Abstract
Autumn picoplankton (Synechococcus, picoeukaryotes and heterotrophic bacteria) and environmental factors have been investigated in a series of reservoirs along the Wujiang River in Guizhou Province, SW China. The average abundances of Synechococcus, picoeukaryotes and heterotrophic bacteria was 104, 102 and 106 cells ml−1, respectively. In autumn meso-eutrophic reservoirs, thermal stratification was clear and abundances of different picoplankton groups in release water was low; whereas these phenomena were not obvious in autumn hypereutrophic reservoir. Picoplankton numbers decreased with increasing water depth and showed a positive correlation with water temperature, which reflected the importance of light and temperature on the picoplankton growth. Contribution of Synechococcus to total phytoplankton production and contribution of picoeukaryotes to total phytoplankton production asynchronous changed with varying trophic states. Synechococcus preferred meso-eutrophic reservoirs over hypereutrophic reservoir and picoeukaryotes showed no preference for the investigated reservoirs in autumn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reservoirs occupy an intermediate position between natural lakes and rivers. They can be considered as a conceptualized continuum of aquatic environments with regard to the environmental factors that control water quality and biological productivity (Kimmel & Groeger, 1984). As a rule, reservoirs become eutrophic more rapidly than natural lakes, because most reservoirs receive higher sediment and nutrient loads than do most natural lakes. Reservoirs are complex and dynamic ecosystems and it is important to understand how they operate and respond to changes for a correct management.
Picoplankton (0.2–2 μm; Sieburth et al., 1978), including heterotrophic bacteria (BACT) and autotrophic picoplankton (APP), are a ubiquitous component of the microbial plankton communities of freshwater ecosystems (Stockner, 1988). Picoplankton play a much greater role in elemental cycling than previously imagined and respond swiftly to environmental conditions. Therefore, they can be effectively used as early indicators of ecosystem change (e.g., Schallenberg & Burns, 2001). Nowadays, it is well known an increase of picoplankton abundance and biomass, and a decrease of its relative importance with the increase of trophic state in freshwater systems (Szelag-Wasielewska, 1997; Stockner et al., 2000; Bell & Kalff, 2001; Callieri & Stockner, 2002). Light and water temperature are also two important factors controlling picoplankton abundance in lakes (Wehr, 1993; Wakabayashi & Ichise, 2004). The absolute abundance and biomass of APP are highly variable from one lake to another (Sommaruga & Robarts, 1997), and few studies have demonstrated the predominance of picoplankton and nanoplankton in eutrophic lake (e.g., Wehr, 1990). Although numerous studies have been conducted with picoplankton in lakes, only few were focused on those in reservoirs.
In this study, we have investigated the abundances of autumn picoplankton (Synechococcus, picoeukaryotes, and heterotrophic bacteria) and examined environmental factors in the reservoirs at different trophic levels along the Wujiang River in Guizhou Province, SW China. Our aim is to understand the relationship between trophic state and abundances of different picoplankton groups, and to discern the factors controlling the distributions of picoplankton in these reservoirs along the same river.
Materials and methods
Study sites
The 1037-km-long Wujiang River is a southern tributary of the Changjiang River, and it has a runoff of 53.4 billion cubic meters with a fall of 2124 m. The river is a major power source for China’s massive West-to-East Power Transmission Project. A series of reservoirs have been constructed for this project since 1971. The Wujiang River drainage basin is located in the karst areas of Guizhou Province. The river water chemistry is controlled by carbonate dissolution by both carbonic and sulfuric acid, and dominated by Ca2+, HCO3 −, Mg2+and SO4 2− (Han & Liu, 2004).
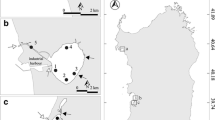
Investigations were carried out at five reservoirs along the Wujiang River (Fig. 1). They are Wujiangdu Reservoir (impounded in 1971), Dongfeng Reservoir (impounded in 1989), Hongjiadu Reservoir (impounded in 2001), Yinzidu Reservoir (impounded in 2001), Puding Reservoir (impounded in 1989). A total of 25 stations were investigated (Fig. 1.). At the following stations, water samples were taken with 5 l Niskin bottles at different depths: A1, A4, and D1 at the depths of 0, 10, 20, 40, 60 m, A5 at the depths of 0, 10, 20, 40, 60, and 80 m, B3 at the depths of 0, 20, 40, 60 m, B4 and C4 at the depths of 0, 20, 40, 60, 80 m, E4 at the depths of 0, 5, 10, 20 m, E6 at the depths of 0, 5, 10, 20, 30 m, respectively. Release water samples were collected at stations A6, B5, C5, D2, and E7 and other water samples were obtained from surface water.
Sampling
Sample collection was carried out on October 23–28, 2006. Water temperature, dissolved oxygen, pH and chlorophyll were measured in situ by an automated multi-parameter profiler (YSI 6920). Immediately after collection, water samples were filtered through 0.22 μm membrane filters (Millipore) and cold stored in darkness for measuring the concentrations of PO4 3−, NO3 − and NO2 − by automatic flow analyzer (Sans plus Systems, SKALAR). At stations A5, B4, C4, D1, and E6, a small portion of each sample was stored for measuring total nitrogen and phosphorus (TN and TP), respectively. TP was determined spectrophotometrically (Unico UV-2000) using the molybdenum blue method after alkaline potassium persulfate digestion. TN was also analyzed spectrophotometrically (Unico UV-2000) after alkaline potassium persulfate digestion. 1.5 l of surface water sample was preserved with Lugol’s Solution for quantitative and qualitative analysis of phytoplankton at stations A5, B4, C4, D1, and E6. Water samples for determining abundances of different picoplankton groups were filtered by 50 μm fabrics in order to remove the bigger impurities and were put aside in darkness for 15 min with paraformaldehyde; then immediately stored in liquid nitrogen till analysis in one month (Pan et al., 2005).
Quantitative and qualitative analysis of phytoplankton
The precipitation method was used for taxon identification and counting (Chen et al., 1998; Yan et al., 2006). Phytoplankton was quantitatively and qualitatively determined by a standard light microscope. Margalef diversity index and simplified Shannon–Weiner index (Llyod et al., 1968) were calculated according to the following equations:
Where S is the number of total species, N is the number of total cells, n i is the cell number of species i, C = 3.322.
Analysis of picoplankton
Picoplankton samples were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with an air-cooled argon laser (488 nm, 15 mW), and the data obtained were processed with CELL-QuestTM software (Becton Dickinson, San Jose, CA, USA). Yellowish green fluorescent beads (1.002 μm) (Polysciences Inc., catelogue # 18660) were added to calibrate cell fluorescence emissions and light scatter signals. Autotrophic picoplankton and heterotrophic bacteria were measured with the published procedures (Pan et al., 2005).
Statistical analysis
Statistical analysis of the data was done with the software SPSS (version 11.5; SPSS Inc.). Pearson’s correlation coefficient analysis and principal component analysis (PCA) was carried out.
Results
Physical and chemical properties
Typical vertical profiles of temperature (T), pH, dissolved oxygen (DO), PO4 3−, NO3 − and NO2 − at stations A1, A4, A5, B3, B4, C4, D1, E4, and E6 were examined in the present works (Fig. 2). The water column of Wujiangdu Reservoir was not thermally stratified at the depth above 60 m (i.e., 20.38–21.24°C). However, the thermal stratification in water column of other four reservoirs was clear (e.g., 10.65–21.93°C at station C4). There was a vertical decrease in pH at Dongfeng Reservoir (e.g., 7.96–6.72 at station B4) and in DO at Hongjiadu Reservoir (e.g., 8.36–3.41 mg l−1 at station C4) with depth. The T, pH and DO of release water showed lower values than those of surface water in Dongfeng Reservoir, Hongjiadu Reservoir, Yinzidu Reservoir, and Puding Reservoir, and these phenomena were not found in Wujiangdu Reservoir. The PO4 3− value of Wujiangdu Reservoir (8.19 ± 11.55 μmol l−1) was about 102 times higher than that of other four reservoirs (0.04 ± 0.03 μmol l−1). The values of NO3 − and NO2 − in these investigated reservoirs (217.66 ± 48.97 μmol l−1 and 1.55 ± 1.71 μmol l−1, respectively) did not show any significant differences.
Trophic conditions
Phytoplankton, TP and TN at the stations A5, B4, C4, D1 and E6 were examined in order to understand trophic condition of each reservoir. For each station, no significant difference was found in the average concentration of TN; however, the average concentration of TP at station A5 was 0.356 mg l−1, about 10 times higher than that at other four stations (Fig. 3), indicating that Wujiangdu Reservoir was hypereutrophic and the other four reservoirs were meso-eutrophic according to Vollenweider & Kerekes (1982). High phosphorus concentration in Wujiangdu Reservior is due to excess phosphorus input by the Xifeng River, a southern tributary of the Wujiang River. Phytoplankton composition and biomass were variable among these reservoirs (Table 1), and a significant positive correlation between chlorophyll concentration and phytoplankton numbers was observed (R 2 = 0.913, P < 0.05). Margalef diversity index and Shannon–Weiner index did not show any significant differences from one reservoir to another (Table 1).
Distributions of different picoplankton groups
Synechococcus, picoeukaryotes and heterotrophic bacteria have been investigated in this study (Fig. 4). The number of Synechococcus [(4.9 ± 6.5) × 104 cells ml−1] was two orders of magnitude higher than that of picoeukaryotes [(2.3 ± 2.8) × 102 cells ml−1] and two orders of magnitude lower than that of heterotrophic bacteria [(1.2 ± 0.9) × 106 cells ml−1]. The cell abundance of picoeukaryotes in these investigated reservoirs was comparable, so was the abundance of heterotrophic bacteria. However, the cell abundance of Synechococcus in Wujiangdu Reservoir [(1.9 ± 1.0) × 103 cells ml−1] was one order of magnitude lower than that in the other four reservoirs [(7.1 ± 6.9) × 104 cells ml−1]. Generally, the abundances of picoplankton groups tended to decrease with depth in Dongfeng Reservoir, Hongjiadu Reservoir, Yinzidu Reservoir and Puding Reservoir, and this phenomenon was rarely detected in Wujiangdu reservoir in autumn. The picoplankton numbers of release water were obviously lower than those of surface water in these reservoirs except for Wujiangdu Reservoir (Fig. 4).
The chlorophyll has been examined to understand the relationship between the autotrophic picoplankton and the photosynthetic autotrophs in these reservoirs. The concentrations of chlorophyll decreased with depth, similar to the distributions of the autotrophic picoplankton. Release water also showed lower chlorophyll values than surface water (Fig. 4). There was a strong positive correlation between picoeukaryotes numbers and chlorophyll concentrations in both meso-eutrophic reservoirs and hypereutrophic reservoir; however, Synechococcus numbers only exhibited a strong positive correlation with chlorophyll concentrations in the meso-eutrophic reservoirs (Fig. 5), indicating that the contribution of Synechococcus to total phytoplankton production and that of picoeukaryotes to total phytoplankton production asynchronously changed with varying trophic states.
Discussion
Phytoplankton
Phytoplankton can be used as an indicator of water quality in reservoirs (Korneva & Mineeva, 1996; Kuang et al., 2004; Miyazaki et al., 2006). We also found some differences in phytoplankton structure and biomass in these reservoirs; however, the phytoplankton diversity indexes did not show significant differences between the hypereutrophic and meso-eutrophic levels (Table 1), and this indicated that in autumn phytoplankton diversity indexes may not be a good indicator of trophic state in these reservoirs. Phytoplankton biomass was variable with time (e.g., Lau & Lane, 2002). Previous study revealed low phytoplankton biomass in October in Hongfeng Lake, which is a eutrophic reservoir located in the tributary of the Wujiang River (Wang et al., 2003). Wujiangdu Reservoir also showed low phytoplankton biomass in this study. However, it was dominated by Cyanophyta (Table 1), suggesting a typical hypertrophic phytoplankton assemblage in Wujiangdu Reservoir (Romo & Miracle, 1994).
Picoplankton abundance in release water
Release water came from the deep water of the reservoir. In the meso-eutrophic reservoirs, the release water possessed similar physical and chemical properties to the deep water, and picoplankton abundance in the release water was also comparable to that in the deep water. So, picoplankton numbers of the release water were found lower than those of the surface water in Dongfeng Reservoir, Hongjiadu Reservoir, Yinzidu Reservoir, and Puding Reservoir. In the hypereutrophic Wujiangdu Reservoir, no clear thermal stratification was observed and the release water had similar physical and chemical characters to the surface water, and as a result, the picoplankton abundance in the release water was comparable to that in the surface water.
Different picoplankton groups
In the meso-eutrophic reservoirs, Synechococcus numbers showed a strong positive correlation with picoeukaryotes numbers and heterotrophic bacteria numbers, respectively; however, these phenomena were not observed in the hypereutrophic Wujiangdu Reservoir (Fig. 6). Cyanobacteria have developed highly efficient uptake and retention mechanisms for phosphate (Ritchie et al., 2001). However, phosphate additions inhibited the growth rates of picocyanobacteria (Ernst et al., 2005). In this study, significant differences were shown in Synechococcus abundance between the hypereutrophic and meso-eutrophic levels (Fig. 4), and this indicated that Synechococcus preferred meso-eutrophic reservoirs over hypereutrophic reservoir in autumn. However, this was not found for picoeukaryotes (Fig. 4), suggesting that it showed no preference for these reservoirs in autumn. Different responses of Synechococcus and picoeukaryotes to trophic state resulted in their different correlations between meso-eutrophic and hypereutrophic reservoirs. Planktonic bacteria and algae are closely related to each other (Bird & Kalff, 1984). This study revealed a positive correlation between heterotrophic bacteria and autotrophic picoplankton (i.e., Synechococcus and picoeukaryotes; Table 2), and this demonstrated the dependence of bacteria on the substrates produced by small primary producers.
Relationships between picoplankton and environmental factors
A principal component analysis (PCA) was conducted to understand the relationships among the investigated physical, chemical, and biological factors. The first three principal components with an eigenvalue higher than 1 were extracted. They explain 43.6%, 17.4%, and 12.6% of the total variance, respectively. The first component (PC1) shows a positive correlation with temperature, pH, dissolved oxygen, chlorophyll, Synechococcus, picoeukaryotes and heterotrophic bacteria, reflecting that they are the coupling factors (Fig. 7a). It is well known that thermal stratification creates a barrier to the transfer of oxygen produced by surface water into deeper water and CO2 uptake by the algal photosynthesis increases pH in surface water (e.g., Malinsky-Rushansky et al. 2002). So, temperature, dissolved oxygen, and pH have a significant positive correlation between each other (Table 2) and often synchronously vary with depth (e.g., Gonzalez et al., 2004). In this study, picoplankton abundance showed a strong positive correlation with temperature (Table 2) and tend to decrease with depth (Fig. 4), which reflected the importance of light and temperature on the picoplankton growth (Andersson et al., 1994; Agawin et al., 2000; Pan et al., 2005). Nutrient supply is an important factor affecting the picoplankton distribution (Stockner & Shortreed, 1994; Vrede et al., 1999). The second component (PC2) and the third component (PC3) both exhibit a negative correlation with Synechococcus and a positive correlation with PO4 3− (Fig. 7), in consistency with the result of the Pearson’s correlation coefficient analysis for Synechococcus and PO4 3− (Table 2), reflecting that high phosphate concentration reduces picocyanobacterial growth rates (Schallenberg & Burns, 2001). Heterotrophic bacteria numbers showed a strong negative correlation with the concentrations of NO3 − and a strong positive correlation with those of NO2 − (Table 2; Fig. 7), probably due to the denitrification of heterotrophic bacteria (Brettar & Hofle, 1993) and the uptake of NO3 − by phytoplankton. Environmental factors (e.g., temperature and PO4 3−) control the picoplankton distribution and picoplankton (e.g., heterotrophic bacteria) also interfere with the variations of environmetal factors (e.g., NO3 − and NO2 −).
References
Agawin, N. S. R., C. M. Duarte & S. Agusti, 2000. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnology and Oceanography 45: 591–600.
Andersson, A., P. Haecky & A. Hagstrom, 1994. Effect of temperature and light on the growth of micro- nano- and pico-plankton: impact on algal succession. Marine Biology 120: 511–520.
Bell, T. & J. Kalff, 2001. The contribution of picophytoplankton in marine and freshwater systems of different trophic status and depth. Limnology and Oceanography 46: 1243–1248.
Brettar, I. & M. G. Hofle, 1993. Nitrous oxide producing heterotrophic bacteria from the water column of the central Baltic: abundance and molecular identification. Marine Ecology Progress Series 94: 253–265.
Bird, D. F. & J. Kalff, 1984. Empirical relationship between bacterial abundance and chlorophyll concentration in fresh and marine waters. Canadian Journal of Fisheries and Aquatic Sciences 41: 1015–1023.
Callieri, C. & J. G. Stockner, 2002. Freshwater autotrophic picoplankton: a review. Journal of Limnology 61: 1–14.
Chen, C., X. Hu, M. Liu, J. Yi & H. Pan, 1998. A preliminary study on the distribution of phytoplankton and water pollution in Hongfeng Lake. Journal of Guizhou Normal University (Natural Science) 16: 5–10 (in Chinese).
Ernst, A., M. Deicher, P. M. J. Herman & U. I. A. Wollenzien, 2005. Nitrate and phosphate affect cultivability of cyanobacteria from environments with low nutrient levels. Applied and Environmental Microbiology 71: 3379–3383.
Gonzalez, E. J., M. Ortaz, C. Penaherrera & A. de Infante, 2004. Physical and chemical features of a tropical hypertrophic reservoir permanently stratified. Hydrobiolgia 522: 301–310.
Han, G. & C. Q. Liu, 2004. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chemical Geology 204: 1–21.
Kimmel, B. L. & A.W. Groeger, 1984. Factors controlling primary production in lakes and reservoirs: a perspective. In Lake and Reservoir Management. Environmental Protection Agency, Washington, DC, US: 277–281.
Korneva, L. G. & N. M. Mineeva, 1996. Phytoplankton composition and pigment concentrations as indicators of water quality in the Rybinsk reservoir. Hydrobiologia 322: 255–259.
Kuang, Q., Y. Bi, Y. Xia & Z. Hu, 2004. Phytoplankton community and algal growth potential in Taipinghu Reservoir, Anhui Province, China. Lakes & Reservoirs: Research and Management 9: 119–124.
Lau, S. S. S. & S. N. Lane, 2002. Biological and chemical factors influencing shallow lake eutrophication: a long-term study. The Science of the Total Environment 288: 167–181.
Llyod, M., J. H. Zar & J. R. Karr, 1968. On the calculation of information-theoretical measures of diversity. American Midland Naturalist 79: 257–272.
Malinsky-Rushansky, N., T. Berman, T. Berner, Y. Z. Yacobi & Z. Dubinsky, 2002. Physiological characteristics of picophytoplankton, isolated from Lake Kinneret: responses to light and temperature. Journal of Plankton Research 24: 1173–1183.
Miyazaki, T., K. Tainaka, T. Togashi, T. Suzuki & J. Yoshimura, 2006. Spatial coexistence of phytoplankton species in ecological timescale. Population Ecology 48: 107–112. .
Pan, L. A., L. H. Zhang, J. Zhang, J. M. Gasol & M. Chao, 2005. On-board flow cytometric observation of picoplankton community structure in the East China Sea during the fall of different years. FEMS Microbiology Ecology 52: 243–253.
Ritchie, R. J., D. A. Trautman & A. W. D. Larkum, 2001. Phosphate uptake in the cyanobacterium Synechococcus R-2 PCC 7942. Plant Cell Physiology 38: 1232–1241.
Romo S. & M. R. Miracle, 1994. Population dynamics and ecology of subdominant phytoplankton species in a shallow hypertrophic lake (Albufera of Valencia, Spain). Hydrobiologia 213: 31–56.
Schallenberg, M. & C. W. Burns, 2001. Tests of autotrophic picoplankton as early indicators of nutrient enrichment in an ultra-oligotrophic lake. Freshwater Biology 46: 27–37.
Sieburth, J. M., V. Smetacek & J. Lenz, 1978. Pelagic ecosystem structure: heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnology and Oceanography 23: 1256–1263.
Sommaruga, R. & R. D. Robarts, 1997. The significance of autotrophic and heterotrophic picoplankton in hypertrophic ecosystems. FEMS Microbiology Ecology 24: 187–200.
Stockner, J. G., 1988. Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnology and Oceanography 33: 765–775.
Stockner, J. G. & K. S. Shortreed, 1994. Autotrophic picoplankton community dynamics in a pre-alpine lake in British Columbia, Canada. Hydrobiologia 274: 133–142.
Stockner, J. G., C. Callieri & G. Cronberg, 2000. Picoplankton and other non-bloom forming cyanobacteria in lakes. In Whitton, B. & M. Potts (eds), Ecology of Cyanobacteria: Their Diversity in Time and Space. Kluwer Academic Publishers: 195–231.
Szelag-Wasielewska, E., 1997. Picoplankton and other size groups of phytoplankton in various shallow lakes. Hydrobiologia 342/343: 79–85.
Vrede, K., T. Vrede, A. Isaksson & A. Karlsson, 1999. Effects of nutrients (phosphorous, nitrogen, and carbon) and zooplankton on bacterioplankton and phytoplankton–a seasonal study. Limnology and Oceanography 44: 1616–1624.
Vollenweider, R. A. & J. Kerekes, 1982. Eutrophication of waters: monitoring assessment and control. Organization for Economic Co-Operation and Development (OECD), Paris, 156 pp.
Wakabayashi, T. & S. Ichise, 2004. Seasonal variation of phototrophic picoplankton in Lake Biwa (1994–1998). Hydrobiologia 528: 1–16.
Wang, B., C. Q. Liu & Y. Wu, 2003. Hydrogeochemistry and microalgae of karst lake in Guizhou province: a case study at Hongfeng Lake and Baihua Lake. In Proceedings of International Symposium on Water Resources and the Urban Environment, Wuhan, China: pp. 112–116.
Wehr, J. D., 1990. Predominance of picoplankton and nanoplankton in eutrophic Calder Lake. Hydrobiologia 203: 35–44.
Wehr, J. D., 1993. Effects of experimental manipulation of light-phosphorus supply on competition among picoplankton and nanoplankton in a oligotrophic lake. Canadian Journal of Fisheries and Aquatic Sciences 50: 936–945.
Yan, N., Y. Wang, H. Pan & C. Chen, 2006. Using phytoplankton community structure and diversity for assessment of water quality of Shatuo Reservior. Guizhou Science 24: 67–72 (in Chinese).
Acknowledgments
This study was financially supported by the Ministry of Science and Technology of China (Grant No. 2006CB403205), and also by National Natural Science Foundation of China (Grant Nos. 40603006, 90610037, and 40571158). We are grateful to Dr. C. Callieri and anonymous reviewers for valuable comments and suggestions on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: L. Naselli-Flores
Rights and permissions
About this article
Cite this article
Wang, B., Liu, CQ., Wang, F. et al. The distributions of autumn picoplankton in relation to environmental factors in the reservoirs along the Wujiang River in Guizhou Province, SW China. Hydrobiologia 598, 35–45 (2008). https://doi.org/10.1007/s10750-007-9138-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9138-6