Abstract
Longitudinal succession of stream fish faunas is commonly related to increasing stream size and stability. However, effects of succession on assemblage morphology are seldom quantified. We used an ecomorphological approach to determine differences in faunal structure among distinct stream types of the Cheyenne River drainage in South Dakota, USA. During May–October 2004 we collected fishes monthly from five streams. We examined 28 morphological traits of the dominant fish species and compared morphological structure among faunas using univariate analysis of variance (ANOVA) tests and multivariate ordination and distance calculation techniques. Species richness and composition varied between smaller creeks and larger rivers. Morphological diversity increased with richness, but richer assemblages were also more tightly packed in morphospace, partly because of increased cyprinid richness. Some morphological differences were predicted by variation in mean discharge and discharge flashiness (flow stability). Fishes of more stable or larger river stations characteristically had smaller heads and mouths and longer intestines. Larger mean standard length was also associated with less flashy flow regimes and higher mean discharge. All assemblages were hyperdispersed in morphological space, consistent with the harsh zoogeographical history of the region and suggesting the presence of open niches. Increasing species and morphological diversity despite increasing discharge flashiness suggests higher niche diversity in Great Plains rivers compared to adjacent creeks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological processes change in transition from headwater streams to river mouths (Vannote et al., 1980; Minshall et al., 1985). However, longitudinal transitions in food web structure and trophic complexity are poorly understood (Power & Dietrich 2002). Longitudinal fish faunal succession is well documented (e.g. Sheldon 1968; Schlosser 1990), but its relation to niche dimensions is less understood. Landmark studies demonstrated that fish species diversity increased downstream if habitat diversity increased (Gorman & Karr, 1978) or habitat stability increased (Horwitz 1978), both of which favor trophic and habitat specialists (Poff & Allan, 1995), perhaps because of increased niche space (Schlosser, 1987).
The multidimensional niche of a fish species is difficult to define, but morphological traits are useful indicators of niche dimensions (Gatz, 1979; Watson & Balon, 1984), because fish morphology partly reflects the evolutionary influence of environmental conditions (Hubbs, 1940; Lamouroux et al., 2002). Findley (1973, 1976; Gatz 1980) pioneered an approach to array species within multidimensional niche space using ordination and distance analyses on a large suite of measurements of trophic and habitat related morphological characters. This approach has been used to estimate niche space among and within fish assemblages (Winemiller, 1991; Adite & Winemiller, 1997), reveal patterns of ecomorphological convergence and divergence (Winemiller, 1991; Winemiller et al., 1995), document habitat associations (Motta et al., 1995), identify functional groups (Piet, 1998), and establish a link between habitat complexity and morphological diversity (Willis et al., 2005). Winemiller (1991) proposed that morphological diversity within fish faunas increases with habitat volume, habitat diversity, environmental stability, and levels of interspecific competition and predation, all of which commonly increase with stream size (Minshall et al., 1985; Schlosser, 1990). Variability in fish size contributes to morphological diversity (Winemiller, 1991). Greater pool depth downstream is critical for supporting larger fishes (Schlosser, 1987; 1990), perhaps because fish size is correlated with habitat volume (Larimore & Smith, 1963). Thus, there appears to be a relation between the stream continuum and fish morphological diversity, as evidenced by higher trophic diversity in larger streams (Bayley & Li, 1992; Poff & Allan, 1995).
Here we assess relations of fish length and morphological diversity to stream type in the Cheyenne River drainage of the North American Great Plains. Stream types are distributed along two confluent stream continua and represent the range of perennial-warmwater streams that is present. We expect fish length and species diversity to increase downstream (Schlosser, 1987; Rahel & Hubert, 1991; Bayley & Li, 1992) and that higher species diversity will correspond to higher morphological diversity (Winemiller, 1991; Willis et al., 2005). Also, species replacements due to faunal zonation (Rahel & Hubert, 1991; Quist et al., 2004a), if present, could cause morphological differentiation among assemblages (Winemiller, 1991).
Study area
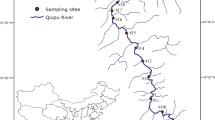
The Cheyenne River watershed lies entirely within the Great Plains physiographic province (Holliday et al., 2002) and has an area of 65,398 km2, primarily in Wyoming and South Dakota, USA. There are two main forks of the Cheyenne River, the Belle Fourche River and Upper Cheyenne River (Fig. 1). Both begin in highlands of the Powder River Basin in northeastern Wyoming, but their courses diverge to encircle the Black Hills with the Belle Fourche River on the north and Upper Cheyenne River on the south. The forks eventually join to form the Lower Cheyenne River. Dams and reservoirs are present on both forks and on streams of the Black Hills, but the combination of perennial tributary streams, the two forks of the Cheyenne River, and the Lower Cheyenne River forms two confluent stream continua within an extensive undammed portion of the drainage, downstream from the Black Hills. Both continua are approximately 360 km in length (stream km estimates from National Hydrography Database 1:100,000 scale data).
Map of the Cheyenne River drainage in South Dakota, with locations of five sampling stations (circles) and five US Geological Survey gaging stations (triangles) on five different streams. Major dams and reservoirs, shown in black, are: A = Belle Fourche Dam, B = Orman Reservoir, C = Deerfield Reservoir, D = Pactola Reservoir, E = Sheridan Reservoir, F = Stockade Reservoir, G = Angostura Reservoir. The dashed line indicates the boundary of the Black Hills
The five streams we studied included Beaver Creek, Whitewood Creek, the Belle Fourche River below Belle Fourche Dam, the Upper Cheyenne River below Angostura Dam, and the Lower Cheyenne River, which represent the variety of perennial-warmwater streams that is present. Our study was conducted during a drought period that began in 2001, so we used US Geological Survey gage data from water years 2002 through 2004 to characterize the flow regime of each stream. In both stream continua, mean discharge and discharge flashiness increased from upstream to downstream (Table 1). The Whitewood Creek––Belle Fourche River––Lower Cheyenne River continuum had a more variable annual flow regime than the Beaver Creek––Upper Cheyenne River––Lower Cheyenne River continuum.
Methods
Fish collection
We sampled one station per stream, each being selected to be representative of a stream type and used one station on the Lower Cheyenne River for both continua (Fig. 1). We conducted fish collections monthly from May through October 2004 and used a mesohabitat approach to representatively sample fishes (Vadas & Orth, 1998). We conducted monthly sampling over a 36-h period at each station, during which we made the maximum number of mesohabitat collections possible, using multiple capture methods depending upon habitat conditions (Table 2; Bramblett & Fausch 1991). We conducted night sampling from May through July and determined that capture composition did not vary between day and night (unpublished data).
Following criteria described by Winemiller (1991), we measured 28 morphological traits using clear plastic rulers and ocular micrometry (Adite & Winemiller, 1997; Table 3). With the exception of mouth position code, gill raker code, and maximum standard length, all traits were converted to ratios of standard length, body depth, body width, or head length (Winemiller, 1991). We conducted measurements on three adult-sized individuals of each species that was dominant at one or more stations (Watson & Balon, 1984; Winemiller, 1991; Willis et al., 2005), with the exception that specimens of large-bodied species were selected to reflect the average size of individuals normally encountered (Appendix 1). Dominant species, summed by order of rank, comprised 99% of each fish fauna (Winemiller, 1991). For each species, the three specimens selected for study were similar in size to reduce allometric variation among individuals (Winemiller, 1991).
Data analyses
All analyses included only the dominant species so that comparisons reflected characteristic station faunas, uninfluenced by incidental species. Relative abundance was based on cumulative standard length rather than the number of individuals collected, to better represent the total biomass of each station fauna. We constructed a presence–absence matrix (stations in columns, species in rows) and used the approach recommended by Gotelli (2000; Gotelli & Entsminger, 2001b, 2003) to determine whether species distributions were random. This analysis uses the C-score (Stone & Roberts, 1990) to quantify species co-occurrence patterns, comparing the empirical C-score with the average of many Monte Carlo simulations. We used EcoSim 7.0 software (Gotelli & Entsminger, 2001a) and selected the Gotelli Swap algorithm, which analyzed a random set of presence–absence matrices (1,000 iterations) with row and column totals held constant.
We also performed descriptive analyses on the presence-absence matrix to examine patterns of faunal similarity among stations in relation to stream size and stability. We determined dominant species richness by station as well as both percent-unshared species (faunal turnover; Russell, 1998) and faunal similarity (Sørensen’s Index; Legendre & Legendre, 1998) between station pairs. Sørensen’s Index varies from 0 to 1, with higher values indicating higher similarity. We also calculated dominant species diversity for each station with Fisher’s α, using cumulative standard lengths as relative abundances, and calculated error bars for use in a statistical comparison (Kempton & Taylor, 1974; Magurran, 2004). Fisher’s α is a rigorous diversity measure that approximates the number of species represented by a single individual and comparisons are unaffected by sample size if the total number of individuals collected per station is above 1,000.
We used multiple linear regression to determine the influence of mean discharge (stream size) and discharge flashiness (R-B index, stream stability) on dominant species richness and species diversity. We considered discharge a superior estimate of stream size to watershed area because upstream dams and diversity confounded the relation of watershed to stream size in our study area. The R-B index is a superior measure of discharge flashiness because it incorporates a temporal component of flow variability into the flashiness estimate (Baker et al., 2004). It is a discharge oscillation to total discharge ratio that typically varies from 0 to 1, with higher values indicating flashier flow regimes.
We compared cumulative mean standard length (SL; all species combined) among stations. We used Analysis of Variance (ANOVA) with Tukey Honestly Significant Difference (HSD) post-hoc tests and multiple linear regression to determine whether SL varied among stations and whether variation was related to mean discharge or discharge flashiness. Standard lengths were loge-transformed. We also used ANOVA to compare standard lengths among sampling stations for each species that was dominant at multiple stations to determine how individual species contributed to the overall trend.
As an exploratory technique, we used ANOVA to individually compare the 28 morphological traits among stations (Winemiller et al., 1995). We conducted a Principal Components Analysis (PCA) on the 22-species correlation matrix of log10-transformed morphological-trait data to reduce the morphological data to fewer dimensions and assess morphological differences among stations by comparing average PC scores (Mahon, 1984; Winemiller, 1991; Piet, 1998). We considered Principal Component axes to be nontrivial if eigenvalues exceeded null eigenvalues calculated using the broken-stick model (Jackson, 1993). The PCA and broken-stick model calculations were performed using PC-ORD for windows, version 4.25 software (MjM Software, Gleneden Beach, Oregon, USA). We calculated means and standard deviations of PCA scores for fishes on each nontrivial axis. Mean score indicated morphological position on each PC axis and standard deviation indicated morphological diversity (Willis et al., 2005). We used multiple linear regression to determine the influence of mean discharge and discharge flashiness on morphological position and morphological diversity.
We calculated Euclidian distances among all species pairs using all 28 morphological traits by station and for all stations (Gatz, 1979; Winemiller, 1991; Adite & Winemiller, 1997). Prior to calculations, the means of all morphological measures were standardized to fit a Gaussian distribution (mean = 0, standard deviation = 1), which adjusts for differing scales among measures (Gatz, 1979; Watson & Balon, 1984; Winemiller, 1991). From the Euclidian distance calculations we determined: (1) distance to nearest neighbor for each species per fauna and mean nearest neighbor distance per fauna, (2) average between-species distance for each species per fauna and mean distance per fauna, (3) distance to the morphological centroid (hypothetical average fish based on the mean values of all species) for each species per fauna and mean distance to centroid per fauna. Distance to nearest neighbor provides an estimate of species dispersion, average between-species distance provides an estimate of ecological similarity among species, and distance to centroid provides an estimate of total niche space occupied (Findley, 1976; Gatz, 1979; Winemiller, 1991; Adite & Winemiller, 1997). We used ANOVA to compare mean distance to nearest neighbor, paired distances among all species, and distance to centroid among stations and simple linear regression to test the relation between species richness and mean nearest-neighbor distance, standard deviation of nearest-neighbor distance, and mean distance to centroid (Winemiller, 1991). Standard deviation of nearest-neighbor distance is an estimate of the evenness of species packing within morphological space (Findley, 1976; Winemiller, 1991). Finally, we investigated the relation between morphological similarity and co-occurrence by determining the nearest neighbor of each species and calculating the percentage of species that co-occurred with their nearest neighbor (Winston, 1995).
Results
We collected a total of 18,721 fish of 28 species. Twenty-two species were dominant at one or more stations (Table 4; Appendix 1). Station fish assemblages had significantly less co-occurrence than expected by chance (Fig. 2). Number of dominant species (richness) was lower in creek stations than river stations (Fig. 3), but was poorly predicted by mean discharge (Q) or discharge flashiness (R-B; b′Q = 0.68, b′R−B = 0.00, r 2 = 0.5, F = 0.9, P = 0.53). Number of unshared dominant species varied from 35% to 89% and was highest between river stations and creek stations (Table 5). Similarly, Sørensen’s index of faunal similarity varied from 0.2 to 0.8 with river stations being most similar (Table 5). Species diversity (Fisher’s α) was lower in creek stations than river stations (Fig. 3), but unrelated to mean discharge or discharge flashiness (b′Q = 0.73, b′R-B = −0.09, r 2 = 0.4, F = 0.8, P = 0.55).
Dominant species richness (top) and species diversity (Fisher’s α; bottom) by sampling station, ordered by mean discharge (Table 1)
Average SL was variable among stations (F = 579.2, P < 0.01) and statistically different among all station pairs (Tukey HSD pairwise mean differences (PMDs) >|0.10|, P < 0.01), except between the Upper Cheyenne River and Lower Cheyenne River (PMD = 0.03, P = 0.14). The mean SL of Beaver Creek fish was highest, followed by the Upper and Lower Cheyenne Rivers, Whitewood Creek, and the Belle Fourche River (Fig. 4). Multiple linear regression indicated that mean discharge and mean discharge flashiness predicted mean SL (b′Q = 0.81, b′R-B = −1.39, r 2 = 1.0, F = 28.0, P = 0.03). Few species increased in mean SL from smaller to larger streams (Fig. 5). Moxostoma macrolepidotum was the primary exception.
All morphological traits were similar among station faunas (Table 6; F < 2.0, df = 4, 47, P > 0.12 for all comparisons) except caudal fin length (F = 3.3, P = 0.02). Caudal fin length was lower in Beaver Creek than in the Lower Cheyenne River (PMD = −0.04, P = 0.03), but similar among other stations (PMDs < |0.04|, P > 0.08). The first three PC axes modeled 51% of the total variation in morphological space among all 22 species (Table 7). Additional PC axes modeled little variation (<8.9%) and were trivial based on broken-stick eigenvalues that exceeded PCA eigenvalues. Multiple regressions of mean PCA scores for fish species indicated that PC1 scores varied with discharge and discharge flashiness among stations, but not PC2 or PC3 scores (Table 8). Multiple regressions of PCA score standard deviation indicated that variation among station scores was unrelated to mean discharge or discharge flashiness (Table 8).
Species with high PC1 scores (Noturus flavus, Fundulus spp., Perciformes) had large, terminal, or superior mouths and short intestines, indicative of their carnivorous habits, whereas species with small, subterminal mouths, and long intestines (Hybognathus argyritis, Carpiodes carpio carpio, Moxostoma macrolepidotum) scored lowest. Species with high PC2 scores were deep-bodied (Hiodon alosoides, Cyprinus carpio, Carpiodes carpio carpio, Centrarchidae) and those scoring lowest were dorso-ventrally compressed (Macrhybopsis gelida, N. flavus). High scoring species on PC3 (Carpiodes carpio carpio, N. flavus, Lepomis cyanellus) had no obvious morphological similarity but, according to eigenvector loadings, shared a combination of wide bodies and heads, high eye position, small eyes, long snouts and dorsal fins, tall pelvic and anal fins, long gill rakers, short swim bladders, and large maximum SL. Species scoring low on PC3 were opposite and also difficult to interpret as a group (H. alosoides, Cyprinella lutrensis lutrensis, Fundulus sciadicus).
The 22 fish species were broadly distributed within morphospace (Fig. 6). Fish assemblages of river stations occupied the majority of the morphospace used by the entire species pool. Assemblages of creek stations, particularly the 5-species assemblage of Whitewood Creek, occupied smaller portions of the total morphospace. River assemblages exhibited more species clustering than creek assemblages. The most notable species cluster included species with negative scores on PC1 and PC2, or PC2 and PC3 (Fig. 6). These were mostly small-bodied cyprinids (C. lutrensis lutrensis, H. argyritis, Hybognathus placitus, Notropis stramineus missuriensis, Pimephales promelas, Platygobio gracilis) along with a catostomid (Catostomus commersonii), an ictalurid (Ictalurus punctatus), and a funduliid (Fundulus kansae).
Euclidian distance measures indicated high morphological similarity among station faunas. Mean nearest-neighbor distance, average pairwise difference, and mean distance to centroid were similar among stations (Figs. 7–9). However, number of species per fauna predicted mean nearest-neighbor distance and mean distance to centroid (Fig. 10). Standard deviation of nearest-neighbor distances was not predicted by species richness, but was comparatively low for Whitewood Creek (Fig. 10). Nineteen of 22 species (86%) cooccurred with their nearest neighbor at one or more stations (Table 9).
Frequency histograms of fish species Euclidian distances to the faunal centroid by sampling station. Mean Euclidian distance to the faunal centroid (black triangle) is also shown with the standard deviation (error bars). Analysis of variance indicated mean distance to the faunal centroid was similar among stations (F = 0.1, P = 0.98)
Frequency histograms of fish species Euclidian distances among all species pairs by sampling station. Mean Euclidian distance among all species pairs (black triangle) is also shown with the standard deviation (error bars). Analysis of variance indicated mean distance among all species pairs was similar among stations (F < 0.1, P = 1.00)
Frequency histograms of fish species similarities to nearest neighbors based on Euclidian distances among species. Mean Euclidian distance to nearest neighbor (black triangle) is also shown with the standard deviation (error bars). Analysis of variance indicated mean distance to nearest neighbors was similar among stations (F = 1.2, P = 0.33)
Three measures of Euclidian distance among fish species plotted against species richness:(A) mean distance to the faunal centroid, (B) mean distance to the nearest neighbor, and (C) standard deviation of mean distance to the nearest neighbor. Results of simple linear regressions are provided for each graph and lines are plotted for statistically significant regressions (P < 0.05)
Discussion
Fish assemblages of river stations were more speciose and, as a result, more morphologically diverse than assemblages in creek stations. Although mean distance to centroid did not vary among stations PCA and regression analyses indicated that richer faunas occupied wider morphospace. Morphologically unique species with high PC scores (H. alosoides, H. argyritis, M. gelida, Carpiodes carpio carpio, M. macrolepidotum) accounted for higher morphological diversity in river stations. Similar mean distance to centroid among stations may have resulted from a relatively small sample size (few species) and high variance (high species dispersion).
However, declining nearest-neighbor distances with increasing species richness indicated that more speciose assemblages were also more tightly packed in morphospace. This could in part result from chance (Ricklefs & Travis, 1980). Nevertheless, the relation between nearest-neighbor distance and species richness was supported by increased richness of small-bodied cyprinids (and a few other taxa) with relatively similar morphologies.
Faunal replacements caused position in morphospace to vary between creeks and rivers. With the notable exception of Sander canadensis, species of larger river stations (Belle Fourche River, Lower Cheyenne River) had smaller heads and mouths, sub-terminal or inferior mouths, and longer intestines compared to species of creek stations, perhaps reflecting a switch from carnivory to omnivory (Hugueny & Pouilly, 1999; Xie et al., 2001; Pouilly et al., 2003). Thus, distinct differences between creek and river fish faunas of the Great Plains (Cross & Moss, 1987; Bramblett & Fausch, 1991) may indicate trophic differences. Carnivores of small, headwater creeks presumably are adapted to prey upon entrained terrestrial invertebrates or large benthic invertebrates associated with coarse substrata, whereas omnivores of larger, mainstem rivers apparently are adapted to use benthic trophic pathways associated with the smaller-sized invertebrate fauna and vegetative material of finer substrata and detritus (sensu Vannote et al., 1980; Minshall et al., 1985).
High mean distance to centroid (> 6.0) for all faunas indicates that fishes were hyperdispersed in morphological space. In comparison, Winemiller (1991) considered the Alaskan faunas he studied to be hyperdispersed with mean distance to centroid values of 2.0 and 2.5. Hyperdispersion suggests that open niches are available and is presumably a result of recent and repeated colonization of fishes from adjacent, more diverse faunas (Winemiller, 1991). Hyperdispersion is common where prehistoric climate patterns and topography have combined to limit species diversity (Mahon, 1984; Moyle & Herbold, 1987; Hugueny, 1989; Oberdorff et al., 1997). This paradigm fits well with fish assemblages of the Cheyenne River drainage because they have been formed largely within the last 12,000 years as the climate has warmed and are subsets of larger faunas found to the southeast (Cross et al., 1986; Hoagstrom & Berry, 2006). Moreover, hyperdispersed faunas are typically unsaturated and invasible (Hugueny & Paugy, 1995; Belkessam et al., 1997), which has been demonstrated for Great Plains fish faunas (Gido & Brown, 1999; Gido et al., 2004).
The hyperdispersed faunas of the Cheyenne River drainage have high morphological diversity because most genera are represented by a single species. Members of hyperdispersed faunas typically segregate among habitats and do not exhibit intense interspecific interactions (Moyle et al., 1982; Moyle & Vondracek, 1985; Oberdorff et al., 1998). There are strong relations between habitat availability and fish distribution and abundance in the Great Plains (Hubert & Rahel, 1989; Quist et al., 2004a; b; Brunger Lipsey et al., 2005), which suggest that interspecific interactions are less important in faunal assembly than habitat conditions. A high level of co-occurrence of morphological nearest neighbors in our study supports this contention.
Relations between larger mean SL, less flashy flow regimes, and larger streams support Schlosser (1987) who emphasized the importance of stable environments for larger fishes. However, length distributions varied among species. Some large-bodied fishes (Cyprinus carpio, H. argyritis, Carpiodes carpio carpio, I. punctatus) had higher mean SL upstream, perhaps because adults migrated upstream to spawn, but eggs or larvae drifted downstream. Similarly, smaller-bodied species that were most abundant upstream (C. lutrensis lutrensis) may have been present downstream as displaced larvae or juveniles (sensu Brown & Armstrong, 1985; Harvey, 1987). Increasing length of M. macrolepidotum downstream was clearly related to reproduction and recruitment. Low mean SL in the Upper Cheyenne River resulted from the abundance of young-of-year, whereas high mean SL in the Lower Cheyenne River resulted from the opposite (Hoagstrom et al., 2007b). Perhaps most striking was that the fauna of the smallest stream (Beaver Creek) had the highest mean SL. This was presumably a result of a very stable flow regime and deep pools that supported fish populations with a greater proportion of larger adults (Hoagstrom et al., 2007b). Hence, a general explanation for increasing mean SL with increased flow stability and discharge is lacking, but likely relates to better growth and survival in more benign habitats and reduced susceptibility to predation in deeper habitats (Schlosser, 1987; Harvey & Stewart, 1991).
The native morphological diversity of river stations was presumably higher than at present. Large-river fishes have declined and disappeared from the Cheyenne River drainage since settlement, but have not been replaced by non-natives (Hoagstrom et al., 2006; Hoagstrom et al., 2007a). Missing species such as Scaphirhynchus platorynchus, Lepisosteus platostomus, Cycleptus elongatus, and Lota lota (Hoagstrom & Berry, 2006) had extreme morphologies and presumably increased historical morphological diversity. In contrast, missing native species from creek stations (Couesius plumbeus, Notropis stramineus missuriensis; Bailey & Allum, 1962) would have increased morphological diversity less because they are relatively similar to extant species (Rhinichthys cataractae cataractae, Semotilus atromaculatus). Hence, hyperdispersion may have been less in native creek faunas.
This study indicates that increasing species diversity downstream in the Great Plains corresponds to increasing morphological diversity. Elsewhere, such increases are commonly attributed to higher habitat diversity (Gorman & Karr, 1978) or higher habitat stability (Horwitz, 1978). In our study, habitat stability decreased downstream, suggesting that increased niche diversity may account for higher morphological diversity (sensu Scarnecchia, 1988; Willis et al., 2005). Further, niche diversity in our river stations may have been underestimated because, although the small-bodied cyprinid guild increased morphological similarity in river stations, such species commonly partition habitats (Douglas, 1987). We did not measure habitat diversity directly, but our observations suggest that habitat diversity declined downstream because riffles and pools became less distinct and increasing channel width resulted in a predominance of run habitat (Hampton & Berry, 1997). Rahel and Hubert (1991) found that habitat diversity declined downstream in Horse Creek, Wyoming, a typical Great Plains stream, and concluded that either increased living space or increased habitat stability accounted for increased species diversity. Thus, higher niche diversity our in river stations may reflect increased living space (sensu Rahel & Hubert, 1991), which presumably refers to increased niche space without a measurable increase in structural complexity. Winemiller (1991) referred to this as increasing habitat volume. Hence, studies that determine how fishes of Great Plains rivers segregate niches in the absence of physical habitat structure will be necessary to further describe trophic relations within these faunas.
References
Adite, A. & K. O. Winemiller, 1997. Trophic ecology and ecomorphology of fish assemblages in coastal lakes of Benin, west Africa. Ecoscience 4: 6–23.
Bailey, R. M. & M. O. Allum, 1962. Fishes of South Dakota. Miscellaneous Publications, Museum of Zoology, University of Michigan, No. 119; 131 pp.
Baker, D. B., R. P. Richards, T. T. Loftus & J. W. Kramer, 2004. A new flashiness index: characteristics and applications to midwestern rivers and streams. Journal of the American Water Resources Association 40: 503–522.
Bayley, P. B. & H. W. Li, 1992. Riverine fishes. In Calow P. & G. E. Petts (eds), The Rivers Handbook, Hydrological and Ecological Principles, Volume 1. Blackwell Scientific Publications, Oxford: 251–281.
Belkessam, D., T. Oberdorff & B. Hugueny, 1997. Unsaturated fish assemblages in rivers of north-western France: potential consequences for species introductions. Bulletin Français de la Pêche et del la Pisciculture 344: 193–204.
Bramblett, R. G. & K. D. Fausch, 1991. Fishes, macroinvertebrates, and aquatic habitats of the Purgatoire River in Piñon Canyon, Colorado. Southwestern Naturalist 36: 281–294.
Brown, A. V. & M. L. Armstrong, 1985. Propensity to drift downstream among various species of fish. Journal of Freshwater Ecology 3: 3–17.
Brunger Lipsey, T. S., W. A. Hubert & F. J. Rahel, 2005. Relationships of elevation, channel slope, and stream width to occurrences of native fishes at the Great Plains-Rocky mountains interface. Journal of Freshwater Ecology 4: 695–705.
Cross, F. B., R. L. Mayden & J. D. Stewart, 1986. Fishes in the western Mississippi Basin (Missouri, Arkansas and Red rivers). In Hocutt C. H. & E. O. Wiley (eds), The Zoogeography of North American Freshwater Fishes. John Wiley & Sons, New York: 363–412.
Cross, F. B. & R. E. Moss, 1987. Historic changes in fish communities and aquatic habitats in plains streams of Kansas. In Matthews W. J. & D. C. Heins (eds), Community and Evolutionary Ecology of North American Stream Fishes. University of Oklahoma Press, Norman: 155–165.
Douglas, M. E., 1987. An ecomorphological analysis of niche packing and niche dispersion in stream-fish clades. In Matthews W. J. & D. C. Heins (eds), Community and Evolutionary Ecology of North American Stream Fishes. University of Oklahoma Press, Norman: 144–149.
Findley, J. S., 1973. Phenetic packing as a measure of faunal diversity. American Naturalist 107: 580–584.
Findley, J. S., 1976. The structure of bat communities. American Naturalist 110: 129–139.
Gatz, A. J. Jr., 1979. Community organization in fishes as indicated by morphological features. Ecology 60: 711–718.
Gatz, A. J. Jr., 1980. Phenetic packing and community structure: a methodological comment. American Naturalist 116: 147–149.
Gido, K. B. & J. H. Brown, 1999. Invasions of North American drainages by alien fish species. Freshwater Biology 42: 387–399.
Gido, K. B., J. F. Schaefer & J. Pigg, 2004. Patterns of fish invasions in the Great Plains of North America. Biological Conservation 118: 121–131.
Gorman, O. T. & J. R. Karr, 1978. Habitat structure and stream fish communities. Ecology 59: 507–515.
Gotelli, N. J., 2000. Null model analysis of species co-occurrence patterns. Ecology 81: 2606–2621.
Gotelli, N. J. & G. L. Entsminger, 2001a. EcoSim: Null models software for ecology. Version 7.0. Acquired Intelligence Inc. & Kesey-Bear. http://homepages.together.net/∼gentsmin/ecosim.htm.
Gotelli, N. J. & G. L. Entsminger, 2001b. Swap and fill algorithms in null model analysis: rethinking the knight’s tour. Oecologia 129: 281–291.
Gotelli, N. J. & G. L. Entsminger, 2003. Swap algorithms in null model analysis. Ecology 84: 532–535.
Hampton, D. R. & C. R. Berry Jr., 1997. Fishes of the mainstem Cheyenne River in South Dakota. Proceedings of the South Dakota Academy of Science 76: 11–25.
Harvey, B. C., 1987. Susceptibility of young-of-the-year fishes to downstream displacement by flooding. Transactions of the American Fisheries Society 116: 851–855.
Harvey, B. C. & A. J. Stewart, 1991. Fish size and habitat depth relationships in headwater streams. Oecologia 87: 336–342.
Hoagstrom, C. W. & C. R. Berry Jr., 2006. Island biogeography of native fish faunas among Great Plains drainage basins: basin scale features influence composition. American Fisheries Society Symposium 48: 221–264.
Hoagstrom, C. W., A. C. DeWitte, N. J. C. Gosch & C. R. Berry, Jr., 2007a. Historical fish assemblage flux in the Cheyenne River below Angostura Dam. Journal of Freshwater Ecology 22: 219–229.
Hoagstrom, C. W., A. C. DeWitte, N. J. C. Gosch & C. R. Berry, Jr., 2007b. Perennial-warmwater fish communities of the Cheyenne River drainage: a seasonal assessment. Proceedings of the South Dakota Academy of Science 85: 213–245.
Hoagstrom, C. W., S. S. Wall, J. P. Duehr & C. R. Berry Jr., 2006. River size and fish assemblages in southwestern South Dakota. Great Plains Research 16: 117–126.
Holliday, V. T., J. C. Knox G. L. Running IV, R. D. Mandel & C. R. Ferring, 2002. The central lowlands and Great Plains. In Orme A. R. (ed.), The Physical Geography of North America. Oxford University Press, Oxford: 335–362.
Horwitz, R. J., 1978. Temporal variability patterns and the distributional patterns of stream fishes. Ecological Monographs 48: 307–321.
Hubbs, C. L., 1940. Speciation of fishes. American Naturalist 74: 198–211.
Hubert, W. A. & F. J. Rahel, 1989. Relations of physical habitat to abundance of four nongame fishes in high-plains streams: a test of Habitat Suitability Index models. North American Journal of Fisheries Management 9: 332–340.
Hugueny, B., 1989. West African rivers as biogeographic islands: species richness of fish communities. Oecologia 79: 236–243.
Hugueny, B. & D. Paugy, 1995. Unsaturated fish communities in African rivers. American Naturalist 146: 162–169.
Hugueny, B. & M. Pouilly, 1999. Morphological correlates of diet in an assemblage of west African freshwater fishes. Journal of Fish Biology 54: 1310–1325.
Jackson, D. A., 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74: 2204–2214.
Kempton, R. A. & L. R. Taylor, 1974. Log-series and log-normal parameters as diversity discriminants for the Lepidoptera. Journal of Animal Ecology 43: 381–399.
Lamouroux, N., N. L. Poff & P. L. Angermeier, 2002. Intercontinental convergence of stream fish community traits along geomorphic and hydraulic gradients. Ecology 83: 1792–1807.
Larimore, R. W. & P. W. Smith, 1963. The fishes of Champaign County, Illinois, as affected by 60 years of stream changes. Illinois Natural History Survey Bulletin 28: 299–382.
Legendre, P. & L. Legendre, 1998. Numerical Ecology, Second English Edition. Elsevier, Amsterdam, 853 pp.
Magurran, A. E., 2004. Measuring Biological Diversity. Blackwell Publishing, Malden, Massachusetts, 256 pp.
Mahon, R., 1984. Divergent structure in fish taxocenes of north temperate streams. Canadian Journal of Fisheries and Aquatic Sciences 41: 330–350.
Minshall, G. W., K. W. Cummins, R. C. Petersen, C. E. Cushing, D. A. Bruns, J. R. Sedell & R. L. Vannote, 1985. Developments in stream ecosystem theory. Canadian Journal of Fisheries and Aquatic Sciences 42: 1045–1055.
Motta, P. J., K. B. Clifton, P. Hernandez & B. T. Eggold, 1995. Ecomorphological correlates in ten species of subtropical seagrass fishes: diet and microhabitat utilization. Environmental Biology of Fishes 44: 37–60.
Moyle, P. B. & B. Herbold, 1987. Life-history patterns and community structure in stream fishes of western North America: comparisons with eastern North America and Europe. In Matthews, W. J. & D. C. Heins (eds.), Community and Evolutionary Ecology of North American Stream Fishes. University of Oklahoma Press, Norman: 25–32.
Moyle, P.B., J. J. Smith, R. A. Daniels, T. L. Taylor, D. G. Price & D. M. Baltz, 1982. Distribution and ecology of stream fishes of the Sacramento-San Joaquin drainage system, California. University of California Publication, Zoology 115: 1–256.
Moyle, P. B. & B. Vondracek, 1985. Persistence and structure of the fish assemblage in a small California stream. Ecology 66: 1–13.
Oberdorff, T., B. Hugueny, A. Compin & D. Belkessam, 1998. Non-interactive fish communities in the coastal streams of North-western France. Journal of Animal Ecology 67: 472–484.
Oberdorff, T., B. Hugueny & J.-F. Guégan, 1997. Is there an influence of historical events on contemporary fish species richness in rivers? Comparisons between Western Europe and North America. Journal of Biogeography 24: 461–467.
Piet, G. J., 1998. Ecomorphology of a size-structured tropical freshwater fish community. Environmental Biology of Fishes 51: 67–86.
Poff, N. L. & J. D. Allan, 1995. Functional organization of stream fish assemblages in relation to hydrological variability. Ecology 76: 606–627.
Pouilly, M., F. Lino, J.-G. Bretenoux & C. Rosales, 2003. Dietary-morphological relationships in a fish assemblage of the Bolivian Amazonian floodplain. Journal of Fish Biology 62: 1137–1158.
Power, M. E. & W. E. Dietrich, 2002. Food webs in river networks. Ecological Research 17: 451–471.
Quist, M. C., W. A. Hubert & F. J. Rahel, 2004a. Elevation and stream-size thresholds affect distributions of native and exotic warmwater fishes in Wyoming. Journal of Freshwater Ecology 19: 227–236.
Quist, M. C., W. A. Hubert & F. J. Rahel, 2004b. Relations among habitat characteristics, exotic species, and turbid-river cyprinids in the Missouri River drainage of Wyoming. Transactions of the American Fisheries Society 133: 727–742.
Rahel, F. J. & W. A. Hubert, 1991. Fishes assemblages and habitat gradients in a Rocky Mountain-Great Plains stream: biotic zonation and additive patterns of community change. Transactions of the American Fisheries Society 120: 319–332.
Ricklefs, R. E. & J. Travis, 1980. A morphological approach to the study of avian community organization. Auk 97: 321–338.
Russell, G. J., 1998. Turnover dynamics across ecological and geological scales. In McKinney, M. L. & J. A. Drake (eds), Biodiversity Dynamics, Turnover of Populations, Taxa, and Communities. Columbia University Press, New York: 377–404.
Scarnecchia, D. L., 1988. The importance of streamlining in influencing fish community structure in channelized and unchannelized reaches of a prairie stream. Regulated Rivers: Research and Management 2: 155–166.
Schlosser, I. J., 1987. A conceptual framework for fish communities in small warmwater streams. In Matthews, W. J. & D. C. Heins (eds), Community and Evolutionary Ecology of North American Stream Fishes. University of Oklahoma Press, Norman: 17–24.
Schlosser, I. J., 1990. Environmental variation, life history attributes, and community structure in stream fishes: implications for environmental management and assessment. Environmental Management 14: 621–628.
Sheldon, A. L., 1968. Species diversity and longitudinal succession in stream fishes. Ecology 49: 193–198.
Stone, L. & A. Roberts, 1990. The checkerboard score and species distributions. Oecologia 85: 74–79.
Trautman, M. B., 1981. The Fishes of Ohio with Illustrated Keys, Revised Edition. Ohio State University Press, Columbus, 782 pp.
Vadas R. L. Jr. & D. J. Orth, 1998. Use of physical variables to discriminate visually determined mesohabitat types in North American streams. Rivers 6: 143–159.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Watson, D. J. & E. K. Balon, 1984. Ecomorphological analysis of fish taxocenes in rainforest streams of northern Borneo. Journal of Fish Biology 25: 371–384.
Willis, S. C., K. O. Winemiller & H. Lopez-Fernandez, 2005. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142: 284–295.
Winemiller, K. O., 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecological Monographs 61: 343–365.
Winemiller, K. O., L. C. Kelso-Winemiller & A. L. Brenkert, 1995. Ecomorphological diversification and convergence in fluvial cichlid fishes. Environmental Biology of Fishes 44: 235–261.
Winston, M. R., 1995. Co-occurrence of morphologically similar species of stream fishes. American Naturalist 145: 527–545.
Xie, S., Y. Cui & Z. Li, 2001. Dietary-morphological relationships of fishes in Liangzi Lake, China. Journal of Fish Biology 58: 1714–1729.
Acknowledgments
Federal Aid in Sport Fish Restoration funds administered by South Dakota Game, Fish and Parks (Project Number F-21-R and F-15-R) supported this research. We thank A. DeWitte, N. Gosch, J. Kral, M. Mangan, R. Sylvester, and R. Rasmus for field assistance and M. Barnes and J. Duehr for logistical support. S. Wall prepared the map (Fig. 1). M. Pyron and M. Collyer provided editorial comments on an early version of this article. This study was only possible through the generosity of five private landowners who allowed us access to their land. An anonymous reviewer provided comments that greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: J. A. Cambray
Rights and permissions
About this article
Cite this article
Hoagstrom, C.W., Berry, C.R. Morphological diversity among fishes in a Great Plains river drainage. Hydrobiologia 596, 367–386 (2008). https://doi.org/10.1007/s10750-007-9110-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9110-5