Abstract
The diversity and geographical distribution of cyanoprokaryotes in the Eurasian Arctic and Hypoarctic were investigated. We combined information from the literature and data from our own research in various parts of high-latitude regions. We collected and studied more than 1000 samples from terrestrial and freshwater habitats. The published data on cyanoprokaryotes include records from about 1500 locations. Both original and published data on biodiversity were used for the analysis. The data were submitted to the CYANOpro database (http://kpabg.ru/cyanopro/). A total of 603 species were recorded. In the Arctic zone, 482 species were found. The Eurasian Hypoarctic flora includes 428 species. The Murmansk region (359 species), Spitsbergen archipelago (314), and Bolshezemelskaya tundra (191) have the highest number of species among the studied territories. The flora is unevenly studied in different areas of the Arctic, and therefore, it is too early to suggest any significant specificity of the algal flora to particular areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanoprokaryota (Cyanobacteria) are widespread and ecologically important organisms of aquatic and terrestrial ecosystems of the Arctic. Their unique abilities to photosynthesize and fix molecular nitrogen make them a special group in production of organic matter in the water bodies and soils of high latitudes.
In Arctic water bodies, cyanoprokaryotes form dominant communities in phytoplankton and benthos. In southern parts of the Arctic, some species can cause «blooming» of the water bodies. In terrestrial habitats of high latitudes, cyanoprokaryotes can form visible growths on the surface of and in the soil. Reduced competition from higher plants allows cyanobacterial mats and films to occupy considerable areas. Cyanoprokaryotes occur on soil surfaces lacking vegetation, and on the surface of, and within cracks penetrating rocky substrata. Growth in cracks in rocks and internally amongst rock crystals provides protection against temperature changes, dehydration, and external physical influences (e.g., destruction by the wind). Cyanoprokaryotes are often the first organisms to inhabit glaciers and moraines (Kaštovská et al., 2005, 2007; Turicchia et al., 2005; Davydov, 2011). Their high abundance is seen in bryophyte communities of moist habitats along the shores of lakes, streams, pools, and the splash zone of waterfalls.
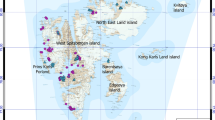
The current state of Cyanoprokaryota biodiversity has not been analyzed in the massive area (58.7 million km2) occupied by the Eurasian Arctic and Hypoarctic (Fig. 1). The studies of cyanoprokaryotes in high latitudes are problematic due to much of the region being remote. The aim of this study was to combine all available published data and results of our own research on diversity of Cyanoprokaryota in the Eurasian sector of the Arctic and Hypoarctic.
Within Eurasia, the Arctic territories usually include continental margins and the entire Arctic Ocean with its archipelagos and islands. Recognition of zones within northern high-latitude regions varies among authors (Polunin, 1951; Aleksandrova, 1980; Bliss, 1981; Elvebakk, 1985; Matveyeva, 1998). The division of the Arctic into High Arctic and Low Arctic is commonly accepted (Bliss, 1975). In Russian research practice, two zones are usually distinguished in the Arctic: polar deserts and tundras which divide into Arctic tundra and Subarctic tundra (Aleksandrova, 1980, 1988). The High Arctic subzone includes polar deserts and Arctic tundras, and the Low Arctic subzone comprises Subarctic tundras.
Here, we assume that the southern boundary of the Arctic corresponds to the southern boundary of the tundra zone (Aleksandrova, 1980; Walker et al., 2005) and the Arctic includes the polar deserts zone and tundra zone. The transition zone from tundra to the forest is considered Subarctic or Hypoarctic (Yurtsev, 1994). According to B.A. Yurtsev (1994), it includes typical and southern tundras, forest tundra, and the northern edge of the boreal forest. Here, we place only transitional ecotone communities of forest tundra and northern taiga in the Hypoarctic. Consequently, we accept the boundary between the northern and middle taiga as the southern border of the Hypoarctic (Yurtsev, 1994).
Historical review of studies on Cyanoprokaryota biodiversity in the Eurasian Arctic and Hypoarctic regions
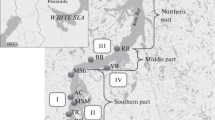
The location of all regions is shown in Fig. 2. Spitsbergen (Svalbard) archipelago has the longest history of study of Cyanoprokaryota in the European sector of the Arctic. The first records were made in the late nineteenth and early twentieth centuries (Table 1). Spitsbergen archipelago has also been a focus for their role in colonization processes following glacial retreat (Kaštovská et al., 2005, 2007; Turicchia et al., 2005; Stibal et al., 2006; Kvíderová et al., 2011) including nitrogen fixation (Liengen & Olsen, 1997; Solheim et al., 2002; Zielke et al., 2002, 2005). Several species from Spitsbergen archipelago were the subject of taxonomic and biogeographic studies (Komárek et al., 2006; Strunecky et al., 2012; Richter & Matuła, 2013).
The regions included in the research: 1 Malozemelskaya tundra, 2 Bolshezemelskaya tundra, 3 Polar Urals, 4 Subpolar Urals, 5 Commander Islands, Ch Chukotka peninsula, FJL Franz Josef Land archipelago, KK Krasnoyarsk kray, KP Kamchatka peninsula, MaR Magadan region, MR Murmansk region, NSI New Siberian Islands, NZ Novaya Zemlya archipelago, RK Karelia republic, RS Sakha (Yakutia) republic, SZ Severnaya Zemlya archipelago, SV Spitsbergen archipelago, T Taimyr peninsula, Y Yamalo-Nenets Autonomous Okrug
There have been few psychological studies on Franz Josef Land archipelago and Novaya Zemlya archipelago (Table 1).
Murmansk region is well studied with 359 species described. This is one of the richest floras in Russia, possibly due to the long history of research in the region starting with Elfving (1895). The history of research and a combined list of species for the region are provided by Davydov (2010a).
Three hundred and twenty species have been recorded from Eastern European tundra. Several studies have examined the tundras of Bolshezemelskaya and Malozemelskaya and the Polar and Subpolar Urals (Table 1).
There have been several studies in the Asian sector of the Arctic. Soil communities in polar deserts have been studied by Patova & Belyakova (2006). At lower latitudes, soil and freshwater species are described for Taimyr Peninsula, Yamalo-Nenets Autonomous Okrug, and rivers of Yakutia. Few studies have been carried out in the Chukotka and in Magadan regions.
Literature survey and data analyses
We combined our new findings with records from our CYANOpro database (http://kpabg.ru/cyanopro/) (Melechin et al., 2013), obtained during an extensive literature analysis, in order to review Cyanoprokaryota diversity in the Eurasian Arctic and Hypoarctic. The database has free and open access to Cyanoprokaryota biodiversity data, it is accessible through the Internet and only registration is needed. For species identification, recent monographs were used (Komárek & Anagnostidis, 1998, 2005; Komárek 2013). We studied 1500 database records of cyanoprokaryotes and our data (both new and published) obtained from 1000 samples collected in terrestrial and aquatic ecosystems. We used a special program code of CYANOpro database system to filter data and select data points for the polar deserts of the Arctic and Hypoarctic.
Floristic similarity was investigated using the Sørensen index (KS) (weighted pair-group method using arithmetic averaging) in the program module GRAPHS (Nowakowskiy, 2004): KS = 2a/(a + b) + (a + c), where a is the number of species common to both sets; c is the number of species unique to the first set; and b is the number of species unique to the second set.
Estimate of the current cyanobacterial biodiversity in the Eurasian Arctic and Hypoarctic and perspectives of further studies
Comparative analysis of species composition along the latitudinal gradient from polar deserts to the Hypoarctic
Within the Eurasian sector of the Arctic and Hypoarctic, 603 species of cyanoprokaryotes were found of which 482 were in the Arctic zone. This diversity belongs to 113 genera, 38 families, and 8 orders.
In the Eurasian sector of polar deserts, 156 species of cyanoprokaryotes were noted. Most species (147) were found in the Barentz province of the polar desert zone which includes North-East Land Island in the Spitsbergen archipelago, Franz Josef Land archipelago, and the northern tip of Novaya Zemlya. Siberian province (Severnaya Zemlya archipelago, the northern tip of Taimyr) had only 45 species.
Flora of polar deserts in Spitsbergen archipelago is presently the best studied among high-latitude regions and had 118 species. Flora of Franz Josef Land, the whole territory of which is entirely within the polar desert zone, had 69 species. Species similarity between archipelagos is low (Sørensen index of 26%) with only 25 common species, most being typical hydrophytes. A more detailed study of Franz Josef Land could result in a lower floristic difference.
Franz Josef Land and Severnaya Zemlya floras are closer (Sørensen index of 33%); however, the number of common species is only 18.
As expected, the number of species increases in the less harsh conditions of the Arctic tundra. 456 species are recorded for the territories of which 129 are common to polar deserts and Arctic tundra (Sørensen index of 41%). 30 species of cyanoprokaryotes were found only in polar deserts but not in the tundra. Most of them were found in southern areas of the Hypoarctic, except for 11 species (Chroococcus obliteratus P. G. Richt., Coleodesmium wrangelii ([C. Ag.] Born. et Flah.) Borzì ex Geitl., Gomphosphaeria cordiformis (Wille) Hansg., Leptolyngbya aeruginea (Kütz. ex Hansg.) Komárek, L. gelatinosa (Voronich.) Anagn. et Komárek, Merismopedia hyalina (Ehrenb.) Kütz., Microchaete calothrichoides Hansg., Phormidium lividum Näg., Symplocastrum aurantiacum (Hansg. ex Hansg.) Anagn., Trichocoleus tenerrimus (Gom.) Anagn., Xenococcus minimus Geitl.) which were not found there. We do not consider these as typical species of polar deserts because they have a worldwide distribution.
Four hundred and twenty-eight species have been recorded from the Eurasian Hypoarctic. The floristic similarity between the tundra zone and Hypoarctic is high (Sørensen index of 65%). A high similarity is also noted between the flora of the polar deserts and the Hypoarctic (Sørensen index of 45%). 307 species are found in the Hypoarctic flora but not in the Arctic zone; a significant number (117) is found in polar deserts and probably will be discovered in the tundra zone. 190 species are found only in the Hypoarctic. Some can be described as boreal species, in particular the representatives of the genera Anabaena (A. aequalis, Anabaena augstumalis, A. catenula (Kütz.) Born. et Flah., A. cylindrica Lemm., A. verrucosa B.-Pet. et al.), Dolichospermum (D. affinis, D. circinale, D. lemmermannii), Hapalosiphon (Hapalosiphon hibernicus W. West et G. S. West, H. intricatus, H. pumilus), Rivularia (R. aquatica De-Wild., R. beccariana [De Not.] Born. et Flah., R. borealis P. G. Richt., R. haematites), as well as the species Aulosira laxa Kirchn. ex Born. et Flah., Gloeotrichia echinulata, G. pisum Thur. ex Born. et Flah., Gomphosphaeria virieuxii (Virieux) Komárek et Hind. and Woronichinia karelica Komárek et Kom.-Legn.
Comparative studies of territorial floras of the Arctic and Hypoarctic
Regions and territories within the Arctic and Eurasian Hypoarctic have received varying intensities of study. The highest number of species has been observed in the well-studied Murmansk region (408 taxa, 359 species). The high number of species is detected on Spitsbergen archipelago (314) and Bolshezemelskaya tundra (191), Karelia Republic. Taimyr peninsula (123), Malozemelskaya tundra (122), Polar Urals (89), Chukotka (84), Subpolar Urals (150), Franz Josef Land archipelago (68), Magadan region (63), Novaya Zemlya archipelago (60), Yamal peninsula (62) have been only partially studied and have lower numbers of species. The smallest number of species was found in Severnaya Zemlya archipelago (41).
The reason for the rich flora of the Murmansk region is the wide diversity of habitats and the long history of studies. Vegetation zones vary from the northern boreal forests to the southern tundra. The number of species that are similar to Spitsbergen flora is high, considering that a large part of Spitsbergen (60%) is covered by glaciers. The floristic diversity of the Spitsbergen archipelago is probably due to a wide range of environmental conditions from mountainous territories with a varied geology to large areas of lowland tundra with many small ponds. Despite the small area of Malozemelskaya tundra, there is a rich flora (122 species). Some parts of the Urals region have been well-studied. The north part (Polar Urals) has 89 species, and the southern part (Subpolar Urals) has 150 species. The species richness could be explained by diverse mountain conditions (considerable altitudinal range and diverse landscapes), and the relatively low latitude of the Urals regions. Taimyr has a large number of species but an increase in species richness would be expected after more detailed studies as in addition to the large area it has several vegetation zones.
Similarities of species composition between different regions are generally quite low (Fig. 3). A high similarity (Sørensen index of >50%) only occurs for relatively well-studied flora of the Murmansk region and Spitsbergen archipelago (54%), and for Franz Josef Land and Novaya Zemlya (51%). The similarities between floras of Polar Urals and Subpolar Urals (30%), Polar Urals and Bolshezemelskaya tundra (43%), and Bolshezemelskaya tundra and Taimyr (38%) may be due to their similar geology.
A complete graph of similarity between Cyanoprokaryota floras in studied areas of the Arctic (gray circle) and Hypoarctic (white circle) (Sørensen index). For clustering, the mean distance between elements of each cluster was used with weighted pair-group method using arithmetic averaging, numbers on the ridges are similarity index shown in percentages where BT Bolshezemelskaya tundra, Ch Chukotka, FJL Franz Josef Land archipelago, MaR Magadan region, MR Murmansk region, MT Malozemelskaya tundra, NZ Novaya Zemlya archipelago, PU Polar Urals, SPU Subpolar Ural, SV Spitsbergen archipelago, SZ Severnaya Zemlya archipelago, T Taimyr peninsula, Y Yamal peninsula
The distribution of species amongst habitats
There are two ecological groups of species according to habitat type: aquatic and terrestrial. The latter can be sub-divided into subaerophytic (at the margins between aquatic and aerophytic habitats) and aerophytic (inhabitants of rocky substrates and soil surfaces).
Aquatic habitats
A reduction of species diversity in freshwater waterbodies Cyanoprokaryota from south to north is one of the main environmental features of high latitudes. This happens since the majority of water bodies in the polar desert zone of the Arctic are oligotrophic and also ultra-oligotrophic as they have a glacial origin and low temperature in the summer and the short vegetation period. Under such conditions, the species diversity and biomass of cyanoprokaryotes in plankton and benthos is low. The most common planktonic species are shown in Table 2.
There is low species diversity in typical planktonic genera (Anabaena, Aphanizomenon, Dolichospermum) in floras of high-latitude regions of Spitsbergen, Franz Josef Land and Novaya Zemlya archipelagos but their diversity increases markedly in the subarctic tundra. For example, a typical widespread species, Aphanizomenon flos-aquae, has not yet been found in any Arctic archipelagos (Spitsbergen, Franz Josef Land and Novaya Zemlya, Severnaya Zemlya) but it is frequently observed in the more southern tundra and Hypoarctic territories (e.g., Murmansk region, Malozemelskaya tundra and Bolshezemelskaya tundra, Taimyr) where it often colors water with its vigorous growths.
Plankton of large lakes of polar deserts usually includes tychoplanktonic species from Pseudanabaena, Leptolyngbya, Jaaginema, and Oscillatoria. Cyanoprokaryota of benthic habitats in Arctic and Hypoarctic lakes are more diverse and abundant. One of the most common benthic species in the lakes of high latitudes on Spitsbergen archipelago, and probably in other Arctic territories, is Oscillatoria tenuis (Table 2). Benthic mats formed by Phormidium uncinatum are also frequent.
In benthos of more southern lakes of the tundra zone species of Tolypothrix and Hapalosiphon are most frequent, whilst in mountain lakes these are replaced by species of Scytonema and Chamaesiphon.
Rivers at high latitudes are fed by meltwater from glaciers and snow fields. Upper reaches of rivers are cold, fast, and have large amounts of suspended mineral particles. These are extremely unfavorable conditions for development of cyanoprokaryotes. Towards the lower reaches, river flow slows but transparency and temperature often remain low. Cyanoprokaryota communities in rivers have similar species composition to those in smaller streams (Table 2).
Watersheds of more southern rivers are located in waterlogged boggy areas, so the transparency of the water is low due to high concentrations of humic acids. Turbulent rapids are often common for Hypoarctic and Arctic rivers. High rocky banks are typical of rivers in the mountains. Epilithon in these diverse rivers comprises species of Merismopedia, Microcystis, Coelospherium, Chamaesiphon, Stigonema, Tolypothrix, and Rivularia (Table 2).
In large rivers, massive growth of cyanoprokaryotes is only observed in their estuaries where phytoplankton blooms are caused by species of Aphanizomenon, Dolichospermum, Nodularia, and Phormidium.
Arctic streams are divided into two types: fast and slow. Fast streams are fed by glaciers. These have water that is cloudy with suspended sediment and only a few degrees above freezing. Algal communities are restricted to epilithon that forms mucous films on the surface of large boulders. Diversity is low being from 1 to 8 species (Table 2).
A greater diversity is found in slow streams. Phormidium uncinatum is the first alga to appear in upper reaches of slow streams which usually begin near snowfields. Further downstream species of Leptolyngbya appear. Small pebbles in the stream bed form a good habitat for Dichothrix gypsophila. This is also common in ephemeral ponds and small lakes.
Epilithon in streams also includes species of Ammatoidea, Aphanocapsa, Chamaesiphon, and Tolypothrix (Table 2). The bed of small streams is often covered by Microcoleus autumnalis which is one of the most common Arctic species.
Streams of southern Hypoarctic regions that are fed by ground springs have a high transparency. These are a good habitat for Tolypothrix tenuis, T. distorta, Nostoc coeruleum, Calothrix braunii Born. et Flah., C. clavata, Calothrix parietina, Dichothrix gypsophila, Gloeotrichia pisum and Rivularia haematites.
Terrestrial habitats
Terrestrial habitats support a greater diversity and abundance of cyanoprokaryotes with progression from South to North. A decrease in competition from higher plants and an increase in the range of ecological niches could explain this.
Subaerophytic habitats
These are transitional between aquatic and aerophytic habitats, typically being banks of water bodies, flooded areas of slopes and terraces and moss tundra wetlands. They are the most frequently occurring habitats in high latitudes and support the highest diversity of cyanoprokaryotes. Extensive mats (from 2 to 3 cm thick) are formed at the bottom of widespread, shallow, ephemeral lakes. These are dominated by Phormidium uncinatum which forms an upper layer and Leptolyngbya cf. gracillima and Pseudanabaena cf. minima, which form a bottom layer (Table 3). Mats dominated by Petalonema species (P. alatum and P. crustaceum) are less frequent.
Continuous snow melt during summer produces abundant runoff which results in waterlogging of upper soil horizons under permafrost conditions. Those habitats are called seepage (Komárek et al., 2012). Here, Gloeocapsa kuetzingiana, G. sanguinea, G. violacea and species that are often recorded in puddles and streams (Chroococcus turgidus, Microcoleus autumnalis, Oscillatoria tenuis, Phormidium kuetzingianum, P. uncinatum) are found.
Epiphytic on mosses in waterlogged moss tundra are Chroococcus turgidus, Symplocastrum friesii, Hapalosiphon pumilus, Fischerella muscicola, Aphanocapsa muscicola, Scytonema ocellatum, S. hofmannii, Microcoleus vaginatus, Nostoc punctiforme.
Nostoc commune is probably the most common species for all tundra habitats and especially in wet tundra. It is one of the most common species of the Arctic which can be found everywhere due to its adaptability to a wide range of habitats from bare ground of glacier nunataks to the bottom of rocky outcrops and small ponds. In wet moss tundras, its colonies can form a continuous cover over several square meters. Nostoc colonies can cover moss surfaces as well as grow within moss cushions. They could also be found in small ponds and water filled depressions. The study on genetic variation using 16S rRNA sequencing and AFLP methods of different colonies in the studied regions confirmed that all the specimens belong to Nostoc commune (Patova et al., 2015).
Aerophytic habitats
Aerophytic cyanoprokaryotes are inhabitants of rocky substrates and soil surfaces. Elevated rocky surfaces of different origins and geology are widespread in the Arctic and Hypoarctic. Combined with a lack of competition from lichens and vascular plants, this stimulates species richness of cyanoprokaryotes. Greatest abundance is observed on wet rocks receiving water from snow fields. Loose rocks are easily drained and remain dry most of the summer which makes them a hostile environment which is not colonized by cyanoprokaryotes. Under typical Arctic conditions, the most common thin crusts on wet rocks are of species of Gloeocapsa and Chroococcus (Table 4). In Hypoarctic regions, the most frequent crusts are of Stigonema ocellatum, S. minutum, and S. informe with associated Gloeocapsopsis magma and Gloeocapsa violascea.
Cyanoprokaryota crusts on soil surfaces vary in composition and can occupy large areas due to the high occurrence of bare spots in the Arctic regions. Those habitats are from 1 to 10 m2 or 20–90% of the area in the communities. Typical species of wet soils are Aphanocapsa grevillei, Leptolyngbya boryana, Microcoleus favosus, M. vaginatus, Nostoc commune, N. punctiforme, Petalonema crustaceum, Stigonema ocellatum, S. minutum, Scytonema ocellatum, Tolypothrix tenuis, and T. distorta.
Species often found at relatively high abundance in soils are Chroococcus cohaerens, Cyanothece aeruginosa, Desmonostoc muscorum, Kamptonema animale, Leptolyngbya boryana, Leptolyngbya foveolarum, Phormidium ambiguum, Nostoc punctiforme, N. linckia, and N. microscopicum. In ornithogenic habitats, such as downslope from bird colonies, species-richness is 4–5 species, but Microcoleus autumnalis is always found.
The presently known diversity of cyanoprokaryotes in the Eurasian Arctic and Hypoarctic comprises 603 species (Table 5). The Arctic has 80% of this total. In the Hypoarctic, 71% are observed. This could be attributed to the smaller area and the reduced range of environmental conditions (Fig. 2).
89% of species in the Arctic are found in the tundra zone while polar deserts have only 32%. The diversity of polar deserts in comparison with the total Arctic and Hypoarctic species is only 26%.
The harsh environment of polar deserts supports fewer species than Arctic tundra in which 456 species have been observed. This increased diversity can be attributed to the wider range of habitats that are favorable for cyanoprokaryotes.
The species richness within subclasses is similar in all zones. Oscillatoriophycidae (40%) is dominant in the total species list whilst Synechococcophycideae (32%) and Nostocophycideae (27%) are similar. The proportion of Nostocophycideae species increases in Hypoarctic. In the tundra zone and the Hypoarctic, species richness within orders is greatest in the Synechococcales and Nostocales. In polar deserts, there is an increase in the proportion of Chroococcales and a decrease in the proportion of Nostocales (Fig. 4). This is due to the low diversity of Anabaena and Dolichospermum and the increase in diversity of Gloeocapsa and Chroococcus species.
Undoubtedly, Cyanoprokaryota floras of the Arctic and Hypoarctic are still unevenly and incompletely studied. Murmansk region (359 species) and Spitsbergen archipelago (314 species) are the most fully studied. They also have the greatest similarity of species composition (Sørensen index of >50%). Relatively well-studied floras of other regions have 100–300 species as well as high similarity in species composition. It can be predicted that important additions to knowledge of species occurrence for all studied regions will result from an expansion of research to new areas. A significant increase in the knowledge of diversity of Arctic Cyanoprokaryota would also result from the application of the techniques of molecular genetics to investigate morphologically similar species and others which are difficult to place within the current taxonomic system. Some researchers discuss a possible endemism of Arctic and bipolar species (e.g., Komárek et al., 2012).
Subaerophytic cyanoprokaryotes had the highest species diversity (300 species) of all ecological groups. Cyanoprokaryota diversity changes differently for aquatic and terrestrial environments in a latitudinal gradient from polar deserts to Hypoarctic tundra. One of the main observed environmental patterns is a notable reduction in diversity of typical aquatic species from south to north, and in contrast, an increase in diversity of subaerophytic and aerophytic species in the north. An increasing area of the terrestrial ecosystem is occupied by cyanoprokaryotes with progression from the Hypoarctic to polar deserts. The main reason appears to be the reduced competition from higher plants.
The most widespread species of the Arctic and Hypoarctic are indicated in the species lists given in Tables 2, 3, and 4.
Spitsbergen and Franz Josef Land archipelagos and Polar and Subpolar Urals are typical mountain areas of the Arctic. Cyanoprokaryota floras of these regions are characterized by epilithic species of Gloeocapsa and Chroococcus as well as by the same species composition of subaerophytic cyanoprokaryotes and the high number of species growing on primitive soils (species of Leptolyngbya spp., Pseudanabaena spp., Microcoleus spp.).
Perspectives and future directions
The diversity of Cyanoprokaryotes in the Eurasian part of the Arctic and Hypoarctic is similar to that of the Antarctic and sub-Antarctic islands (537 species) (https://data.aad.gov.au), of Sweden (558) and of the Czech Republic (505), all being regions where detailed studies have been made (Willen, 2001; Kaštovský et al., 2009). However, these regions all have less environmental diversity than the studied regions of the Arctic and Hypoarctic. We suggest that further research on Cyanoprokaryota in the Arctic and Hypoarctic, including modern molecular studies, would significantly increase the known species and indicate which of these are endemic.
This review has indicated the high diversity of Cyanoprokaryota in the Arctic and Hypoarctic and that species occupy a wide range of aquatic and terrestrial habitats where they form dominant communities. To the south, the pattern is reversed and aquatic cyanoprokaryotes grow in abundance and cause blooms but in terrestrial habitats cyanoprokaryotes are of greatly reduced importance.
A special feature of the Eurasian Arctic and Hypoarctic is the limited number of dominant species. However, these are not special species for high-latitude regions as they are also found in the southern boreal zone.
Further study is required of the ecological preferences of individual species and their role in the formation of microbial communities in aquatic and terrestrial ecosystems of high latitudes. An integrated study of Cyanoprokaryota biodiversity, ecology, and geography in the Arctic and Hypoarctic will enlarge knowledge of the structural and functional diversity of ecosystems and history of biota in the Eurasian region.
References
Aleksandrova, V. D., 1980. The Arctic and Antarctic: Their Division into Geobotanical Areas. Cambridge University Press, Cambridge.
Aleksandrova, V. D., 1988. Vegetation of Soviet Polar Deserts. Cambridge University Press, Cambridge.
Batov, V. A., M. A. Vine-Rib & M. A. Sokolova, 1978. To the Study of Thermophilic Algae Flora of the High-Latitude Fluid Chukotka. Flora and Vegetation of Chukotka, Vladivostok: 15–22.
Belyakova, R. N., 2001. Blue-green algae of area Kukunski (Lorinski) hot springs (Chukotka peninsula). Novosti sistematiki nizshikh rastenii 34: 10–21. (in Russian).
Bliss, L. C., 1975. Tundra grasslands, herblands, and shrublands and the role of herbivores. Geoscience and Man 10: 51–79.
Bliss, L. C., 1981. North American and Scandinavian tundras and polar deserts. In Bliss, L. C., O. W. Heal & J. J. Moore (eds), Tundra Ecosystems: A Comparative Analysis. Cambridge University Press, Cambridge: 8–24.
Bogdanov, V. D., E. N. Bogdanova, A. L. Gavrilov, I. P. Melnichenko, L. N. Stepanov & M. I. Yarushina, 2004. Bioresources Aquatic Ecosystems of the Polar Urals. Urals Dep. RAS, Yekaterinburg. (in Russian).
Bogdanov, V. D., E. N. Bogdanova, I. P. Melnichenko, S. M. Melnichenko, L. N. Stepanov & M. I. Yarushina, 1991. Biology of Aquatic Ecosystems Mordy-Yakha River. Sverdlovsk. Submitted to VINITI, 06.06.91, N 2367-B91: 1–76. (in Russian).
Bondarenko, N. A. & L. A. Schur, 2007. The Cyanophyta’s plankton of small reservoirs of Eastern Siberia. Algologia 17: 26–41. (in Russian).
Borge, O., 1899. Süsswasseralgen von Franz Josefs-Land: gesammelt von der Jackson-Harmsworth’schen Expedition. Öfversigt af Kongl. Vetenskaps-akademiens förhandlingar, Stockholm 7: 751–766.
Borge, O., 1911. Die Süsswasseralgenflora Spitzbergens. Videnskapsselskapets Skifter. I. Mat.-Natur. Klasse 11: 1–38.
Davydov, D., 2005. Terrestrial cyanobacteria of east coast of Grønfjord (West Spitsbergen Island). Complex investigations of Spitsbergen Nature [Kompleksnye issledovaniya prirody Shpitsbergena] 5: 377–382. (in Russian).
Davydov, D., 2008. Cyanoprokaryota. In Koroleva, N. E., N. A. Konstantinova, O. A. Belkina, D. A. Davydov, A Yu Likhachev, A. N. Savchenko & I. N. Urbanavichiene (eds), Flora and Vegetation of Grønfjord Area (Spitsbergen Archipelago). K&M, Apatity: 93–102.
Davydov, D., 2010a. Cyanoprokaryota and their role in the process of nitrogen fixation in terrestrial ecosystems of the Murmansk region. GEOS, Moscow. (in Russian).
Davydov, D., 2010b. Cyanoprokaryota of the Spitsbergen archipelago: the state of study. Botanicheskij zhurnal [Russian Botanical Journal] 95: 169–176. (in Russian).
Davydov, D., 2011. Diversity of the Cyanoprokaryota of the Grønfjord western coast (Spitsbergen, Svalbard). Botanicheskiy zhurnal [Russian Botanical Journal] 96: 1409–1420. (in Russian).
Davydov, D., 2013. Cyanoprokaryota in polar deserts of Rijpfjorden east coast, North-East Land (Nordaustlandet) Island, Spitsbergen. Algological Studies 142: 29–44. doi:10.1127/1864-1318/2013/0082.
Davydov, D., 2014. Diversity of the Cyanoprokaryota of the area of settlement Pyramiden, West Spitsbergen Island, Spitsbergen archipelago. Folia Cryptogamica Estonica 51: 13–23.
Davydov, D., 2016. Diversity of the Cyanoprokaryota in polar deserts of Innvika cove North-East Land (Nordaustlandet) Island, Spitsbergen. Czech Polar Reports 6: 66–79.
Dorogostaiskaya, E. V., 1959. To the question of soil algal flora spotted tundra of the Far North. Botanicheskij zhurnal [Russian Botanical Journal] 44: 312–321. (in Russian).
Elfving, F., 1895. Anteckningar om Finlands Nostochaceae heterocysteae. Meddelanden af Societas pro Fauna et Flora Fennica 21: 25–50.
Elvebakk, A., 1985. Higher phytosociological syntaxa on Svalbard and their use in subdivision of the Arctic. Nordic Journal of Botany 5: 273–284.
Gabyshev, V.A., 2015. The phytoplankton the major rivers of Yakutia and adjacent areas of Eastern Siberia. Thesis of Doctor of Science, Yakutsk. (in Russian).
Getsen, M. V., A. S. Stenina & E. N. Patova, 1994. Algoflora of Bolshezemelskaya tundra in the conditions of anthropogenic pollution. Komi Science Centre Publishers, Syktyvkar. (in Russian).
Kaštovská, K., J. Elster, M. Stiball & H. Šantrůčková, 2005. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (High Arctic). Microbial Ecology 50: 396–407.
Kaštovská, K., M. Stiball, M. Šabacká, B. Černá, H. Šantrůčková & J. Elster, 2007. Microbial community structure and ecology of subglacial sediments in two polythermal Svalbard glaciers characterized by epifluorescence microscopy and PLFA. Polar Biology 30: 277–287.
Kaštovský, J., T. Hauer, J. Komárek & O. Skácelová, 2009. The list of cyanobacterial species of the Czech Republic to the end of 2009. Fottea 10: 245–249.
Kim, G. H., T. A. Klochkova & S. H. Kim, 2008. Notes on freshwater and terrestrial algae from Ny-Ålesund, Svalbard (high Arctic sea area). Journal of Environmental Biology 29: 485–491.
Kim, G. H., T. A. Klochkova, J. W. Han, S.-H. Kang, H. G. Choi, K. W. Chung & S. J. Kim, 2011. Freshwater and terrestrial algae from Ny-Ålesund and Blomstrandhalvøya Island (Svalbard). Arctic 64: 25–31. doi:10.14430/arctic4077.
Komárek, J. & K. Anagnostidis, 1998. Cyanoprokaryota, 1. Teil: Chroococcales. In Ettl, H., G. Gärtner, G. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa 19/1. G. Fischer Verlag, Jena.
Komárek, J. & K. Anagnostidis, 2005. Cyanoprokaryota, 2. Teil: Oscillatoriales. In Büdel, B., G. Gärtner, L. Krienitz & M. Schlager (eds), Süsswasserflora von Mitteleuropa 19/2. Elsevier, München.
Komárek, J., 2013. Cyanoprokaryota, 3. Teil: Heterocytous genera. In Büdel, B., G. Gärtner, L. Krienitz & M. Schlager (eds), Süsswasserflora von Mitteleuropa 19/3. Springer, Berlin.
Komárek, J., L. Kovacik, J. Elster & O. Komárek, 2012. Cyanobacterial diversity of Petuniabukta, Billefjorden, central Spitsbergen. Polish Polar Research 33: 347–368. doi:10.2478/v10183-012-0024-1.
Komárek, J., A. Taton, J. Sulek, A. Wilmotte, K. Kaštovská & J. Elster, 2006. Ultrastructure and taxonomic position of two species of the cyanobacterial genus Schizothrix. Cryptogamie, Algologie 27: 53–62.
Komulainen, S. F., T. A. Chekryzheva & I. G. Vislyanskaya, 2006. Algoflora of lakes and rivers of Karelia. Taxonomic composition and ecology. Karelian Research Center of the Russian Academy of Sciences, Karelia. (in Russian).
Kosheleva, I. T. & L. N. Novichkova, 1958. About the spotty tundra of Western Siberia and their algoflora. Botanicheskij zhurnal [Russian Botanical Journal] 43: 1478–1485. (in Russian).
Kosinskaya, E. K., 1933. The critical freshwater algae list collected V.P. Savich in the Arctic government expedition of 1930 year [Kriticheskiy spisok presnovodnykh vodorosley, sobrannykh V. P. Savichem v Arkticheskoy pravitel’stvennoy ekspeditsii 1930]. Proceedings Botanical Institute of Academy of Science USSR 1: 35–51. (in Russian).
Kuzmin, G. V., 1986. By the flora of algae downstream Yama river. (Magadan region). Botanicheskij zhurnal [Russian Botanical Journal] 71: 513–527. (in Russian).
Kvíderová, J., J. Elster & M. Šimek, 2011. In situ response of Nostoc commune s.l. colonies to desiccation in Central Svalbard. Norwegian High Arctic. Fottea 11: 87–97.
Lagerheim, G., 1894. Ein Beitrag zur Schneeflora Spitzbergens. La nuova notarisia 650–654.
Liengen, T. & R. A. Olsen, 1997. Seasonal and site-specific variations in nitrogen fixation in a high Arctic area, Ny-Alesund, Spitsbergen. Canadian Journal of Microbiology 43: 759–769.
Matuła, J., 1982. Investigations on the algal flora of West Spitsbergen. Acta Universitatis Wratislaviensis 525: 173–194.
Matuła, J., M. Pietryka, D. Richter & B. Wojtun, 2007. Cyanoprokaryota and algae of Arctic terrestrial ecosystems in the Hornsund area, Spitsbergen. Polish Polar Research 28: 283–315.
Matveyeva, N. V., 1998. Zonation of plant cover in the Arctic [Zonalnost’ v rastitelnom pokrove Arktiki]. Proceedings Komarov Botanical Institute of Russian Academy of Science 21: 1–219. (in Russian).
Melechin, A.V., D.A. Davydov, S.S. Shalygin, & E.A. Borovichev, 2013. Open information system on biodiversity cyanoprokaryotes and lichens CRIS (Cryptogamic Russian Information System). Bulleten MOIP. Department of Biology [Bulleten MOIP. Otdel biologicheskiy] 118: 51–56. (in Russian).
Naumenko, Yu V & L. A. Semenova, 1996. By studying some algae ponds Yamal Peninsula (Western Siberia). Novosti sistematiki nizshikh rastenii 31: 46–52. (in Russian).
Novichkova-Ivanova, L. N., 1963. Changes of soil algae community of on Franz Josef Land archipelago [Smeny sinuziy pochvennykh vodorosley Zemli Frantsa-Iosifa]. Botanicheskij zhurnal [Russian Botanical Journal] 48: 42–53. (in Russian).
Novichkova-Ivanova, L.N., 1972. Soil and aerial algae of polar deserts and Arctic tundra. In Wielgolaski, F.E. & T. Rosswall (eds), Proceedings IV International Meeting on the Biological, Leningrad: 261–265.
Nowakowskiy, A. B., 2004. Features and principles of the program module «GRAPHS» [Vozmozhnosti i principy programnogo modulya «GRAPHS»]. Institut Biologii, Syktyvkar. (in Russian).
Oleksowicz, A. S. & M. Luścińska, 1992. Occurrence of algae on tundra soils in Oscar II Land, Spitsbergen. Polish Polar Research 13: 131–147.
Palibin, I.V.,1903. Botanical results icebreaker “Ermak” voyage in the Arctic Ocean of the summer of 1901 year. [Botanicheskiye rezul’taty plavaniya ledokola “Yermak” v Severnom Ledovitom okeane letom 1901 g.] Petersburg.
Patova, A.D., E.N. Patova, D.M. Schadrin & I.N. Egorova, 2015. Morphological and molecular chracteristics of Nostoc commune Vauch. ex Born. & Flah.populations in mountain and arctic habitats. Abstracts of 6th International Conference on Polar and Alpine Microbiology. České Budějovice. 199–200.
Patova, E. N. & R. N. Belyakova, 2006. Terrestrial cyanoprokaryota of Bolshevik island (Severnaya Zemlya Archipelago). Novosti sistematiki nizshikh rastenii 40: 83–91. (in Russian).
Patova, E. N. & I. V. Demina, 2008. Algae in anthropogenically unaffected water bodies of the Polar Urals. Inland Water Biology 1: 54–63.
Patova, E. N. & I. N. Sterlyagova, 2016. Cyanoprokaryota in different types of water bodies in Subpolar Ural (Kosyu River Basin). Transaction of Kola Science Centrum. Applied Ecology of the North 7: 24–40.
Patova, E. N., 2001. The first data of blue-green algae Nenets reserve. Novosti sistematiki nizshikh rastenii 34: 34–38. (in Russian).
Patova, E. N., 2004. Cyanophyta in waters and soils of Eastern European tundra. Botanicheskij zhurnal [Russian Botanical Journal] 89: 1403–1419. (in Russian).
Perminova, G. N., 1990. Soil algae of some areas of northern Eurasia and the Far East [Pochvennye vodorosli nekotoryh rajonov severa Evrasii i Dalnego Vostoka]. Kirov, Submitted to VINITI, № 4471-B90: 1–41. (in Russian).
Pivovarova, Zh F, 1987. Algal groups steppe kastanozems soils of Kolyma. Botanicheskij zhurnal [Russian Botanical Journal] 71: 888–891. (in Russian).
Plichta, W. & M. Luścińska, 1988. Blue-green algae and their influence on development of tundra soils in Kaffiöyra, Oscar II Land, Spitsbergen. Polish Polar Research 9: 475–484.
Polunin, N., 1951. The real Arctic: suggestions for its delimitation, subdivision and characterization. Journal of Ecology 39: 308–315.
Raabová, L., J. Elster & L. Kováčik, 2016. Phototrophic microflora colonizing substrates of man-made origin in Billefjorden Region, Central Svalbard. Czech Polar Reports 6: 21–30.
Richter, D. & J. Matuła, 2013. Leptolyngbya sieminskae sp. n. (Cyanobacteria) from Svalbard. Polish Polar Research 34: 151–168.
Richter, D., J. Matuła & M. Pietryka, 2009. Cyanobacteria and algae of selected tundra habitats in the Hornsund fi ord area (West Spitsbergen). Oceanological and Hydrobiological Studies 38: 65–70.
Richter, D., M. Pietryka & J. Matuła, 2015. Relationship of cyanobacterial and algal assemblages with vegetation in the high Arctic tundra (West Spitsbergen, Svalbard Archipelago). Polish Polar Research 36: 239–260.
Sdobnikova, N. V., 1986. Soil algae in the southern tundra Taimyr. In Chernov, Y. I. & N. V. Matveyeva (eds), Southern Taimyr Tundra. Nauka, Leningrad: 68–79. (in Russian).
Shirshov, P. P., 1935. Ecological and geographical essay of freshwater algae of Novaya Zemlya and Franz Josef Land [Ekologo-geograficheskiy ocherk presnovodnykh vodorosley Novoy Zemli i Zemli Frantsa-Iosifa]. Proceedings of Arctic Institute 14: 73–162. (in Russian).
Skulberg, O. M., 1996. Terrestrial and limnic algae and cyanobacteria. In Elvebakk, A. & P. Prestrud (eds), A Catalogue of Svalbard Plants, Fungi, Algae and Cyanobacteria. Norsk Polarinstitutt, Skrifer, Oslo: 383–395.
Solheim, B., U. Johanson & T. V. Callaghan, 2002. The nitrogen fixation potential of Arctic cryptogram species is influenced by enhanced UV-B radiation. Oecologia 133: 90–93.
Stenina, A.S. & E.N. Patova, 2007. Algae. In: Systematic Lists of Species of Flora and Fauna of the State Nature Reserve “Nenetskij” in 2001-2006 years. St. Petersburg University Publishers, St. Petersburg: 3–21. (in Russian).
Stibal, M., M. Šabacká & K. Kaštovská, 2006. Microbial communities on glacier surfaces in Svalbard: impact of physical and chemical properties on abundance and structure of Cyanobacteria and Algae. Microbial Ecology 52: 644–654.
Stockmayer, S., 1906. Kleiner Beitrag zur Kenntnis der Süsswasseralgenflora Spitzbergens. Oesterreichische botanische Zeitschrift 56: 47–53.
Strǿm, K.M. 1921. Some algae from hot springs in Spitzbergen. Botaniska notiser 17–21.
Strunecky, O., J. Komárek & J. Elster, 2012. Biogeography of Phormidium autumnale (Oscillatoriales, Cyanobacteria) in western and central Spitsbergen. Polish Polar Research 33: 369–382.
Summerhayes, V. S. & C. S. Elton, 1923. Contributions to the ecology of Spitsbergen and Bear Island. Journal of Ecology 11: 214–286.
Thomasson, K., 1958. Zur planktonkunde Spitzbergens, 1. Hydrobiologia 12: 226–236.
Thomasson, K., 1961. Zur planktonkunde Spitzbergens, 2. Hydrobiologia 18: 192–198.
Turicchia, S., S. Ventura, U. Schütte, E. Soldati, M. Zielke & B. Solheim, 2005. Biodiversity of the cyanobacterial community in the foreland of the retreating glacier Midtre Lovènbreen, Spitsbergen, Svalbard. Algological Studies 117: 427–440. doi:10.1127/1864-1318/2005/0117-0427.
Vasilieva-Kralina, N. I., P. A. Remigaylo, V. A. Gabyshev, L. I. Kopyrina, E. V. Pshennikova, A. P. Ivanova & L. A. Pestryakova, 2005. Algae. The Diversity of Yakutia Plants. Siberia Dep. RAS, Novosibirsk: 150–272.
Voronikhin, N. N., 1930. Algae Polar and Northern Urals. Proceedings Leningrad’s Society of Naturalists 60: 1–71.
Voronkov, N. V., 1911. Plankton reservoirs of Yamal Peninsula. Annual of the Zoological Museum of the Imperial Academy of Sciences 16: 180–214. (in Russian).
Walker, D. A., M. K. Raynolds, F. J. A. Daniëls, E. Einarsson, A. Elvebakk, W. A. Gould, A. Katenin, S. Kholod, C. Markon, E. Melnikov, N. Moskalenko, S. Talbot & B. A. Yurtsev, 2005. The circumpolar Arctic vegetation map. Journal of Vegetation Science 16: 267–282.
Wille, N., 1879. Ferskvandsalger fra Novaja Semlja samlede af Dr. F. Kjellman paa Nordenskiölds Expedition 1875. Öfversigt af kongl. Vetenskaps-akademiens forhandlingar 5: 13–74.
Willen, E., 2001. Checklist of Cyanobacteria in Sweden. ArtDatabanken. SLU, Upsala.
Willen, T., 1980. Phytoplankton from lakes and ponds on Vestspitsbergen. Acta Phytogeographica Suecica 68: 173–188.
Wittrock, V. B., 1883. Über die Schnee – und Eisflora, besonders in den arktischen Gegenden. In Nördenskiold, A.E. (ed), Studier och Forskningar Föranledda af Mina Resor i Höga Norden. Stockholm: 2–3.
Wittrock, V.B. & O. Nordstedt, 1882. Algae aquae dulcis exsiccatae. Upsaliae, Lundae et Stockholmiae: 21.
Yermolaev, V.I., G.D. Levadnaya & T.A. Safonova, 1971. Algae ponds neighborhoods Taimyr station. In: Tikhomirov, B.A. (ed) Biogeocoenosis Taimyr Tundra and Their Productivity: 116–129. (in Russian).
Yurtsev, B. A., 1994. Floristic division of the Arctic. Journal of Vegetation Science 5: 765–776.
Zakharova, V. I., L. V. Kuznetsova, E. I. Ivanova, et al., 2005. The Diversity of the Vegetative World of Yakutia/Ed. N. S. Danilova. Siberian Branch of RAS, Novosibirsk. (in Russian).
Zielke, M., A. S. Ekker, R. A. Olsen, S. Spjelkavik & B. Solheim, 2002. The influence of abiotic factors on biological nitrogen fixation in different types of vegetation in the High Arctic, Svalbard. Arctic, Antarctic and Alpine Research 34: 293–299.
Zielke, M., B. Solheim, S. Spjelkavik & R. A. Olsen, 2005. Nitrogen fixation in the high Arctic: role of vegetation and environmental conditions. Arctic, Antartic and Alpine Research 37: 372–378.
Acknowledgements
This study was conducted with the support of grants from Russian Foundation for Basic Research Nos. 15-04-06346, 15-29-02662. We thank Anna Patova (Ludwig-Maximillian University, Germany) for help in translation of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Eugen Rott, Allan Pentecost & Jan Mares / Aspects of cyanobacterial biogeography, molecular ecology, functional ecology and systematics
Rights and permissions
About this article
Cite this article
Davydov, D., Patova, E. The diversity of Cyanoprokaryota from freshwater and terrestrial habitats in the Eurasian Arctic and Hypoarctic. Hydrobiologia 811, 119–137 (2018). https://doi.org/10.1007/s10750-017-3400-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3400-3