Abstract
Picophytoplankton (Pico) seasonal dynamics and ecology were investigated in three eutrophic coastal lagoons (Calich, Santa Giusta, and Corru S’Ittiri) located in the same geographic region (Sardinia, Mediterranean Sea). Pico dynamics were examined to determine a relationship with variability in environmental conditions characterizing the three sites, and identify which parameters affected observed Pico dynamics. Results showed Pico density reached 108 cells L−1 and biomass values did not exceed 72.8 μg C L−1. Statistical analyses detected significant differences in hydrographic and nutrient conditions among lagoons, which were also characterized by variable Pico seasonal dynamics and composition. Calich and Santa Giusta were dominated all year around by picocyanobacteria. Maximum picocyanobacteria abundance was observed during summer in both lagoons, associated with the highest temperatures recorded. Picocyanobacteria abundance and nutrient concentrations showed a significant negative and positive correlation in Calich and Santa Giusta, respectively. In contrast, picoeukaryotes dominated Pico assemblages in Corru S’Ittiri, where the highest transparency and salinity and the lowest seasonal variation in salinity were recorded. In Corru S’Ittiri, the maximum Pico abundance was observed in autumn, when lower temperature and the highest nutrient concentrations were observed. Our study provides information on Pico assemblage ecology, which remains scarce in coastal and transitional environments, particularly in Mediterranean climate regimes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prokaryotic and eukaryotic autotrophic cells in the picoplankton size fraction (0.2–2.0 μm) are given the general classification of picophytoplankton (Pico) (Magazzù and Decembrini 1995). For more than two decades, Pico have been recognized as important contributors to total phytoplankton biomass and primary production in marine and freshwater environments (Stockner 1988; Bernardi Aubry et al. 2006). Specifically, Pico have long been considered typical biological components of oceans and common in oligotrophic ecosystems. More recently, Caroppo (2015) reported that Pico were increasingly observed in eutrophic environments. Explanations for the expansion of Pico remain largely elusive, but might involve alterations in physical variables, nutrients, and/or grazers (Sorokin et al. 1996). The importance of small phytoplankton in productive waters has previously been overlooked due to methodological limitations or because small phytoplankton were not examined during routine assessments in these environments (Carrick and Schelske 1997). Small phytoplankton should typically outcompete large phytoplankton under low nutrient concentration conditions and vice versa in high nutrient concentrations (Harris 1994), based on size dependencies of nutrient uptake kinetics (Hein et al. 1995) and diffusion limitations of nutrient transport (Thingstad and Rassoulzadegan 1999). However, in a study on phytoplankton variability based on long-term data of chlorophyll a from a large number of estuarine-marine coastal sites, Cloern and Jassby (2010) provided evidence that the competitive success of phytoplankton groups was determined by many factors acting simultaneously, and not solely nutrients. Schapira et al. (2010) reviewed the environmental factors controlling Pico distribution and composition, including meteorological conditions, nutrient levels, grazing, and viral lysis. However, Pico in eutrophic coastal waters have received little attention (Siokou-Frangou et al. 2010; Caroppo 2015). In these enriched environments, many factors influence the composition and dynamics of the phytoplankton community (nutrient and light availability, turbulence, and predation, among others). These factors exhibit high temporal and spatial variability in coastal waters; therefore, it is often difficult to interpret the ecological role of Pico in the food web, their spatial and seasonal dynamics, and interactions with environmental variables (Calvo-Díaz et al. 2008; Gaulke et al. 2010).
Compared with a notable number of results published worldwide on Pico ecology, information on spatial and temporal distribution, production, and biomass in Mediterranean climate ecosystems is scarce, particularly in coastal lagoons (Magazzù and Decembrini 1995; Siokou-Frangou et al. 2010), with the exception of several reports on bloom formation (Paoli et al. 2007). The literature reports variable Pico composition (e.g., picocyanobacteria or picoeukaryote dominance) in Mediterranean climate coastal ecosystems worldwide, and indicates different drivers of Pico seasonal dynamics, including temperature, salinity, nutrient availability, grazing, and larger phytoplankton dynamics (e.g., Caroppo 2000; Ning et al. 2000; Worden et al. 2004; Paoli et al. 2007; Collos et al. 2009; Schapira et al. 2010). Conflicting results reported in a small number of studies require further investigation into Pico assemblage ecology in Mediterranean coastal ecosystems. Even Bec et al. (2011) indicated that changes in Pico community structure (i.e., picocyanobacteria or picoeukaryote dominance) would reflect picoplanktonic responses to natural changes and anthropogenic environmental perturbations, such as eutrophication, habitat transformations, or fishing among others, suggesting the need to clarify the primary drivers regulating their distribution in Mediterranean coastal lagoons.
In this study, we investigated Pico seasonal dynamics, including picocyanobacteria and picoeukaryotes. The study was conducted over an annual cycle in three Mediterranean eutrophic lagoons, which differ in shape, size, number and type of inlets and outlets, and hydrodynamics, but are located in the same geographic area (Sardinia, North Western Mediterranean Sea) (Bazzoni et al. 2013; Satta et al. 2014). The objectives of the study were as follows: (1) determine if Pico spatial and seasonal dynamics reflect differences in the seasonal environmental conditions of the three sites, (2) identify which environmental variables affect the observed Pico dynamics, and (3) compare our results with other records on the ecology of Pico assemblages in comparable ecosystems worldwide in order to contribute to improve the knowledge on the ecology of Pico assemblages in Mediterranean climate coastal ecosystems.
Materials and Methods
Study Areas
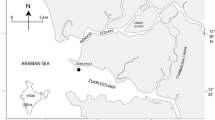
The three lagoons are located along the western coast of Sardinia (North Western Mediterranean Sea), Calich Lagoon in the north and connected to the Gulf of Alghero and Santa Giusta and Corru S’Ittiri lagoons in the center, connected to the Gulf of Oristano (Fig. 1). Calich and Santa Giusta lagoons are exploited for fishing and Corru S’Ittiri for shellfish harvesting. Their catchments are affected by urban, agricultural, and zootechnical activities; consequently, the lagoons are highly eutrophic (Bazzoni et al. 2013; Satta et al. 2014). Furthermore, several hydraulic interventions have been conducted in the lagoons over the years, considerably altering their connections with the sea.
Calich Lagoon
The lagoon is elongated in shape from northwest to southeast; it is the smallest lagoon among the three investigated (Table 1; Fig. 1a). Oruni channel in the northern part of the lagoon, Rio Calvia in the south, and Rio Barca in the center deliver freshwater to the lagoon (Fig. 1a). Rio Barca drains 70 % of the catchment. The lagoon is permanently connected to the sea through a natural channel, which was highly modified by human activities. A tourist harbor, very popular in summer, is located in this channel (Fig. 1a).
Santa Giusta Lagoon
Santa Giusta Lagoon is circular in shape and is the largest study site (Table 1; Fig. 1b). Rio Pauli Maiori and Rio Pauli Figu are the main freshwater inputs, both located on the lagoon’s east side (Fig. 1). The lagoon has undergone several modifications, which affect the hydrology and hydraulics of the area (Sechi et al. 2001; Lugliè et al. 2002; Satta et al. 2014). Today, exchanges with the sea occur permanently through the Pesaria Channel, on the west coast of the lagoon and via a secondary canal, controlled by bulkheads that connect the lagoon with an industrial harbor further south (Fig. 1b). A fish catch system operates in the final section of the Pesaria Channel. Extensive fish mortalities, frequently associated with hypoxic conditions and potentially harmful algal blooms, have frequently occurred during summer months throughout the lagoon (Bazzoni et al. 2013).
Corru S’Ittiri Lagoon
This lagoon is elongated in shape, and unlike the others originated from a coastal area, which was confined by a natural shore line-parallel sand spit, the spit is artificially connected to the land along its southern end (Table 1; Fig. 1c). Freshwater input from the catchment occurs from the small Pauli Pirastru Pond located to the north. In addition, freshwater from a nearby irrigation area drainage system flows into the lagoon via six separate canals along the eastern coastline. The sea connects to the lagoon permanently through two inlets; the larger via the southern end and a smaller northern inlet (Fig. 1c).
Sampling Activities
Monthly sampling was conducted from June 2011 to May 2012 in all lagoons, with the exception of summer 2011, when sampling was performed bimonthly in Santa Giusta lagoon (SG) and Corru S’Ittiri lagoon (CO). For each season, months were defined as follows: summer = July–September, autumn = October–December, winter = January–March, and spring = April–June. At each site, samples were collected at three stations in Calich lagoon (CA) and CO, and five in SG (Fig. 1a–c). Water samples (1 L) were transferred directly into clean polyethylene bottles at a depth of 0.3 m below the surface, taken immediately to the laboratory, and stored under cold, dark conditions for nutrient analyses. Pico were examined after collection of 100-mL water samples and subsequent preservation in dark bottles with buffered formaldehyde (2 % final concentration).
Chemical and Physical Analyses
In situ water transparency was measured with a Secchi disk, while water temperature and salinity were determined with a multi-parameter probe (YSI 6600V2). In the laboratory, water samples were analyzed spectrophotometrically for ammonium (N-NH4), nitrite (N-NO2), nitrate (N-NO3), and orthophosphate (P-PO4) following Strickland and Parsons (1972). Dissolved inorganic nitrogen (DIN) was calculated as the sum of N-NH4, N-NO2, and N-NO3.
Picophytoplankton
Formaldehyde-fixed water samples were maintained in the dark at 4 °C and analyzed within 24 h. Duplicate slides were prepared by filtering 2–5 mL (depending on cell density) of water from each sample onto 0.2-μm black-stained polycarbonate membranes (Nucleopore). Cell counts were made using a Zeiss Axiovert 100 microscope, equipped with green (BP520–560 nm/FT580 nm/LP590 nm) and blue (BP450–490 nm/FT510 nm/LP520 nm) filters set at ×1000 magnification. Each optical field was observed with both filter sets to distinguish autotrophic picocyanobacteria (P-Cyan) and autotrophic picoeukaryotes (P-Euk). For each slide, at least 20 randomly selected fields and a minimum of 100 randomly selected cells of each group type were counted. Total Pico counts were the sum of the two groups (MacIsaac and Stockner 1993). Cell sizes of ~200 randomly selected individuals from each Pico group (~400 total) were measured on each slide (Bratbak 1993), and biovolumes were calculated using formulas provided by Bratbak (1985). Cell carbon content was determined applying the conversion factors 250 fg C μm−3 for P-Cyan and 220 fg C μm−3 for P-Euk for mean cell volume (Tamigneaux et al. 1995). Pico biomass was calculated by multiplying cell carbon content by cell abundance.
Data Analyses
In order to compare transparency values among the studied lagoons and due to the notable differences in depth across lagoons (Table 1), mean depth of each lagoon was set equal to 1 and each transparency value obtained with the Secchi disk measure has been converted in proportion to this unit. This conversion was deemed necessary because transparency values were often coincident with the bottom, which represented the possible maximum transparency in each lagoon, but they differed because of the different shallowness of the sites (Table 1).
Statistical analyses were performed using R 2.15.2 software (R Core Team 2012). An a priori level of significance was established at P < 0.05, i.e., 95 % confidence interval.
Considering that the three studied lagoons belong to the same climate region (Mediterranean) and are relatively close to each other, we assumed that the Mediterranean climate seasonality should result in comparable patterns of seasonal variability of environmental conditions and Pico abundance and composition across all the lagoons. Other local drivers (e.g., level of eutrophication or other human activities in the catchments) or site-specific characteristics (e.g., seawater exchanges, freshwater inputs, morphometry) can act on the general climate patterns of variability, resulting in the significant differences among lagoons in the seasons. In order to ascertain seasonal differences in environmental conditions (transparency, temperature, salinity, DIN, P-PO4) and Pico biomass (Pico, P-Cyan, P-Euk) across all the lagoons, two-way analysis of variance (ANOVA) was performed considering two fixed factors (the seasons and the lagoons), individually and combined. In addition, one-way ANOVA was performed for each lagoon to detect significant differences among sampling stations for each of the aforementioned variables. Prior to ANOVA analyses, the two assumptions were confirmed for all pooled data: normal distribution (Kolmogorov-Smirnov test) and homogeneity of variance (Bartlett test). All data required logarithmic [ln(x)] transformation to meet ANOVA assumptions. Furthermore, even after log and several other transformations, assumptions were not met by temperature and DIN in the case of two-way ANOVA. Therefore, the untransformed temperature and DIN data were subjected to a more conservative significance level (P < 0.01) (Underwood 1997). When significant differences in the dependent variables based on factors were observed, post hoc Tukey’s pairwise comparisons test was performed.
For each lagoon, significant correlations between environmental variables, i.e., transparency, temperature, salinity, DIN, P-PO4, and Pico biomass, i.e., Pico, P-Cyan, and P-Euk, were also assessed using Spearman’s rank correlation.
Results
Environmental Conditions
The combined effects of seasonality and lagoons showed significant differences in transparency, salinity, DIN, and P-PO4, except for temperature (Table 2). However, significant differences in temperature in the different seasons were observed across all lagoons (Table 2). Specific results are described in the following paragraphs.
Temperature was higher in summer and lower in winter in all lagoons. CO showed the highest temperatures in all seasons, CA the lowest, and SG exhibited intermediate values (Online Resource 1; Fig. 2). Spring temperature values in CA resulted significantly different from those in CO and SG (Tukey’s tests: P < 0.01).
Transparency was higher in winter in all the lagoons, lower in summer in SG and CO, and in spring in CA (Online Resource 1; Fig. 2). Comparing the relative transparency of the three lagoons, CO showed the highest transparency values in all seasons except in autumn (annual mean of 0.88), followed by CA (annual mean of 0.86) and SG (annual mean of 0.75) (Online Resource 1; Fig. 2). The summer transparency significantly diversified SG from CO (Tukey’s test: P = 0.005) and from CA (Tukey’s test: P = 0.013).
Maximum salinity was detected in summer in all lagoons, with minimum values in autumn in CA and CO and in winter in SG (Online Resources 1; Fig. 2). CO exhibited the highest salinity in all seasons (annual mean of 35.7 ‰), CA the lowest (annual mean of 17.9 ‰), and SG showed intermediate values (annual mean of 32.2 ‰) (Online Resources 1; Fig. 2). CA values significantly differed from CO and SG values in all seasons (Tukey’s test: P < 0.01). In CO and SG, salinities were significantly different in winter and spring (Tukey’s test: P < 0.05). In addition, CO salinity never dropped below 26.96 ‰, and the CO seasonal variation in salinity was marked lower than in CA and SG. Tukey’s test showed significant differences only between the summer and autumn in CO (P = 0.015). CA summer salinity differed significantly from CA autumn and spring values (Tukey’s test: P < 0.001), whereas SG summer and SG autumn salinity values differed significantly from SG spring and winter values (Tukey’s test: P < 0.001) and SG winter values (Tukey’s test: P = 0.035), respectively.
The highest DIN and P-PO4 concentrations were observed in autumn in CA and CO. In contrast, maximum DIN and P-PO4 values were detected in summer and spring in SG (Online Resource 1; Fig. 3). The DIN and P-PO4 concentrations were the highest in CA (81.3 and 2.9 μM annual means, respectively), followed by CO (14.1 and 1.6 μM annual means, respectively). In SG, values were much lower (2.2 μM for DIN and 0.2 μM for P-PO4 annual means). The lower P-PO4 concentrations recorded in SG, resulted in significant differences with CA in autumn, winter, and spring and with CO in autumn and spring (Tukey’s tests: P < 0.001). CA and CO significantly differed in winter P-PO4 concentrations (Tukey’s test: P < 0.05). High significant differences in DIN concentrations occurred between CA and SG and between CA and CO values in all seasons (Tukey’s tests: P < 0.001). CO and SG significantly differed in autumn DIN values (Tukey’s test: P < 0.001).
Significant differences in environmental variables were not detected among sampling stations in each lagoon (data not shown).
Picophytoplankton Seasonal Dynamics
Significant differences in Pico and P-Cyan abundances occurred considering the combined effects of seasonality and site (Table 2). Furthermore, significant differences in P-Euk abundance occurred among the lagoons (Table 2).
P-Cyan were the dominant Pico group in CA and SG, contributing >90 % to total density and biomass in all seasons, and determining the maximum summer Pico abundance in these two lagoons (Online Resource 2; Figs. 4 and 5). Significant seasonal differences in P-Cyan abundance were observed between CA and CO summer values (Tukey’s test: P = 0.007) and between CA and SG spring values (Tukey’s test: P = 0.011). In contrast, P-Euk dominated at CO, contributing >80 % to Pico total density and biomass in autumn, winter, and spring, and determining the maximum autumn Pico abundance in the lagoon (Online Resource 2; Figs. 4 and 5). P-Euk were only observed in CA during spring in one sampling, contributing <4 % to total density and biomass. P-Euk were also detected at <4 % total density and <6 % total biomass in summer, autumn, and spring at SG (Online Resource 2; Figs. 4 and 5). Tukey’s test showed significant differences in P-Euk abundance between CO and CA (P = 0.002), CO and SG (P = 0.041), and CA and SG (P = 0.040).
Significant differences in biotic variables were not detected among sampling stations in each lagoon (data not shown).
Picophytoplankton Interactions with Environmental Variables
Results of Spearman’s analysis (Table 3) indicated that P-Cyan was significantly and positively correlated with temperature and salinity in all lagoons, and negatively correlated with relative transparency in SG and CO. A significant and negative correlation occurred also between P-Cyan and DIN and P-PO4 in CA and CO. In contrast, P-Cyan was positively correlated with P-PO4 in SG.
Spearman’s analysis highlighted that P-Euk exhibited a significant and negative correlation with relative transparency and a positive correlation with temperature in SG, and a negative correlation with temperature in CO. A significant and negative correlation also occurred between P-Euk and DIN in CA, a positive correlation between P-Euk and DIN and P-PO4 in SG, and a positive correlation between P-Euk and P-PO4 in CO.
Discussion
The present work provides new data on Pico, P-Cyan, and P-Euk seasonal dynamics in eutrophic Mediterranean lagoons. Our results contribute to improved knowledge on the ecology of Pico assemblages, which remains scarce in coastal and transitional environments, particularly in Mediterranean climatic regimes. Despite the similarity of the general seasonal pattern of the main climate proxies, such as temperature, and the common high trophic level of the studied lagoons, our data revealed that Pico seasonality varied significantly in both abundance and composition (i.e., P-Cyan and P-Euk) depending on different environmental factors.
Results from this study show Pico density values similar to those detected in other coastal lagoons worldwide (Gaulke et al. 2010; Schapira et al. 2010; Caroppo 2015 and references therein). Noteworthy, biomass values observed in one of the studied lagoons (CA) were much higher than in other coastal waters worldwide (Calvo-Díaz et al. 2008; Caroppo 2015 and references therein).
Maximum Pico abundance was recorded in summer in two of the lagoons (CA and SG). These lagoons were dominated all year around by P-Cyan, whose dynamics, consequently, can be considered as representative of Pico. A strong positive correlation between P-Cyan and temperature is well documented in the literature, where temperature is indicated as a key factor controlling P-Cyan growth (Ning et al. 2000; Bec et al. 2005; Collos et al. 2009). Coherently, in our data, P-Cyan showed a significant positive correlation with temperature in all lagoons. Interestingly, contrasting behaviors between P-Cyan and analyzed nutrients were observed in the two P-Cyan-dominated lagoons, supporting the hypothesis that the link between P-Cyan abundance and nutrient availability is more complex than previously thought (Caroppo 2015). In one case (CA), significant negative correlations between P-Cyan and DIN and P-PO4 values were found, as it was established in several studies in other Mediterranean climate coastal environments (Ning et al. 2000; Bernardi Aubry et al. 2006; Collos et al. 2009). In a second case (SG), we identified significant positive correlation between P-Cyan and P-PO4. Also, Gaulke et al. (2010) reported maximum P-Cyan abundance coincident with high summer P-PO4 values in the Neuse River Estuary, North Carolina, USA. Overall, the role of nutrients seems to have a limited influence on the P-Cyan dynamics, in agreement with the P-Cyan ability to adapt to a wide range of nutrient concentrations (Flombaum et al. 2013). In particular, the interpretation of the relationship between P-Cyan and nutrient dynamics is difficult if the water nutrient levels and inorganic compounds are only considered, leaving out the fluxes of water nutrients and the dissolved organic matter (Flombaum et al. 2013). Moreover, studies on eutrophic areas show grazing is one of the main drivers behind P-Cyan seasonal dynamics, surpassing the role of nutrient availability in structuring communities (Caroppo, 2015 and references therein).
Interestingly, in the present study, P-Euk prevailed strongly in the third lagoon (CO), with maximum abundance in autumn. In a previous study on dinoflagellate cyst assemblages, CO phytoplankton composition resulted significantly different from other two lagoons, including SG, located in the same geographical area (Satta et al. 2014). In this case, salinity was a fundamental variable in determining the diverse results among the study sites. In addition, there is evidence that the peculiar Pico results observed for CO in the present work reflected significant differences in environmental conditions among lagoons, especially regarding relative transparency and salinity. Compared to CA and SG, CO exhibited the highest relative transparency and salinity values and the lowest seasonal variation in salinity. The diverse environmental conditions likely reflected other differences among the three lagoons, such as the diverse origin, extent of the catchment areas and typology and level of human activities in these territories. Actually, CO is the shallowest lagoon, condition that probably favored the highest relative transparency. CO has originated from a modified marine coastal area, confined by a natural shore line-parallel sand spit and closed artificially, assuring, however, a direct and continuous seawater exchange. CO catchment area is the smallest of all the lagoons (22 km2 CO catchment area, 432 km2 CA catchment area, 173 km2 SG catchment area) and is uninhabited (49,473 inhabitants in CA catchment area, 38,041 inhabitants in SG catchment area), although it is strongly exploited for intensive agriculture. Further, freshwater input to the lagoon is really lower (5 × 106 m3 year−1) compared to those of CA (28.6 × 106 m3 year−1) and SG (38.5 × 106 m3 year−1), and the, inputs are less localized than in the other lagoons, occurring at least at six different points along its right side via drainage canals. The lowest seasonal variation and the highest salinity values in CO may be related to the synergic effect of all these factors.
To date, little is known about the drivers that lead to the dominance of Pico communities by P-Euk species due to the few and scattered data on their distribution patterns and their relative contribution to Pico biomass (Vaquer et al. 1996; Siokou-Frangou et al. 2010). In general, P-Cyan outnumbers P-Euk in Mediterranean climate coastal waters worldwide (Ning et al. 2000; Bernardi Aubry et al. 2006). However, throughout different sampling programs in the marine Thau lagoon, France (Mediterranean Sea), P-Euk dominated the Pico assemblage in summer (Vaquer et al. 1996; Bec et al. 2005). P-Euk success was related to P-Euk ability to use solar radiation more efficiently than P-Cyan, determining lower deleterious effects of high irradiance on P-Euk (Vaquer et al. 1996; Bec et al. 2005). On the contrary, in our data, P-Euk were not observed in summer in CO. In addition, assuming transparency as an index of underwater light regime, we did not ascertain any significant relationship between P-Euk and relative transparency in CO. However, we found that P-Cyan resulted significantly and negatively correlated with transparency in this lagoon, suggesting a possible disadvantage of this group in respect to P-Euk by the shallowness and the high relative transparency values in CO. In agreement with the hypotheses that P-Cyan growth is more temperature dependent than P-Euk growth (Kuosa 1991), our results showed a strong P-Euk proliferation at lower temperature in CO, especially in autumn, with the presence of P-Cyan only in summer. Actually, a significant and positive correlation between P-Cyan and temperature, and a significant and negative correlation between P-Euk and temperature were detected in CO. Similar observations were reported for the Neuse River Estuary, North Carolina, USA, where P-Euk dominated in winter (Gaulke et al. 2010), and for the Baltic Sea, where P-Euk proliferated in autumn (Kuosa 1991). In our study, the maximum P-Euk abundance observed in autumn in CO coincided with the maximum DIN and P-PO4 concentrations detected in the lagoon, even if P-Euk abundance resulted significantly and positively correlated only with P-PO4. To date, there have been few studies on the nutrient uptake physiology of coastal Pico communities, especially at the taxa level, and no clear causal relationship has emerged in literature to relate nutrients with P-Euk growth (Vaquer et al. 1996; Gaulke et al. 2010).
The recent literature on photosynthetically active radiation (PAR) and grazing provides more information on P-Cyan and P-Euk dynamics, but unfortunately data from the geographic sites we investigated are currently unavailable. Further, we acknowledge annual observations from several consecutive years are particularly useful in highly sensitive and stressful ecosystems, such as coastal lagoons, where intrinsic complexity in phytoplankton communities is common (Collos et al. 2009).
Conclusion
Our results show different behaviors of Pico in the studied Mediterranean lagoons, at both seasonal and composition levels. Among the environmental variables investigated, temperature represented an important driver for Pico growth, especially for P-Cyan. In contrast, transparency and salinity appeared relevant for P-Euk development. Responses of Pico to seasonal variation in DIN and P-PO4 concentrations resulted, instead, more variable.
The high Pico abundances detected in our study support the idea that Pico importance cannot be overlooked in time-series monitoring programs. Our work supports the hypotheses that Pico ecology is influenced by many factors, acting simultaneously. We believe that, to fully understand the Pico ecology in lagoon ecosystems, further studies are needed considering multiple trophic levels, biological interactions, and additional possible environmental drivers, such as light availability and turbulence.
References
Bazzoni, A.M., S. Pulina, B.M. Padedda, C.T. Satta, A. Lugliè, N. Sechi, and C. Facca. 2013. Water quality evaluation in Mediterranean lagoons using the Multimetric Phytoplankton Index (MPI): study cases from Sardinia. Transitional Waters Bulletin 7(1): 64–71.
Bec, B., Y. Collos, P. Souchu, A. Vaquer, J. Lautier, A. Fiandrino, L. Benau, V. Orsoni, and T. Laugier. 2011. Distribution of picophytoplankton and nanophytoplankton along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Aquatic Microbial Ecology 63: 29–45.
Bec, B., J. Husseini-Ratrema, Y. Collos, P. Souchu, and V. Vaquer. 2005. Phytoplankton seasonal dynamics in a Mediterranean coastal lagoon: emphasis on the picoeukaryote community. Journal of Plankton Research 27(9): 881–894.
Bernardi Aubry, F., F. Acri, M. Bastianini, A. Pugnetti, and G. Socal. 2006. Picophytoplankton contribution to phytoplankton community structure in the Gulf of Venice (NW Adriatic Sea). International Review of Hydrobiology 91: 51–70.
Bratbak, G. 1985. Bacterial biovolume and biomass estimation. Applied and Environmental Microbiology 49: 1488–1493.
Bratbak, G. 1993. Microscope methods for measuring bacterial biovolume: epifluorescence microscopy, scanning electron microscopy, and transmission electron microscopy. In Handbook of methods in aquatic microbial ecology, eds. P.F. Kemp, B.F. Sherr, E.B. Sherr, and J.J. Cole, 309–317. USA: Lewis Publisher.
Calvo-Díaz, A., X. Anxelu, G. Morán, and L.Á. Suárez. 2008. Seasonality of picophytoplankton chlorophyll a and biomass in the central Cantabrian Sea, southern Bay of Biscay. Journal of Marine Systems 72: 271–281.
Caroppo, C. 2000. The contribution of picophytoplankton to community structure in a Mediterranean brackish environment. Journal of Plankton Research 22(2): 381–397.
Caroppo, C. 2015. Ecology and biodiversity of picoplanktonic cyanobacteria in coastal and brackish environments. Biodiversity and Conservation 24: 949–971.
Carrick, H.J., and C.L. Schelske. 1997. Have we overlooked the importance of small phytoplankton in productive waters? Limnology and Oceanography 42: 1613–1621.
Cloern, J.E., and D.J. Jassby. 2010. Patterns and scales of phytoplankton variability in estuarine–coastal ecosystems. Estuaries and Coasts 33: 230–241.
Collos, Y., B. Bec, C. Jauzein, E. Abadie, T. Laugier, A. Pastoureaud, P. Souchu, A. Vaquer, and J. Lautier. 2009. Oligotrophication and emergence of picocyanobacteria and a toxic dinoflagellate in Thau lagoon, southern France. Journal of Sea Research 61: 68–75.
Flombaum, P., J.L. Gallegos, R.A. Gordillo, J. Rincón, L.L. Zabala, D.M. Karl, W.K.W. Li, M.W. Lomas, D. Veneziano, N. Jiao, C.S. Veraand, A.C. Martiny, and J.A. Vrugt. 2013. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proceedings of the National Academy of Sciences 110(24): 9824–9829.
Gaulke, A.K., M.S. Wetz, and H.W. Paerl. 2010. Picophytoplankton: a major contributor to planktonic biomass and primary production in a eutrophic, river-dominated estuary. Estuarine, Coastal and Shelf Science 90: 45–54.
Harris, G.P. 1994. Pattern, process and prediction in aquatic ecology. A limnological view of some general ecological problems. Freshwater Biology 32: 143–160.
Hein, M., M.F. Pedersen, and K. Sand-Jensen. 1995. Dependent nitrogen uptake in micro- and macroalgae. Marine Ecology Progress Series 118: 247–253.
Kuosa, H. 1991. Picoplanktonic algae in the northern Baltic Sea: seasonal dynamics and flagellate grazing. Marine Ecology Progress Series 73: 269–276.
Lugliè, A., N. Sechi, G. Oggiano, G. Sanna, and A. Tapparo. 2002. Ecological assessment of Santa Giusta lagoon (Sardinia, Italy). Annali di Chimica 92: 239–247.
MacIsaac, E.A., and J.G. Stockner. 1993. Enumeration of phototrophic picoplankton by autofluorescence microscopy. In Handbook of methods in aquatic microbial ecology, eds. P.F. Kemp, B.F. Sherr, E.B. Sherr, and J.J. Cole, 187–197. USA: Lewis Publisher.
Magazzù, G., and F. Decembrini. 1995. Primary production, biomass and abundance of phototrophic picoplankton in the Mediterranean Sea: a review. Aquatic Microbial Ecology 9: 97–104.
Ning, X., J.E. Cloern, and B.E. Cole. 2000. Spatial and temporal variability of picocyanobacteria Synechococcus sp. in San Francisco Bay. Limnology and Oceanography 45(3): 695–702.
Paoli, A., M. Celussi, A. Valeri, C. Larato, A. Bussani, S. Fonda Umani, M.R. Vadrucci, C. Mazziotti, and P. Del Negro. 2007. Picocyanobacteria in Adriatic transitional environments. Estuaries and coastal shelf. Science 75: 13–20.
R Core Team. 2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna. ISBN 3–900051–07-0. http://www.R-project.org/.
Satta, C.T., S. Anglès, E. Garcés, N. Sechi, S. Pulina, B.M. Padedda, D. Stacca, and A. Lugliè. 2014. Dinoflagellate cyst assemblages in surface sediments from three shallow Mediterranean lagoons (Sardinia, north western Mediterranean Sea. Estuaries and Coasts 37: 646–663.
Schapira, M., M.J. Buscot, T. Pollet, S.C. Leterme, and L. Seuront. 2010. Distribution of picophytoplankton communities from brackish to hypersaline waters in a south Australian coastal lagoon. Saline Systems 6: 2.
Sechi, N., F. Fiocca, A. Sannio, and A. Lugliè. 2001. Santa Giusta lagoon (Sardinia): phytoplankton and nutrients before and after waste water diversion. Journal of Limnology 60(2): 194–200.
Siokou-Frangou, I., U. Christaki, M.G. Mazzocchi, M. Montresor, M. Ribera D’Alcalá, D. Vaqué, and A. Zingone. 2010. Plankton in the open Mediterranean Sea: a review. Biogeosciences 7: 1543–1586.
Sorokin, Y.I., P.Y. Sorokin, and A. Gnes. 1996. Structure and functioning of the anthropogenically transformed Comacchio lagoonal ecosystem (Ferrara, Italy). Marine Ecological Progressive Series 133: 57–71.
Stockner, J.G. 1988. Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnology and Oceanography 33(4): 765–775.
Strickland, J.D.H., and T.R. Parsons. 1972. A practical handbook of seawater analysis. Vol. 167. Ottawa: Fisheries Research Board of Canada.
Tamigneaux, E., E. Vasquez, M. Mingelbier, B. Klei, and L. Legendre. 1995. Environmental control of phytoplankton assemblages in nearshore waters, with special emphasis on phototrophic ultraplankton. Journal of Plankton Research 17: 1421–1447.
Thingstad, T.F., and F. Rassoulzadegan. 1999. Conceptual models for the biogeochemical role of the photic zone microbial food web, with particular reference to the Mediterranean Sea. Progress in Oceanography 44: 271–286.
Underwood, A.J. 1997. Experiments in ecology: their logical design and interpretation using analysis of variance, 504. Cambridge: Cambridge University Press.
Vaquer, A., M. Troussellier, C. Courties, and B. Bibent. 1996. Standing stock and dynamics of picophytoplankton in the Thau lagoon (Northwest Mediterranean coast. Limnology and Oceanography 41: 1821–1828.
Worden, A.Z., J.K. Nolan, and B. Palenik. 2004. Assessing the dynamics and ecology of marine picophytoplankton: The importance of the eukaryotic component. Limnology and Oceanography 49(1): 168–179.
Acknowledgments
The authors thank colleagues of the Aquatic Ecology group of University of Sassari for nutrient analyses, Fabrizio Bernardi-Aubry for his support in Pico analysis, and Silvia Anglès for her contribution to the linguistic revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Charles Simenstad
Electronic supplementary material
Online Resource 1
(DOC 41 kb)
Online Resource 2
(DOC 47 kb)
Rights and permissions
About this article
Cite this article
Pulina, S., Satta, C.T., Padedda, B.M. et al. Picophytoplankton Seasonal Dynamics and Interactions with Environmental Variables in Three Mediterranean Coastal Lagoons. Estuaries and Coasts 40, 469–478 (2017). https://doi.org/10.1007/s12237-016-0154-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0154-5