and temperature of each mesocosm with a YSI 63/25 FT meter and dissolved oxygen with a YSI 550 DO meter. We analyzed all water chemistry variables using ANOVAs with water level and block as factors on log-transformed data (except pH). Temperature was included as a covariate in the dissolved oxygen analysis. All analyses used α = 0.05 and R v.3.3.1 (R Core Team 2016), and block was rolled into the error term when P > 0.20. Data are available in figshare (Pintar and Resetarits 2017b).
Results
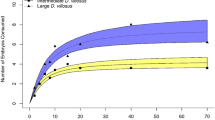
Hyla chrysoscelis laid 59,213 eggs in our mesocosms on 18 nights from 16-May until 21-June with the number of eggs laid per night ranging from 1474 to 6377. The MF treatment received 44,782 eggs (2634.2 ± 338.9, mean ± SE), ML 7965 (468.5 ± 161.2), TF 6348 (373.4 ± 116.2), and TL 118 (9.8 ± 9.8) (Fig. 1). We estimate that this represents the reproductive output of around 45–65 females based on the previous estimates of H. chrysoscelis clutch sizes and site-specific observational data (Resetarits and Wilbur 1989; Resetarits 2005). The effects of predator species and water depth were both significant, as was the interaction between them (Table 1). The initial 2 × 2 × 2 design produced similar results, but the additional competitors had no effect (Table 2; Fig. 2). Block (distance south to north from a tree row) was not significant in both analyses (P > 0.5), so it was rolled into the error term. In our reduced 2 × 2 design, species and water depth had large, similar effect sizes. All but one of the pairwise comparisons (ML–TF) were significantly different from each other (Table 1). Ambystoma maculatum metamorphosed from 12-May until 12-July, with 95% of surviving individuals metamorphosing before 21-June, the last day we collected eggs in this experiment. Ambystoma talpoideum began metamorphosing on 2-June, but only eight metamorphs (3% of surviving individuals) emerged before 21-June. However, oviposition patterns did not change over time as the number of remaining A. maculatum larvae decreased in the mesocosms (r 2 = 0.0042, P = 0.132). There was no relationship between the proportion of eggs occurring in MF mesocosms and the total number of eggs laid on a night (r 2 = 0.0234, P = 0.253). In low mesocosms, we observed significantly higher conductivity and higher temperature, but no differences in pH, whereas DO significantly covaried with temperature but was not independently affected by water level (Table 3).
Discussion
In natural systems, there is often a trade-off between avoiding predators to reduce predation risk and selecting habitats with greater permanence to reduce mortality due to drastic environmental changes or energy expenditures in search of better habitats (Menge and Sutherland 1987; Jackson et al. 2001). Our data show that H. chrysoscelis simultaneously assess and select habitats to minimize risks of both desiccation and predation. The lowest risk habitat (MF) was the preferred oviposition site and highest risk habitat (TL) almost completely avoided (except for 118 eggs in one mesocosm on a single night). The equivalence of ML and TF suggests that the difference in desiccation risk between low and high mesocosms is similar to the difference in predation risk between A. talpoideum and A. maculatum, which is supported by the similar effect sizes. Nevertheless, A. maculatum is a predator of larval Hyla (Walters 1975), and evidence shows that H. chrysoscelis has reduced oviposition in habitats containing A. maculatum when predator-free controls are present (Resetarits and Wilbur 1989).
Studies of oviposition responses to predators typically include predator-free controls, which are almost universally preferred, but the natural landscape may seldom provide that option (Resetarits and Wilbur 1989; Blaustein et al. 2004; Vonesh et al. 2009; Resetarits and Binckley 2013). When given moderate-to-poor choices in oviposition sites, ovipositing mosquitoes not constrained to mesocosms often choose to seek higher quality natural pools (Kiflawi et al. 2003; Silberbush and Blaustein 2011), whereas ovipositing female Salamandra adjust their egg production based on a breeding site’s volume (Segev et al. 2011). Given that there are often diverse arrays of predators in natural systems and their occurrence can be related to variation in habitat quality, it is important to understand how species respond independently to variation in the specific identity of predators and variation in habitat quality. By creating a landscape without predator-free controls, we were able to force female H. chrysoscelis to reveal any preferences with respect to the two species of Ambystoma. Unlike the aforementioned mosquito studies, there were few, if any, better (e.g. fishless) options within ~500 m of our experimental array, providing additional pressure on H. chrysoscelis to select among our mesocosms.
When presented with a landscape of oviposition sites that varied in both desiccation and predation risk, H. chrysoscelis selected sites with lower risks of both desiccation and predation. Our full mesocosms (3× deeper than low) represented a much lower desiccation risk than low mesocosms. Ponds as shallow as our low mesocosms could dry in 1 to 2 months or less during summer in our system, within the typical larval period of H. chrysoscelis (Altig and McDiarmid 2015). It is important to note that none of our mesocosms ever actually dried, and after final filling on 21-Apr, established water levels were maintained by rain throughout the remainder of the oviposition period. The lack of relationship between the proportion of eggs laid in MF patches and the total of number of eggs laid per night could be due to relatively low activity levels with a maximum of 6377 eggs collected on a single night. This represents the reproductive output of about six females, and there were 17 available MF patches on each night, providing adequate space for breeding in the preferred patch type without interference from other frogs. It is unlikely that reduced oviposition in low mesocosms was due to a higher cue density (no. individuals or biomass per volume) as detection/avoidance thresholds for H. chrysoscelis are low and were well exceeded in all of our mesocosms (Rieger et al. 2004). Furthermore, effects are not additive above this threshold, and we observed no difference between our four treatment groups when considering those that received five additional competitors.
Ambystoma talpoideum are facultatively paedomorphic and can persist in ponds for a year or more, whereas A. maculatum are obligately metamorphic, and 95% of A. maculatum metamorphosed in our system by the end of the experiment. Furthermore, A. talpoideum typically grows faster and reaches larger larval sizes than A. maculatum does, and A. talpoideum is competitively dominant over A. maculatum (Walls 1996; Petranka 1998). Thus, A. talpoideum poses a greater predation threat to larval H. chrysoscelis both immediately, because of their larger size, and in the long term due to their longer larval period (Anderson et al. 2013). Responses of ovipositing H. chrysoscelis to a diverse array of fish with a range of predatory capabilities have produced functionally equivalent responses (Resetarits and Wilbur 1989; Binckley and Resetarits 2003; Resetarits and Binckley 2013), whereas oviposition/colonization by other taxa, including beetles and mosquitoes, have produced more diverse responses to predators (Růžička 2001; Vonesh and Blaustein 2010; Resetarits and Pintar 2016). Interestingly, our two Ambystoma species produced functionally unique responses among ovipositing H. chrysoscelis, with much stronger avoidance of A. talpoideum. This may be explained by the greater co-occurrence of H. chrysoscelis with Ambystoma in temporary ponds than with fish in permanent ponds. Both Ambystoma and H. chrysoscelis are typically found in fishless ponds, with H. chrysoscelis and some Ambystoma species exhibiting strong oviposition preferences for sites without fish (Resetarits and Wilbur 1989; Kats and Sih 1992). Therefore, we would predict stronger selection for species-specific responses by H. chrysoscelis to salamanders and other predators common in temporary ponds, in contrast to nearly uniform responses to most fish species.
Although hydroperiod is a strong correlate of fish presence in ponds, pond permanence per se has a large, direct, non-lethal effect on community assembly that rivals predator identity. Frogs selected habitats with greater perceived permanence, the same habitats in natural systems that are more likely to support more dominant predators (Wellborn et al. 1996; Wilbur 1997). Our data support the idea that colonizing organisms are directly and simultaneously assessing patch quality based not only on predator cues, but on other patch characteristics (Binckley and Resetarits 2003). Although we have limited water chemistry data, the relation of lower water depth to higher conductivity and higher temperature would both be expected, as is the covariance of DO with temperature. Other studies have observed preferential oviposition by H. chrysoscelis in mesocosms with lower conductivity and higher dissolved oxygen (Pintar and Resetarits 2017a), so these characteristics could be used as indicators of patch quality, as they are for other ovipositing organisms such as mosquitoes (Spencer and Blaustein 2001). Direct assessment of multiple indicators of patch quality stands in contrast to using specific patch characteristics (depth/volume) as indicators of potential long-term predation risk, allowing more refined responses if more complex communities with stronger predators develop in more permanent habitats (Saward-Arav et al. 2016). The functionally diverse responses of H. chrysoscelis to Ambystoma and water depth provide further support for the idea that direct, non-lethal effects of patch characteristics (predation/desiccation risk), mediated through habitat selection behaviors of colonizing animals, are major determinants of community structure in freshwater systems.
Females from an array of taxa, including frogs (Resetarits and Wilbur 1989; Touchon and Warkentin 2008), mosquitoes (Vonesh and Blaustein 2010; Silberbush and Blaustein 2011), beetles (Messina et al. 1992; Resetarits 2001), damselflies (McGuffin et al. 2006), and butterflies (Thompson and Pellmyr 1991), make oviposition decisions in complex environments. These oviposition decisions have both differential fitness consequences through varying offspring mortality and performance (Jaenike 1978; Rieger et al. 2004; Gripenberg et al. 2010) and form an important component of the myriad of factors driving patterns of species distributions, species abundances, and community composition (Blaustein et al. 1995; Wellborn et al. 1996). The ability to assess habitat quality across multiple gradients not only helps to maximize fitness, but may also affect the persistence of species as environmental change alters habitats across similar gradients through shifts in species distributions or changes in habitat duration from shifting precipitation (Martin 2001; Both et al. 2006).
References
Altig R, McDiarmid RW (2015) Handbook of larval amphibians of the United States and Canada. Cornell University Press, Ithaca
Anderson TL, Mott CL, Levine TD, Whiteman HH (2013) Life cycle complexity influences intraguild predation and cannibalism in pond communities. Copeia 2013:284–291. doi:10.1643/CE-12-034
Binckley CA, Resetarits WJ Jr (2003) Functional equivalence of non-lethal effects: generalized fish avoidance determines distribution of gray treefrog, Hyla chrysoscelis, larvae. Oikos 102:623–629. doi:10.1034/j.1600-0706.2003.12483.x
Binckley CA, Resetarits WJ Jr (2008) Oviposition behavior partitions aquatic landscapes along predation and nutrient gradients. Behav Ecol 19:552–557. doi:10.1093/beheco/arm164
Blaustein L, Kotler BP, Ward D (1995) Direct and indirect effects of a predatory backswimmer (Notonecta maculata) on community structure of desert temporary pools. Ecol Entomol 20:311–318. doi:10.1111/j.1365-2311.1995.tb00462.x
Blaustein L, Kiflawi M, Eitam A et al (2004) Oviposition habitat selection in response to risk of predation in temporary pools: mode of detection and consistency across experimental venue. Oecologia 138:300–305. doi:10.1007/s00442-003-1398-x
Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in a long-distance migratory bird. Nature 441:81–83. doi:10.1038/nature04539
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. Bioscience 35:634–639. doi:10.2307/1309989
DeWitt TJ, Robinson BW, Wilson DS (2000) Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evol Ecol Res 2:129–148
Grabowski JH, Hughes AR, Kimbro DL (2008) Habitat complexity influences cascading effects of multiple predators. Ecology 89:3413–3422. doi:10.1890/07-1057.1
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference-performance relationships in phytophagous insects. Ecol Lett 13:383–393. doi:10.1111/j.1461-0248.2009.01433.x
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities—the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170. doi:10.1139/f00-239
Jaenike J (1978) On optimal oviposition behavior in phytophagous insects. Theor Popul Biol 14:350–356. doi:10.1016/0040-5809(78)90012-6
Kats LB, Sih A (1992) Oviposition site selection and avoidance of fish by streamside salamanders (Ambystoma barbouri). Copeia 1992:468–473. doi:10.2307/1446206
Kiflawi M, Blaustein L, Mangel M (2003) Oviposition habitat selection by the mosquito Culiseta longiareolata in response to risk of predation and conspecific larval density. Ecol Entomol 28:168–173. doi:10.1046/j.1365-2311.2003.00505.x
Kraus JM, Vonesh JR (2010) Feedbacks between community assembly and habitat selection shape variation in local colonization. J Anim Ecol 79:795–802. doi:10.1111/j.1365-2656.2010.01684.x
Martin TE (2001) Abiotic vs. biotic influences on habitat selection of coexisting species: climate change impacts? Ecology 82:175–188. doi:10.1890/0012-9658(2001)082[0175:AVBIOH]2.0.CO;2
McGuffin MA, Baker RL, Forbes MR (2006) Detection and avoidance of fish predators by adult Enallagma damselflies. J Insect Behav 19:77–91. doi:10.1007/s10905-005-9013-0
Menge BA, Sutherland JP (1987) Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am Nat 130:730–757. doi:10.1086/284741
Messina FJ, Kemp JL, Dickinson JA (1992) Plasticity in the egg-spacing behavior of a seed beetle: effects of host deprivation and seed patchiness (Coleoptera: Bruchidae). J Insect Behav 5:609–621. doi:10.1007/BF01048008
Morin PJ, Lawler SP, Johnson EA (1988) Competition between aquatic insects and vertebrates: interaction strength and higher order interactions. Ecology 69:1401–1409. doi:10.2307/1941637
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75
Petranka JW (1998) Salamanders of the United States and Canada. Smithsonian Institution Press, Washington, DC
Petranka JW, Kats LB, Sih A (1987) Predator-prey interactions among fish and larval amphibians: use of chemical cues to detect predatory fish. Anim Behav 35:420–425. doi:10.1016/S0003-3472(87)80266-X
Pintar MR, Resetarits WJ Jr (2017a) Out with the old, in with the new: oviposition preference matches larval success in Cope’s gray treefrog, Hyla chrysoscelis. J Herpetol 51:186–189. doi:10.1670/16-019
Pintar MR, Resetarits WJ Jr (2017b) Data for “Relative predation risk and risk of desiccation co-determine oviposition preferences in Cope’s gray treefrog, Hyla chrysoscelis”. Figshare. doi:10.6084/m9.figshare.4887020
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Resetarits WJ Jr (2001) Colonization under threat of predation: avoidance of fish by an aquatic beetle, Tropisternus lateralis (Coleoptera: Hydrophilidae). Oecologia 129:155–160. doi:10.1007/S004420100704
Resetarits WJ Jr (2005) Habitat selection behaviour links local and regional scales in aquatic systems. Ecol Lett 8:480–486. doi:10.1111/j.1461-0248.2005.00747.x
Resetarits WJ Jr, Binckley CA (2009) Spatial contagion of predation risk affects colonization dynamics in experimental aquatic landscapes. Ecology 90:869–876. doi:10.1890/08-0613.1
Resetarits WJ Jr, Binckley CA (2013) Is the pirate really a ghost? Evidence for generalized chemical camouflage in an aquatic predator, pirate perch Aphredoderus sayanus. Am Nat 181:690–699. doi:10.1086/670016
Resetarits WJ Jr, Chalcraft DR (2007) Functional diversity within a morphologically conservative genus of predators: implications for functional equivalence and redundancy in ecological communities. Funct Ecol 21:793–804. doi:10.1111/j.1365-2435.2007.01282.x
Resetarits WJ Jr, Pintar MR (2016) Functional diversity of non-lethal effects, chemical camouflage, and variation in fish avoidance in colonizing beetles. Ecology 97:3517–3529. doi:10.1002/ecy.1593
Resetarits WJ Jr, Wilbur HM (1989) Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology 70:220–228. doi:10.2307/1938428
Resetarits WJ Jr, Wilbur HM (1991) Calling site choice by Hyla chrysoscelis: effect of predators, competitors, and oviposition sites. Ecology 72:778–786. doi:10.2307/1940580
Rieger JF, Binckley CA, Resetarits WJ Jr (2004) Larval performance and oviposition site preference along a predation gradient. Ecology 85:2094–2099. doi:10.1890/04-0156
Rudolf VHW, Rödel M-O (2005) Oviposition site selection in a complex and variable environment: the role of habitat quality and conspecific cues. Oecologia 142:316–325. doi:10.1007/s00442-004-1668-2
Růžička Z (2001) Oviposition responses of aphidophagous coccinellids to tracks of ladybird (Coleoptera: Coccinellidae) and lacewing (Neuroptera: Chrysopidae) larvae. Eur J Entomol 98:183–188. doi:10.14411/eje.2001.034
Saward-Arav D, Sadeh A, Mangel M et al (2016) Oviposition responses of two mosquito species to pool size and predator presence: varying trade-offs between desiccation and predation risks. Isr J Ecol Evol. doi:10.1080/15659801.2015.1069113
Schmitz OJ (2009) Effects of predator functional diversity on grassland ecosystem function. Ecology 90:2339–2345. doi:10.1890/08-1919.1
Schmitz OJ, Sokol-Hessner L (2002) Linearity in the aggregate effects of multiple predators in a food web. Ecol Lett 5:168–172. doi:10.1046/j.1461-0248.2002.00311.x
Schneider DW, Frost TM (1996) Habitat duration and community structure in temporary ponds. J N Am Benthol Soc 15:64–86. doi:10.2307/1467433
Scott GR, Sloman KA (2004) The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68:369–392. doi:10.1016/j.aquatox.2004.03.016
Segev O, Mangel M, Wolf N et al (2011) Spatiotemporal reproductive strategies in the fire salamander: a model and empirical test. Behav Ecol 22:670–678. doi:10.1093/beheco/arr029
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355. doi:10.1016/S0169-5347(98)01437-2
Silberbush A, Blaustein L (2011) Mosquito females quantify risk of predation to their progeny when selecting an oviposition site. Funct Ecol 25:1091–1095. doi:10.1111/j.1365-2435.2011.01873.x
Skelly DK (1996) Pond drying, predators, and the distribution of Pseudacris tadpoles. Copeia 3:599–605. doi:10.2307/1447523
Spencer M, Blaustein L (2001) Hatching responses of temporary pool invertebrates to signals of environmental quality. Isr J Zool 47:397–418. doi:10.1560/23F2-2XBW-252B-DU5C
Thompson JN, Pellmyr O (1991) Evolution of oviposition behavoir and host preference in Lepidoptera. Annu Rev Entomol 36:65–89. doi:10.1146/annurev.en.36.010191.000433
Touchon JC, Warkentin KM (2008) Reproductive mode plasticity: aquatic and terrestrial oviposition in a treefrog. Proc Natl Acad Sci 105:7495–7499. doi:10.1073/pnas.0711579105
Touchon JC, Warkentin KM (2009) Negative synergism of rainfall patterns and predators affects frog egg survival. J Anim Ecol 78:715–723. doi:10.1111/j.1365-2656.2009.01548.x
Tumlison R, Serviss B (2013) Novel Food habits of branchiate mole salamanders (Ambystoma talpoideum) from Southwestern Arkansas. Southeast Nat 12:579–588. doi:10.1656/058.012.0312
Vonesh JR, Blaustein L (2010) Predator-induced shifts in mosquito oviposition site selection: a meta-analysis and implications for vector control. Isr J Ecol Evol 56:263–279. doi:10.1560/IJEE.56.3-4.263
Vonesh JR, Kraus JM, Rosenberg JS, Chase JM (2009) Predator effects on aquatic community assembly: disentangling the roles of habitat selection and post-colonization processes. Oikos 118:1219–1229. doi:10.1111/j.1600-0706.2009.17369.x
Walls SC (1996) Differences in foraging behaviour explain interspecific growth inhibition in competing salamanders. Anim Behav 52:1157–1162. doi:10.1006/anbe.1996.0262
Walters B (1975) Studies of interspecific predation within an amphibian community. J Herpetol 9:267–279. doi:10.2307/1563191
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363. doi:10.1146/annurev.ecolsys.27.1.337
Wilbur HM (1997) Experimental ecology of food webs: complex systems in temporary ponds. Ecology 78:2279–2302. doi:10.1890/0012-9658(1997)078[2279:EEOFWC]2.0.CO;2
Wilbur HM, Alford RA (1985) Priority effects in experimental pond communities: responses of Hyla to Bufo and Rana. Ecology 66:1106–1114. doi:10.2307/1939162
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314. doi:10.1126/science.182.4119.1305
Woodward BD (1983) Predator-prey interactions and breeding-pond use of temporary-pond species in a desert anuran community. Ecology 64:1549–1555. doi:10.2307/1937509
Acknowledgements
J. Bohenek and L. Eveland assisted with fieldwork. T. Breech provided helpful comments on the manuscript. Support was provided by the University of Mississippi and the Henry L. and Grace Doherty Foundation. This research was approved by the University of Mississippi’s Institutional Animal Care and Use Committee (14-027) and the Mississippi Department of Wildlife, Fisheries, and Parks (0624141). The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Contributions
MRP conceived, designed, and conducted the experiment. MRP analyzed the data and wrote the manuscript with input from WJR. Both authors gave final approval for publication.
Corresponding author
Additional information
Communicated by Howard Whiteman.
Rights and permissions
About this article
Cite this article
Pintar, M.R., Resetarits, W.J. Relative predation risk and risk of desiccation co-determine oviposition preferences in Cope’s gray treefrog, Hyla chrysoscelis . Oecologia 184, 423–430 (2017). https://doi.org/10.1007/s00442-017-3875-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3875-7