Abstract
This study investigated the fish fauna of a stream in southeastern Brazil that runs through sugar cane-growing and urban areas that are subject to sewage discharge. It aimed to determine the importance of spatial and environmental factors on fish distributions and to assess the use of fish as indicators of the environmental conditions. A longitudinal pattern of chemical water quality was observed, with more disturbed conditions at the urban sites. Several opportunistic or tolerant and omnivorous species such as Astyanax altiparanae, Poecilia reticulata, and Astyanax fasciatus were numerically abundant at these urban sites, which resulted in a consistent increase in species density and biomass along the stream. Redundancy analysis extracted three axes, identifying a nutrient–productivity gradient associated with different species composition patterns, as also detected by the indicator species analysis. No species could be considered a potential indicator of the agricultural area in the dry season, whereas four different species were judged potential indicators of three urban sites. In order to explain species distribution, variation in species abundance was partitioned into spatial and environmental components. The latter was more important, explaining 25.4% of the variation. We concluded that the fish assemblage of the Guamium stream is undergoing the initial stages of disturbance, with pronounced effects at the urbanized area, but no detectable changes at the sugar cane sites. Therefore, fish assemblages might be used as ecological indicators to detect urban impacts in similar rivers. However, the potential use of fish to diagnose stressors in areas of sugar cane plantations should be complementary to more comprehensive studies aimed at understanding the influence of this crop on freshwater resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities at the landscape scale have been increasingly recognized as major threats to the ecological integrity of river ecosystems, impacting habitat, water quality and the biota via numerous and complex pathways (Allan et al., 1997). Several studies have shown that not only do non-point sources from agriculture cause long-term, cumulative harm to stream ecosystems (Richards et al., 1996; Skinner et al., 1997), but also that increasing urbanization, which may release toxicants and excess nutrients, alters stream channels, increases storm-water runoff, and accelerates channel erosion (Klein, 1979).
The effects of these chemical and physical changes, especially organic enrichment, on biological communities along river systems have been investigated by several authors (Bartsch, 1948; Hynes, 1960; Rörig et al., 2007). These studies observed changes in community composition and species abundance below the point of discharge. Fish have also been used as indicators of pollution for many decades (Doudoroff & Warren, 1957) and were later incorporated into ecologically based indexes that monitor water quality using fish assemblages (Karr, 1981; Hugues & Gammon, 1987; Ganasan & Hugues, 1998), once their presence, absence, and proportionate abundance may indicate changes in the physical, chemical, or biological conditions in which they live.
The effects of urbanization on fish have been investigated more thoroughly in temperate regions and are generally characterized by increases in tolerant and introduced species, decline of sensitive species (Onorato et al., 2000; Scott & Helfman, 2001) and decreases in diversity or fish multimetric indexes (Klein, 1979, Steedman, 1988). Scott et al. (1986) found that urban development resulted in a restructuring of the fish community, and Pompeu & Alves (2005) and Cunico et al. (2006) reported profound changes in fish community structure in two different Brazilian urban rivers. However, despite these few studies on fish assemblage responses to urbanization, a general response model does not exist (Paul & Meyer, 2001), especially for the neotropical region where relevant information is even more scarce.
At the local scale, several studies have focused on the longitudinal variations in fish communities (Matthews et al., 1992; Mazzoni & Lobón-Cerviá, 2000; Petry & Schulz, 2006; Suárez & Petrere, 2006) as well as how community structure supports the qualitative aspect of the River Continuum Concept (Vannote et al., 1980). A consistent change in community structure from upstream to downstream areas seems to result from changes in physical gradients, with species richness increasing as a result of addition processes, followed by shifts in trophic structure and sizes of individuals (Schlosser, 1982), which are collectively attributed to gradual increases in habitat diversity (Gorman & Karr, 1978; Reyes-Gavilán et al., 1996) and physical and chemical alterations of the water (Matthews & Styron, 1981).
The influence of environmental variables in determining fish distributions in Brazilian streams has been assessed in several studies (Abes & Agostinho, 2001; Mazzoni et al., 2006; Suárez & Petrere, 2006), some of which also considered the effects of anthropogenic perturbations (Cunico et al., 2006; Ferreira & Casatti, 2006; Pinto et al., 2006; Araújo & Tejerina-Garro, 2009). Few studies, however, have attempted to identify fish species that characterize particular regions. This information might help to explain distribution patterns along environmental gradients in rivers and be useful in management and determining areas for conservation (Matthews et al., 1992).
Assessment of how anthropogenic perturbations affect fish communities is complicated by several factors, since local fish assemblage richness and structure are influenced by processes occurring both at a large scale, such as geomorphology and climate (Hugues et al., 1987; Nelson et al., 1992), and a small scale or local level, including land use, soil type and natural terrain (Tejerina-Garro et al., 2005; Allan, 2004). Furthermore, interactions between the many factors that affect spatial structuring of natural communities make it difficult to determine the relative contribution of each factor, as these interactions often result in an overlaid effect in space (Borcard et al., 1992).
In southeastern Brazil, the most common causes of biodiversity loss in inland waters are large urban areas, industry, and agriculture (Tundisi, 2003). In the State of São Paulo, urban areas account for 11.3% of the landscape and agriculture 76.2%, with sugar cane representing 20.1% of the planted area (Gonçalves et al., 2009). The Piracicaba River is one of the most urbanized watersheds of the State, with the second greatest area covered by sugar cane (34%) (Silva et al., 2007). Although the effects of this crop on water bodies and specifically on fish fauna are very poorly understood, there is abundant evidence that widespread environmental degradation results from soil erosion in sugar cane fields (Martinelli & Filoso, 2007). Moreover, sugar cane receives much more fertilizer than pasture, with values varying from 80–100 kg nitrogen/ha (Ometto et al., 2000), which increases the likelihood of having a high input of applied fertilizers to streams (Filoso et al., 2003). These fertilizers are known to contain different concentrations of lead, nickel, chromium, cadmium and zinc, which can affect water quality, bioassimilation and bioaccumulation by the aquatic organisms (Corbi et al., 2008).
In this study, the distribution of fish assemblages was investigated in a stream within a rural–urban gradient where sugar cane crops are abundant. The objectives were to determine which sources of variation (spatial and/or environmental) could explain fish distributions and to determine whether different species could be used as bioindicators to indicate environmental conditions of rural and urban impacts. This approach is a first step toward understanding the influence of different land uses on fish communities in a tropical river in southeast Brazil, which will enable better management of rivers under similar conditions.
Study area
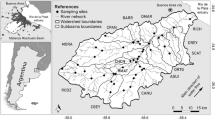
The Piracicaba River basin is a meso-scale watershed (12,400 km2) located predominantly in the State of São Paulo, southeastern Brazil (Fig. 1), and is composed of three main sub-basins (Jaguari, Atibaia, and Piracicaba). The basin encompasses 45 municipalities with a population of approximately five million inhabitants (SMA, 2007). Population and industry are concentrated in the central part of the basin, where most of the sewage load, both domestic and industrial, is generated (Martinelli et al., 1999). Recent land use studies for this basin have shown that pasture and sugar cane dominate; the former covering 43% of the total area and the latter 34%. The urban basin occupies 6% of the area, given the significant number of densely populated cities located in the central part of the basin (Silva et al., 2007). The climatic classification of the area, according to Köppen, is Cwah, i.e., tropical humid with summer rains and dry winter. Average annual precipitation in the basin is 1,400 mm, most of which falls between October and March (Williams et al., 2001).
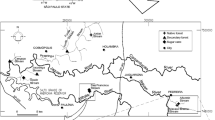
The Guamium stream is a tributary of the right margin of the Piracicaba River, draining an area of approximately 70 km2 in the municipality of Piracicaba. Three dams occur along the 17.9 km course, which crosses an area of heavy sugar cane cultivation and urban influences, especially in its final third, where several industries, including foundries, metal works, ceramic, steel, cardboard packing, and alcohol production, are located. Overall, the dominant land cover types in the basin are sugar cane (78%), exotic forest and urban area (7%), primary forest (5%), and pasture (3%) (Barreto et al., 2005).
Ten sites distributed along the Guamium stream were selected for this study, with distances varying from 2.5 to 17.9 km from the headwaters. They were located between S 22° 33′995″, W 47° 36′206″ and S 22° 41′414″, W 47° 40′380″ with most sites (2–10) classified as third order and site 1 as second order (Fig. 1). Sites 1 to 5 are situated in an area where the dominant land cover is sugar cane crops, with some sparse corn plantations and pasture; riparian vegetation is shrubby and the exotic Leucaena sp. prevails. Sites 6 to 10 are within the urban area, where the influence of industries, residences, irregular occupations, and public and domestic effluents are frequently observed. Vegetation is present and composed mainly of pasture and secondary forest.
Materials and methods
Sampling procedure
Samples of fish, water, and environmental variables of the 10 collection sites were obtained in the dry (August–September/2005) and rainy (January–February/2006) seasons. Fish samples were collected using stationary electrofishing equipment (HONDA EX 1000 generator, 120 V, 1,000 W, 60 Hz, <2.5 A, AC). At each site, three successive catches with constant unit of effort (CPUE) were conducted over a stretch of 50 m. Sampled fish were fixed in formalin and preserved in 70% alcohol, and individual fish were measured and weighed. Identification was based on Britski (1972) and Britski et al. (1986) and confirmed by specialists at the Museum of Zoology of the University of São Paulo. Sampled area was calculated for each sampling site, based on several transversal transects from the lower to the upper section.
At each sampling site, temperature (°C), dissolved oxygen (mg l−1), turbidity (NTU), pH, electrical conductivity (μS cm−1) and total dissolved solids (mg l−1) were measured with a multi-parameter water quality monitoring system (HORIBA U-22). Other analyzed physicochemical parameters were 5-day biological oxygen demand (BOD) (mg l−1), ammonium (N–NH4 +) (mg l−1), orthophosphate (PO4 3−) (μg l−1), chlorophyll-a (μg l−1), alkalinity (mg l−1), following APHA (1989), nitrate (mg l−1) (CETESB, 1978), nitrites (FEEMA, 1981), total nitrogen (mg l−1) and total phosphorus (mg l−1) (Strickland & Parsons, 1968). Measurements of habitat structure included depth (m), water velocity (m s-1), percent tree canopy shading, trunk density (%), proportion of pools (%) and dominant substrate—categorized as sand (<3 mm), fine gravel (≅3–49 mm), coarse gravel (≅50–149 mm), small boulders (≅150–300 mm), or large boulders (>300 mm).
Data analysis
Physicochemical water parameters and biological data on total fish biomass (kg ha−1), density (ind. ha−1), Shannon–Wiener diversity (H′), Simpson’s dominance (D) (Krebs, 1989) and richness (Odum, 1988) were analyzed along the Guamium stream for both seasons. Spearman Rank Correlation, a nonparametric measure of association, was used to relate these parameters at the different sampling points to their distance from the headwaters using Bioestat 5.0 (2007). Correlations were considered significant when P < 0.05.
To analyze species–environment relationships, a DCA was first used to decide between a linear or a unimodal species response, which resulted in the choice of a redundancy analysis (RDA). RDA is a canonical ordination technique that extends principal components analysis to explain variations in attributes using a matrix of environmental variables while preserving the Euclidean distance among the objects (Legendre & Legendre, 1998). For this analysis, fish abundance was transformed into Hellinger distance (Legendre & Gallagher, 2001) and environmental data into log. In each analysis, step-wise forward selection was used to reduce the environmental variables to those most correlated with the axes (Ter Braak, 1987). A cutoff point of P = 0.05 was used to incorporate variables into the final models. The statistical significance of axes derived by each analysis was tested with a Monte Carlo permutation test (Hope, 1968).
In order to understand the effects of the environmental variables and the spatial structure on fish distribution, a method which partitions the variation of species abundance data into independent components was used (Borcard et al., 1992). It considers the idea that some species and environmental variables may share a common spatial structuring, and is based on pre-existing methods of canonical ordination [RDA (van den Wollenberg, 1977) and CCA (ter Braak, 1986)] and the software CANOCO. Total variation in species is then explained as (a) the nonspatial environmental variation in the species data, (b) the spatial structuring in the species data that is shared by the environmental data, (c) the spatial patterns in the species data that are not shared by the environmental data, and (d) unexplained variation and stochastic fluctuations (Borcard et al., 1992). The variable used to describe the spatial component was the distance in kilometers of each site from the headwaters. All analyses were performed in CANOCO version 4.5.
An indicator species analysis (Dufrêne & Legendre, 1997) and a Monte Carlo test with 1,000 permutations were run on PcOrd 4.0 (McCune & Mefford, 1999), to verify non-random species distribution in the sampled sites for the dry and rainy seasons. This method combines information on the concentration of species abundance in a particular group and the fidelity of occurrence of a species in a particular group. The indicator values range from zero (no indication) to 100 (perfect indication). Species with probability of type I error <5% were considered as potential indicators.
Results
Major habitat characteristics
The physical and chemical features of the sampling sites are presented in Table 1 and Fig. 2. Major physical characteristics that differed among the longitudinal axis were related to dominant land cover, as the upper section of the river (sites 1–5) was surrounded by sugar cane and patches of corn cultures and the lower sites (6–10) fell within the range of urban influences. Riparian vegetation, substrate, current speed, and point source pollution also varied along the river, with sand and fine gravel occurring at the upper sections and coarse gravel and large boulders at the urban sites, where sewage entry was commonly observed.
Longitudinal variation of different physicochemical parameters along the Guamium stream for the dry and rainy seasons, indicating significant results of Spearman’s correlations (P < 0.05). T temperature, DO dissolved oxygen, EC electrical conductivity, BOD biochemical oxygen demand, TDS total dissolved solids, TSS total suspended solids, Chl-a chlorophyll-a, TP total phosphorus, Alk alkalinity, TN total nitrogen. Filled circles rainy season, open circles dry season, rs rainy season, ds dry season
Differences in physicochemical and biological parameters between the two seasons were well defined for most sites, with higher values observed in the dry season for all parameters, with the exceptions of temperature and turbidity. The latter was the only parameter that was negatively correlated with the distance from the headwaters, especially in the rainy season. All other parameters were indicative of increasing eutrophication along the river, with significant positive Spearman correlations found with distance for pH, total dissolved solids, conductivity, alkalinity, nitrite, nitrate, total nitrogen, ammonium, total phosphorus, total suspended solids, orthophosphate, chlorophyll-a, and biological oxygen demand during the rainy season.
Fish assemblages and environmental characteristics
In the Guamium stream, 2,731 individuals of 38 fish taxa were captured. Eight species were found exclusively during the dry season: Serrasalmus maculatus Kner, 1858, Leporinus octofasciatus (Steindachner, 1915), Hypostomus sp.2, Hypostomus sp.3, Hypostomus sp.4, Astyanax bockmanni Vari & Castro, 2007, Astyanax sp.1 and Astyanax sp.2. Species that occurred exclusively in the rainy season were Oreochromis niloticus Linnaeus, 1758, Hyphessobrycon eques (Steindachner, 1882), Leporinus obtusidens (Valenciennes, 1836) and Moenkhausia intermedia (Eigenmann, 1908).
The four most abundant species, which contributed 74.37% of the total density in the ichthyocenosis, were predominantly omnivorous and tolerant species (sensu Karr, 1981), including Astyanax altiparanae Garutti & Britski, 2000, Poecilia reticulata Peters, 1859, Astyanax fasciatus (Cuvier, 1819) and Geophagus brasiliensis (Quoy & Gaimard, 1824) (Table 2, Table 3). These species contributed 65.3% of the total fish biomass, with A. altiparanae presenting the highest values (44.4%), followed by G. brasiliensis (14.0%). Astyanax altiparanae and P. reticulata, although present at all sites, showed well-differentiated density patterns along the longitudinal gradient, with a marked increase at site 10 (Table 3).
Species richness, Shannon diversity, and Simpson’s dominance did not present a marked longitudinal pattern, though total density and biomass showed positive correlations with distance (Fig. 3). This pattern was also observed for the most abundant species A. altiparanae and P. reticulata, especially during the rainy season (ρ = 0.7256; P = 0.0175 and ρ = 0.7781; P = 0.0080, respectively).
Redundancy analysis (RDA) of the species data with step-wise forward selection reduced the environmental dataset to three variables. The ordination produced three significant axes with eigenvalues of 0.104, 0.084 and 0.067. The sum of all canonical eigenvalues was 0.254. The species-environment correlations for the three axes were 0.917, 0.876, and 0.868, with the first axis concentrating 40.7% of the variation, the second axis 33% and the third axis 26.3%.
The first RDA identified a gradient primarily associated with changes in nutrient levels, separating the upper (sugar cane) and lower (urban) sites (7, 8, 9, and 10) (Fig. 4). Species that had negative scores and were associated with higher eutrophication included some exclusive species such as Apareiodon affinis (Steindachner, 1915), H. eques, Parodon nasus (Kner, 1959), and O. niloticus, among others. Callichthys callichthys (Linnaeus, 1758) and Phalloceros spp. had positive scores and were associated with site 1, which showed lower nutrient levels.
Biplots of the first and second RDA axes for the fish communities of the Guamium stream. Grey vector lines indicate the relationship of the significant environmental variables to the ordination axis; the line’s length is proportional to its relative significance. Filled circles rainy season, open circles dry season. Nitr nitrite, De depth, Tur turbidity. For species codes, see Table 3
Variables correlated with the second RDA axis described a seasonality gradient related to depth and turbidity, which separated the rainy and dry seasons. Hypostomus ancistroides (Ihering, 1911) and P. reticulata were associated with the deeper sites 4, 5, and 6 during the rainy season, while A. fasciatus and Imparfinis sp. correlated with shallower sites 1 and 3 during the dry season.
The amount of variation in the species matrix explained by each step in the variation-partitioning process revealed that the overall amount of explained variation (in percentage of the total variation of the species matrix) was 31.8%. The entire variation of the species matrix was further explained by (a) environmental variables (25.4% of the variation), of which only about one-tenth of this amount (2.6%) was due to spatial variation; (b) pure spatial variation (not shared by the environmental variables) (6.4% of the total variation); and (c) unexplained variation (68.2%).
According to the calculated indicator values (IV) (Table 4), no species could be considered as potential indicators for the agricultural area in the dry season, whereas the characiform Parodon nasus and the siluriform Rhamdia quelen (Quoi & Gaimard, 1824) were indicators of three urban sites (7, 8, and 9). In the rainy season, three species were defined as potential indicators for the urban sites (7, 8, and 9): A. altiparanae, R. quelen and Hypostomus regani (Ihering, 1905). The gymnotiform Gymnotus sp. and cyprinodontiform Phalloceros spp. were associated with sites 1 and 6, located in the agricultural and urban areas, respectively. Site 10, which formed a single group, could not be included in the analysis, but exclusively accommodated three species: the exotic O. niloticus and the small-sized A. affinis and H. eques. Taxa that had high positive or negative scores on the first RDA axis can also be used as diagnostic indicators of the gradients, confirming the results obtained with the Indicator Species Analysis.
Discussion
Regional problems of water quality in the Piracicaba River Basin have been mainly caused by raw sewage and industrial waste and, to a lesser extent, by sugar cane burning and leaching of fertilizers (Williams et al., 2001). These activities result in increases in dissolved organic and inorganic carbon and conductivity (Daniel et al., 2002). The Guamium stream can be considered moderately impacted, given that studies conducted in other streams and rivers of this basin showed that conductivity values in the most impacted rivers reached 590 μS cm−1 (Daniel et al., 2002). A marked seasonality has also been noted, resulting in higher nutrient, conductivity, and total suspended solids in the dry season. In the Guamium stream, most of the physicochemical parameters were in accordance with the guidelines proposed by the Brazilian regulatory arm of the Ministry of Environment (CONAMA, Resolution no. 357, 2005) for aquatic life preservation but ammonium and total phosphorus were above the established values, indicating increasing eutrophication at the final urban stretches.
Although limnological characteristics of rivers are strongly determined by physiographic aspects such as altitude, geology, and vegetation, some studies conducted in Brazilian rivers report that organic effluents strongly influence the chemical characteristics of these waters (Camargo et al., 1996; Rörig et al., 2007). Spatial variability in water quality has been documented mainly for urbanized (Cunico et al., 2006; Rörig et al., 2007) and physically degraded streams (Casatti et al., 2006), with little information available regarding streams in agricultural areas, especially sugar cane (Smeets et al., 2006).
Despite the moderate pollution levels of the Guamium stream, 38 fish species belonging to 13 families were reported, similar to a study conducted in preserved rivers of the same basin, where 35 species were identified (Cetra & Petrere, 2006). However, dominant species differed, as the Guamium stream fish fauna was predominantly composed of the tolerant and omnivorous A. altiparanae, P. reticulata, and A. fasciatus, which have been associated with altered environmental characteristics such as high organic loads (Schulz & Martins, 2001; Cunico et al., 2006).
The contributions of land use changes to loss of aquatic species and degradation of stream quality have been well documented (Booth & Jackson, 1997; Bledsoe & Watson, 2001), with individuals of many species responding directly to the amount of suspended silt, turbidity, dissolved oxygen, and canopy cover (Karr, 1981; Scott & Hall, 1997). However, some studies show that changes in fish assemblages do not occur immediately following anthropogenic impacts, but may take many years, with gradual replacement of species (Fitzgerald et al., 1998).
Low diversity and high abundance of few species have been associated with degraded southeastern Brazilian urban streams, where P. reticulata, H. ancistroides, and R. quelen seem to be numerically dominant (Cunico et al., 2006). This condition was only partially replicated in the Guamium stream, leading us to propose that, although fish assemblage changes were not evident, the dominance of a few opportunistic and small-bodied species such as P. reticulata suggests that water quality alterations and/or physical habitat degradation are disturbing the community. In fact, exogenous disturbance of a community often favors opportunistic species with small bodies and shorter lifespans, which become numerically dominant (Clarke & Warvwick, 1994).
According to the River Continuum Concept (RCC) (Vannote et al., 1980), a gradual increase in species richness is expected along longitudinal gradients in lotic systems, associated with temporal changes in resource availability, channel morphology, and flow regime. In tropical streams, addition of species is usually related to an increase in physical and structural complexity of the environment (Casatti, 2005; Braga & Andrade, 2005) as a result of increased habitat diversity and stability (Horwitz, 1978). Zonation patterns of fish assemblages can also be explained by several factors, including microhabitat suitability (Matthews, 1986), physiographic characteristics (Gorman & Karr, 1978), and biotic interactions (Gilliam et al., 1993). However, according to Naiman et al. (1988), the RCC is more difficult to apply to river systems where natural zones are well defined or that have endured anthropogenic impacts.
In the Guamium stream, the most evident pattern of fish community changes manifested as an increase in total fish biomass and density from upstream to downstream, with less evident changes in richness and diversity, possibly indicating a disruption of the usual pattern of fish addition. The dominance of A. altiparanae and P. reticulata at the final urban sites, which exhibited higher eutrophication, confirms the high adaptability of these species to the more deteriorated conditions, as also shown for temperate rivers, where the abundance of tolerant taxa increases with increasing urbanization (Onorato et al., 2000). Despite a similar pattern of richness during the dry season, species addition at the terminal stretches occurred during the rainy season, probably due to the movement of species from the Piracicaba River to the mouth of the Guamium stream combined with better water quality conditions at this time of year. In addition, several species, including Hypostomus sp.1, Bryconamericus sp., H. eques, and H. malabaricus, were not recorded at site 10 when water quality was more critical (dry season), possibly indicating the effects of more concentrated sewage in this season on fish fauna composition. In polluted streams, it has been suggested that environmental stresses simplify diverse ecosystems by reducing the number of species present (Odum, 1969). In some cases, however, an increase in richness may occur due to the presence of exotic species, which may be able to persist at sites where native species could not (Kennard et al., 2005).
Considering that gradients of anthropogenic land use are frequently superimposed on underlying gradients of natural factors such as geology, soil type, topography, etc. (Allan, 2004), community changes were analyzed both in relation to changes in water quality and spatial structure, partitioning the variation of species abundance. As the fraction explained by pure spatial variation was low, most of the longitudinal changes in the species matrix could be attributed to the changes in water quality. However, the amount of unexplained variation was high (68.2%), which means that other underlying factors could not be identified from the available data. Other biotic or abiotic regulatory variables may have been missed because they require more complex functions to be described, a situation shown for other communities by Borcard et al. (1992). We did not quantify other substances typically found in urban sewage effluents, including metals, phenols, drugs and carcinogenic compounds (Helawell, 1989), nor agrochemicals heavily used during the production of sugar cane that can potentially be harmful to the environment (Smeets et al., 2006), nor minor physical habitat changes.
The RDA-based assessment of the relationships between fish assemblages and environmental variables indicated that the most important ecological gradient in the Guamium stream was a productivity-nutrient gradient, which defined species associated with the upper agricultural and lower urban zones, as also shown by the indicator species analysis. Although the occurrence of indicator species, such as P. caudimaculatus, in the upper and medium-lower sites of the Guamium stream has been related to the presence of filamentous algae in deforested segments (Abes & Agostinho, 2001), it seems that in this study, this species was instead associated with sites of better water quality, as indicated by the RDA. Gymnotus species have been found close to marginal banks of vegetation and shallow silted streams (Ferreira & Casatti, 2006), tolerating broad variations in environmental conditions such as pH, temperature, oxygen, and conductivity (de Resende, 2006). As demonstrated in other studies (Casatti et al., 2006), microhabitat characteristics of site 1 such as the presence of grasses and lower oxygen levels due to shallow conditions may favor the presence of Gymnotus. Indicator species of the urbanized sites were predominantly bottom dwellers, with the exception of A. altiparanae. Parodon nasus and H. regani are known to be periphyton scrapers, which show preference for stony substrates where algae and bryophytes are abundant (Shibatta et al., 2007). The latter species has been found in the disturbed Pardo River, where it is one of the few fish that survived after the establishment of the great sugar mills (Favaretto et al., 1981). At the sites where P. nasus and H. regani occurred, the substrate was composed of coarse gravel and large boulders, indicating that it was an important component determining the presence of these species, where algal productivity was probably favored by the increased nutrient levels.
Although the indicator species analysis showed which species were closely related to each habitat, the results cannot be attributed solely to the anthropogenic impacts, as ecological indicators at the population or community levels are not tightly coupled to the primary biological effects of stressors (Jenkins & Sanders, 1992). In fact, as stated by Niemi & McDonald (2004), communities and populations respond to many other factors, some of which are not necessarily stress related.
Considering that sugar cane plantations produce various wastes that pollute freshwater streams (Smeets et al., 2006), our results showed that the sites within the agricultural areas where sugar cane fields were dominant supported biologically diverse fish communities, with relatively little disturbance. This observation can possibly be explained by the presence of riparian forest on several sites, which may have helped to buffer landscape disturbances on the stream and determined long-term patterns of colonization, reproduction and survival of fish, as shown by other studies (Hupp, 1982; Pusey & Arthington, 2003). However, the effects of the urbanized area were more pronounced, suggesting that the fish communities were experiencing the initial stages of disturbance, like other similar rivers subjected to high organic loads (Cunico et al., 2006).
Our findings are also consistent with other studies which indicated that urbanization has disproportionately large effects on biota compared with agriculture (Paul & Meyer, 2001) and that agriculture has little influence on habitat quality in watersheds where it occupies less than 50% of the total area (Wang et al., 1997). Although some studies have shown the contribution of sugar cane to tropical streams, particularly to larger predatory fish (Bunn et al., 1997), the processes by which this crop causes habitat and water composition changes need to be better understood. This information, coupled with the use of ecological indicators, might serve as an important tool to assess the condition of the environment (e.g., as an early-warning system) or to diagnose the cause of environmental changes.
References
Abes, S. S. & A. A. Agostinho, 2001. Spatial patterns in fish distributions and structure of the ichthyocenosis in the Água Nanci stream, upper Paraná River basin, Brazil. Hydrobiologia 445: 217–227.
Alkins-Koo, M., 2000. Reproductive timing of fishes in a tropical intermittent stream. Environmental Biology of Fishes 57: 49–66.
Allan, J. D., 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annual Review of Ecology and Systematics 35: 257–284.
Allan, J. D., D. L. Erickson & J. Fay, 1997. The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biology 37: 149–161.
APHA, 1989. Standard methods for the examination of water and wastewater. American Public Health Association, Washington.
Araújo, N. B. & F. Tejerina-Garro, 2009. Influence of environmental variables and anthropogenic perturbations on stream fish assemblages, Upper Paraná River, Central Brazil. Neotropical Ichthyology 7: 31–38.
Barreto, A. G. O. P., G. Sparovek & M. Giannotti, 2005. Atlas rural de Piracicaba. IPEF, Piracicaba.
Bartsch, A. F., 1948. Biological aspects of stream pollution. Sewage Works Journal 20: 292–302.
BIOESTAT, 2007. Aplicações Estatísticas nas Áreas das Ciências Bio-Médicas, v.5.0. Belém.
Bledsoe, B. P. & C. C. Watson, 2001. Effects of urbanization on channel instability. Journal of the American Water Resources Association 37: 255–270.
Booth, D. B. & C. R. Jackson, 1997. Urbanization of aquatic systems: degradation thresholds, stormwater detection and the limits of mitigation. Journal of the American Water Resources Association 33: 1077–1090.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055.
Bozzetti, M. & U. H. Schulz, 2004. An index of biotic integrity based on fish assemblages for subtropical streams in southern Brazil. Hydrobiologia 529: 133–144.
Braga, F. M. S., 2004. Habitat, distribuição e aspectos adaptativos de peixes na microbacia do Rio Grande, Estado de São Paulo, Brasil. Acta Scientarium 26: 31–36.
Braga, F. M. S. & P. M. Andrade, 2005. Distribuição de peixes na microbacia do Ribeirão Grande, Serra da Mantiqueira Oriental, São Paulo, Brasil. Iheringia 95: 121–126.
Britski, H. A., 1972. Peixes de água doce do estado de São Paulo: Sistemática. In Comissão Interestadual da Bacia Paraná-Uruguai, Poluição e Piscicultura. Faculdade de Saúde Pública da USP e Instituto de Pesca, São Paulo.
Britski, H. A., Y. Sato & A. B. S. Rosa, 1986. Manual de identificação de peixes da região de três Marias (com chaves de identificação para peixes da bacia do São Francisco). CODEVASF, Brasília.
Bunn, S. E., P. M. Davies & D. M. Kellaway, 1997. Contributions of sugar cane and invasive pasture grass to the aquatic food web of a tropical lowland stream. Marine and Freshwater Research 48: 173–179.
Camargo, A. F. M., R. A. R. Ferreira, A. Schiavetti & L. M. Bini, 1996. Influence of physiography and human activity on limnological characteristics of lotic ecosystems of the south coast of São Paulo, Brazil. Acta Limnologica Brasiliensia 8: 231–243.
Casatti, L., 2005. Fish assemblage structure in a first order stream, southeastern Brazil: longitudinal distribution, seasonality, and microhabitat diversity. Biota Neotropica 5: 1–9.
Casatti, L., F. Langeani & C. P. Ferreira, 2006. Effects of physical habitat degradation on the stream fish assemblage structure in a pasture region. Environmental Management 38: 974–982.
Casatti, L., C. P. Ferreira & F. Langeani, 2009. A fish-based biotic integrity index for assessment of lowland streams in southeastern Brazil. Hydrobiologia 623: 173–189.
Castro, R. M. C. & L. Casatti, 1997. The fish fauna from a small forest stream of the Upper Paraná River basin, southeastern Brazil. Icthyological Exploration of Freshwaters 7: 337–352.
CETESB, 1978. Normatização Técnica. Companhia de Tecnologia e Saneamento Ambiental de São Paulo, São Paulo.
Cetra, M. & M. Petrere Jr., 2006. Fish-assemblage structure of the Corumbataí River basin, São Paulo State, Brazil: characterization and anthropogenic disturbances. Brazilian Journal of Biology 66: 431–439.
Clarke, K. R. & R. M. Warwick, 1994. Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth, UK.
CONAMA, 2005. Conselho Nacional de Meio Ambiente. Resolução no 357 de 17 de março de 2005. Diário Oficial da União, Brasília.
Corbi, J. J., S. Trivino-Strixino & A. dos Santos, 2008. Environmental evaluation of metals in sediments and dragonflies due to sugar cane cultivation in neotropical streams. Water, Air and Soil Pollution 195: 325–333.
Cunico, A. M., A. A. Agostinho & J. D. Latini, 2006. Influência da urbanização sobre as assembléias de peixes em três córregos de Maringá, Paraná. Revista Brasileira de Zoologia 23: 1101–1110.
Daniel, M. H. B., A. A. Montebelo, M. C. Bernardes, J. P. H. B. Ometto, P. B. Camargo, A. V. Krusche, M. V. Ballester, R. L. Victoria & L. A. Martinelli, 2002. Effects of urban sewage on dissolved oxygen, dissolved inorganic and organic carbon, and electrical conductivity of small streams along a gradient of urbanization in the Piracicaba River Basin. Water, Air, and Soil Pollution 136: 189–206.
de Resende, E. K., 2006. Biologia da tuvira, Gymnotus cf. carapo (Pisces, Gymnotidae) no Baixo Rio Negro, Pantanal, Mato Grosso do Sul, Brasil. Boletim de Pesquisa e Desenvolvimento Embrapa Pantanal 67.
Doudoroff, P. & C. E. Warren, 1957. Biological indices of water pollution with special reference to fish populations. In Tarzwell, C. M. (ed.), Biological Problems in Water Pollution. R.A.Taft Sanitary Engineering Center, U.S.Public Health Service, Cincinnati, OH.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Favaretto, A. L. V., S. O. Petenusci & R. A. Lopes, 1981. Effects of exposure to air on haematological parameters in Hypostomus regani (Pisces: Loricariidae) teleost with aquatic and aerial respiration. I. Red cells. Copeia 198: 918–920.
FEEMA, 1981. Métodos de análise físico-química da água. Fundação Estadual de Engenharia do Meio Ambiente, Rio de Janeiro.
Ferreira, C. P. & L. Casatti, 2006. Influência da estrutura do habitat sobre a ictiofauna de um riacho em uma micro-bacia de pastagem, São Paulo, Brasil. Revista Brasileira de Zoologia 23: 642–651.
Filoso, S., L. A. Martinelli, M. R. Williams, L. B. Lara, A. Krusche, M. V. Ballester, R. Victoria & P. B. Camargo, 2003. Land use and nitrogen export in the Piracicaba River basin, Southeast Brazil. Biogeochemistry 65: 275–294.
Fitzgerald, D. G., E. Kott, R. P. Lanno & D. G. Dixon, 1998. A quarter century of change in the fish assemblages of three small streams modified by anthropogenic activities. Journal of Aquatic Ecosystem Stress and Recovery 6: 111–127.
Ganasan, V. & R. Hugues, 1998. Application of an index of biological integrity (IBI) to fish assemblages of the rivers Khan and Kshipra (Madhya Pradesh) India. Freshwater Biology 40: 367–383.
Gilliam, J. F., D. F. Fraser & M. Alkins-Koo, 1993. Structure of a tropical stream fish community: a role for biotic interactions. Ecology 74: 1856–1870.
Gonçalves, J. S., E. P. Castanho-Filho & S. A. M. Souza, 2009. São Paulo’s Legal Forest Reserve: compliance obligation, impact and legislation improvement proposal. Textos para Discussão 6: 1–74.
Gorman, O. T. & J. R. Karr, 1978. Habitat structure and stream fish communities. Ecology 59: 507–515.
Graham, J. B., 1985. Seasonal and environmental effects on the blood hemoglobin concentrations of some Panamanian air-breathing fishes. Environmental Biology of Fishes 12: 291–301.
Helawell, J. M., 1989. Biological Indicators of Freshwater Pollution and Environment Management. Elsevier Applied Science, London/New York.
Hope, A. C. A., 1968. A simplified Monte Carlo significance test procedure. Journal of the Royal Statistical Society B 30: 582–598.
Horwitz, R. J., 1978. Temporal variability patterns and the distributional patterns of stream fishes. Ecological Monographs 48: 307–321.
Hugues, R. M. & J. R. Gammon, 1987. Longitudinal changes in fish assemblages and water quality in the Willamette River, Oregon. Transactions of the American Fisheries Society 116: 196–209.
Hugues, R. M., E. Rexstad & C. E. Bond, 1987. The relationship of aquatic ecoregions, river basins and physiographic provinces to the ictiogeographic regions of Oregon. Copeia 2: 423–432.
Hupp, C. R., 1982. Stream-grade variation and riparian forest ecology along Passage Creek, Virginia. Bulletin of the Torrey Botanical Club 109: 488–499.
Hynes, H. B. N., 1960. The Biology of Polluted Waters. Liverpool University Press, Liverpool.
Jenkins, K. D. & B. M. Sanders, 1992. Monitoring with biomarkers: a multitiered framework for evaluating the ecological impacts of contaminants. In McKenzie, D. H., D. E. Hyat & V. J. McDonald (eds), Proceedings of the international symposium on ecological indicators, Elsevier Applied Sciences, New York: 1279–1293.
Karr, J. R., 1981. Assessment of biotic integrity using fish communities. Fisheries 6: 21–27.
Kennard, M. J., A. H. Arthington, B. J. Pusey & B. D. Harch, 2005. Are alien fish a reliable indicator of river health? Freshwater Biology 50: 174–193.
Klein, R. D., 1979. Urbanization and stream quality impairment. Water Resource Bulletin 15: 948–963.
Krebs, C. J., 1989. Ecological Methodology. Harper Collins Publishers, New York.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Legendre, P. & L. Legendre, 1998. Numerical Ecology. Elsevier Scientific, Amsterdam.
Martinelli, L. A. & S. Filoso, 2007. Polluting effects of Brazil’s sugar-ethanol industry. Nature 445: 364.
Martinelli, L. A., A. V. Krusche, R. L. Victoria, P. B. de Camargo, M. Bernardes, E. S. Ferraz, J. M. de Moraes & M. C. Ballester, 1999. Effects of sewage on the chemical composition of Piracicaba River, Brazil. Water, Air, and Soil Pollution 110: 67–79.
Matthews, W. J., 1986. Fish faunal ‘breaks’ and stream order in the eastern and central United States. Environmental Biology of Fishes 7: 81–92.
Matthews, W. J. & J. T. Styron, 1981. Tolerance of headwater vs. mainstream fishes for abrupt physichochemical changes. American Midland Naturalist 105: 149–158.
Matthews, W. J., D. J. Hough & H. W. Robison, 1992. Similarities in fish distribution and water quality patterns in streams of Arkansas: congruence of multivariate analyses. Copeia 47: 5–19.
Mazzoni, R. & J. Lobón-Cerviá, 2000. Longitudinal structure, density and production rates of a neotropical stream fish assemblage: the river Ubatiba in the Serra do Mar, southeast Brazil. Ecography 23: 588–602.
Mazzoni, R., N. Fenerich-Verani, E. P. Caramaschi & R. Iglesias-Rios, 2006. Stream-dwelling fish communities from an Atlantic Rain Forest Drainage. Brazilian Archives of Biology and Technology 49: 249–256.
McCune, B. & M. J. Mefford, 1999. Multivariate Analysis of Ecological Data, Version 4.10. MjM Software, Gleneden Beach, OR.
Naiman, R. J., H. Décamps, J. Pastor & C. A. Johnston, 1988. The potential importance of boundaries to fluvial ecosystems. Journal of the North American Benthological Society 7: 289–306.
Nelson, R. L., W. S. Platts, D. P. Larsen & S. E. Jensen, 1992. Trout distribution and habitat in relation to geology and geomorphology in the North Gork Humboldt river drainage, northeastern Nevada. Transactions of the American Fisheries Society 121: 405–426.
Niemi, G. J. & M. E. McDonald, 2004. Application of ecological Indicators. Annual Review of Ecology and Systematics 35: 89–111.
Odum, E. P., 1969. The strategy of ecosystem development. Science 164: 262–270.
Odum, E. P., 1988. Ecologia. Editora Guanabara Koogan, Rio de Janeiro.
Ometto, J. P., L. A. Martinelli, M. V. Ballester, A. Gessner, A. V. Krusche, R. L. Victoria & M. Williams, 2000. Effects of land use on water chemistry and macroinvertebrates in two streams of the Piracicaba river basin, south-east Brazil. Freshwater Biology 44: 327–337.
Onorato, D., R. A. Angus & K. R. Marion, 2000. Historical changes in the ichthyofaunal assemblages of the Upper Cahaba River in Alabama associated with extensive urban development in the watershed. Journal of Freshwater Ecology 15: 47–63.
Paul, M. J. & J. L. Meyer, 2001. Streams in the urban landscape. Annual Review of Ecology and Systematics 32: 333–365.
Petry, A. C. & U. H. Schulz, 2006. Longitudinal changes and indicator species of the fish fauna in the subtropical Sinos River, Brazil. Journal of Fish Biology 69: 272–290.
Piana, P. A., L. C. Gomes & E. M. Cortez, 2006. Factors influencing Serrapinnus notomelas (Characiformes, Characidae) populations in upper Paraná river floodplain lagoons. Neotropical Icthyology 4: 81–86.
Pinto, B. C. T., M. G. Peixoto & F. G. Araújo, 2006. Effects of the proximity from an industrial plant on fish assemblages in the rio Paraíba do Sul, southeastern Brazil. Neotropical Ichthyology 4: 269–278.
Pompeu, P. S. & C. B. M. Alves, 2005. The effects of urbanization on biodiversity and water quality in the Rio das Velhas Basin, Brazil. American Fisheries Society Symposium 47: 11–22.
Pusey, B. J. & H. Arthington, 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54: 1–16.
Reis, R. E., S. O. Kullander & C. J. Ferraris Jr., 2003. Check List of the Freshwater Fishes of South and Central America. EDIPUCRS, Porto Alegre.
Reyes-Gavilán, F. G., R. Garrido, A. G. Nicieza, M. M. Toledo & F. Brama, 1996. Fish community variation along physical gradients in short streams of northern Spain and the disruptive effect of dams. Hydrobiologia 321: 155–163.
Richards, C., L. B. Johnson & G. E. Host, 1996. Landscape-scale influences on stream habitats and biota. Canadian Journal of Fisheries and Aquatic Sciences 53: 295–311.
Rörig, L. R., J. G. Tundisi, C. A. F. Schettini, J. A. Pereira-Filho, J. T. Menezes, T. C. M. Almeida, S. R. Urban, C. M. Radetski, R. C. Sperb, C. A. Stramosk, R. S. Macedo, M. A. Castro-Silva & J. A. A. Perez, 2007. From a water resource to a point pollution source: the daily journey of a coastal urban stream. Brazilian Journal of Biology 67: 597–609.
Schlosser, I. J., 1982. Fish community structure and function along two habitat gradients in a headwater stream. Ecological Monographs 52: 395–414.
Schulz, U. H. & H. Martins Jr., 2001. Astyanax fasciatus as bioindicator of water pollution of Rio dos Sinos, RS, Brazil. Brazilian Journal of Biology 61: 615–622.
Scott, M. C. & L. W. Hall Jr., 1997. Fish assemblages as indicators of environmental degradation in Maryland coastal plain streams. Transactions of the American Fisheries Society 129: 1118–1133.
Scott, M. C. & G. S. Helfman, 2001. Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries 26: 6–15.
Scott, J. B., C. R. Steward & Q. J. Stober, 1986. Effects of urban development on fish population dynamics in Kelsey Creek, Washington. Transactions of the American Fisheries Society 115: 555–567.
Shibatta, O. A., A. M. Gealh & S. T. Bennemann, 2007. Ichthyofauna from the middle and upper stretches of rio Tibagi basin, Paraná, Brazil. Biota Neotropica 7: 125–134.
Silva, A. M., M. A. Nalon, F. J. N. Kronka, C. A. Alvares, P. B. Camargo & L. A. Martinelli, 2007. Historical land-cover/use in different slope and riparian buffer zones in watersheds of the State of São Paulo, Brazil. Scientia Agricola 64: 325–335.
Skinner, J. A., K. A. Lewis, K. S. Bardon, P. Tucker, J. A. Catt & B. J. Chambers, 1997. An overview of the environmental impact of agriculture in the U.K. Journal of Environmental Management 50: 111–128.
SMA, 2007. Relatório de Qualidade Ambiental do Estado de São Paulo. CD-ROM.
Smeets, E., M. Junginger, A. Faaij, A. Walter & P. Dolzan, 2006. Sustainability of Brazilian bio-ethanol [available on internet at http://www.chem.uu.nl/nws/www/general/personal/smeets_a_files/nieuw/E2006-110]. Report NWS-E-2006-110, ISBN 90-8672-012-9.
Steedman, R. J., 1988. Modification and assessment of an index of biotic integrity to quantify stream quality in southern Ontario. Canadian Journal of Fisheries and Aquatic Sciences 45: 492–501.
Strickland, J. D. H. & T. R. Parsons, 1968. A Practical Handbook Of Seawater Analysis. Fisheries Research Board of Canada Bulletin, Ottawa: 167.
Suárez, Y. R. & M. Petrere Jr., 2006. Gradientes de diversidade nas comunidades de peixes da bacia do Rio Iguatemi, Mato Grosso do Sul, Brasil. Iheringia 96: 197–204.
Tejerina-Garro, F. L., M. Maldonado, C. Ibañez, D. Pont, N. Roset & T. Oberdorff, 2005. Effects of natural and anthropogenic environmental changes on riverine fish assemblages: a framework for ecological assessment of rivers. Brazilian Archives of Biology and Technology 48: 91–108.
ter Braak, C. F. J., 1986. Canonical Correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
ter Braak, C. J. F., 1987. CANOCO—a FORTRAN Program for Canonical Community Ordination by Partial, Detrended, Canonical Correspondence Analysis, Principal Components Analysis, and Redundance Analysis. Agricultural Mathematics Group, Wageningen, The Netherlands.
Tundisi, J. G., 2003. Água no século XXI: enfrentando a escassez. RiMa/IIE, São Carlos.
van den Wollenberg, A. L., 1977. Redundancy analysis. An alternative for canonical correlation analysis. Psychometrika 42: 207–219.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Vazzoler, A. E., H. I. Suzuki, E. E. Marques, M. Los Angeles & P. Lizama, 1997. Primeira maturação gonadal, períodos e áreas de reprodução. In Vazzoler, A. E., A. A. Agostinho & N. S. Hahn (eds), A planície de inundação do Rio Paraná. EDUEM, Maringá: 249–265.
Wang, L., J. Lyons, P. Kanehl & R. Gatti, 1997. Influences of watershed land use on habitat quality and biotic integrity in Wisconsin streams. Fisheries 22: 6–12.
Williams, M. R., S. Filoso, L. A. Martinelli, L. B. Luciene & P. B. Camargo, 2001. Precipitation and river chemistry of the Piracicaba River basin in southeastern Brazil. Journal of Environmental Quality 30: 967–981.
Winemiller, K. O., 1989. Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 81: 225–241.
Acknowledgments
The authors thank the Instituto de Pesca (APTA/SAA) for facilities, FAPESP (Proc.05/02182-2) for financial support, José Roberto Ferreira for supervision of the general project financed by CNPq (Proc. 480764/04-2), Heraldo A. Britski and Osvaldo T. Oyakawa for fish identification and Sérgio Luis da Silva for help with the field work. We are also grateful to Eduardo Pereira Cabral Gomes for help in statistical analysis and Rosana Mazzoni Buchas for suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editors: Jim Cambray and Luis Gomes
Rights and permissions
About this article
Cite this article
Alexandre, C.V., Esteves, K.E. & de Moura e Mello, M.A.M. Analysis of fish communities along a rural–urban gradient in a neotropical stream (Piracicaba River Basin, São Paulo, Brazil). Hydrobiologia 641, 97–114 (2010). https://doi.org/10.1007/s10750-009-0060-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0060-y