Abstract
The Lower Mekong River and its three major tributaries, the Sekong, Sesan, and Srepok rivers, are systems with high aquatic biodiversity that now face impacts from new hydroelectric dams. Despite the ecological, economic, and cultural importance of the freshwater biota, knowledge about aquatic ecology in this region remains poor. We used morphological and stable isotope data to explore how fish functional and trophic diversity vary between the four rivers that comprise the Mekong-3S river system. During our field surveys, the Sesan had experienced greatest flow alteration from dams and had lowest taxonomic and functional diversity, with species less packed and less evenly dispersed within morphological space compared to the other rivers. The Sekong had greatest functional diversity, with species less packed in morphological space. Species in the Mekong and Srepok were more evenly distributed in morphological space and had intermediate levels of functional diversity. Isotopic niche diversity in the Sesan did not appear to be significantly different from the Srepok and Sekong rivers. Conversely, the more species-rich Mekong fish assemblage encompassed a greater isotopic space with species less packed and evenly distributed. Greater trophic redundancy was observed amongst fishes of the 3S rivers than the Mekong. Species functional redundancy could buffer river food webs against species loss caused by dams; however, additional traits and niche dimensions should be evaluated to test this hypothesis. Overall, morphological and isotopic evidence from the Mekong and 3S rivers indicate that river impoundment and flow regulation function as an environmental filter that reduces fish functional diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical floodplain-rivers are dynamic and spatially heterogeneous systems with seasonal hydrological regimes and high phylogenetic and functional diversity (Lévêque et al. 2008; Agostinho et al. 2016; Toussaint et al. 2016; Chea et al. 2017). Nevertheless, tropical rivers and their biotas face growing threats from changing water and land uses, hydropower development, and poor governance (Winemiller et al. 2016; Pelicice et al. 2017; Oliveira et al. 2018). What remains poorly understood is how species loss caused by anthropogenic impacts affects functional diversity and ecosystem services in these aquatic systems. Dams modify hydrology, including less predictable flows during dry and wet seasons (Whyatt and Baird 2007; Pokhrel et al. 2018). Dams also change patterns of ecosystem production and the distribution of biodiversity in space and time (Nilsson et al. 2005; Agostinho et al. 2016). Alteration of the natural flow regime affect fish populations to varying degrees depending on their life history strategies (e.g., timing and amount of reproductive effort) and ecological niches (e.g., diet, habitat use) (Mims and Olden 2013; Lima et al. 2018; Oliveira et al. 2018). Agostinho et al. (2016) suggested that dams in Paraná River Basin, Brazil, act as strong environmental filters on reproductive guilds, and this filter strongly affects fishes that undergo seasonal migrations to spawning habitats located upstream, and consequently requires free-flowing stretches. Populations of large migratory species (e.g., dourado Salminus brasiliansis, pacu Piaractus mesopotamicus, and large catfishes such as pintado Pseudoplatystoma corruscans and jaú Zugaro jahu) in the Tietê, Grande and Paranapanema rivers, Paraná Basin, essentially were extirpated after dam construction (Agostinho et al. 2007). Although river damming is expected to affect aquatic fauna as a whole, impacts to large migratory species generally are apparent when their yields decline in commercial and subsistence fisheries (Petrere 1996). Furthermore, environmental changes caused by dams often promote the established of non-native species, particularly those well-adapted to lentic conditions (Johnson et al. 2008).
The Mekong River is the largest river in Southeast Asia and supports one of the world’s most important inland fisheries, providing livelihoods and food security for millions of people (Baran et al. 2013; Hap et al. 2016). Recent research indicates that hydropower development in Southeast Asia is changing the river’s flow regimes with negative impacts to fish stocks in the lower Mekong Basin, which includes three major tributaries referred to as the 3S rivers (Srepok, Sekong, and Sesan) (Whyatt and Baird 2007; Ziv et al. 2012; Piman et al. 2013). The 3S rivers support at least 89 migratory fishes (Baran et al. 2013) and although studies evaluating the status of fish diversity of the lower Mekong and 3S river system before dam operations are lacking, some early reports suggest that the Yali Falls Dam constructed on the upper Sesan River began to modify river hydrology and water quality as early as mid-1996 (Baird et al. 2002; Hirsch and Wyatt 2004). Baird and Meach (2005) reported a dramatic decline in fish catches in the Sesan River following construction of the Yali Falls Dam, with large migratory species most affected. Lack of scouring floods during the wet season resulted in infilling of deep channel pools, important habitats for many of the large native fishes (Whyatt and Baird 2007). Recent modeling studies (Ziv et al. 2012; Piman et al. 2013; Pokhrel et al. 2018) have estimated impacts of proposed dams on hydrology, fish production and biodiversity in the Lower Mekong Basin. Results from these models indicate that flow regulation by upstream dams will disrupt floodplain ecology in the Lower Mekong, including the Tonle Sap River and Lake and tributaries within the Mekong Delta (Pokhrel et al. 2018).
Whereas assemblage taxonomic composition and diversity commonly have been shown to change in response to flow alteration, assessments based on functional traits could improve our understanding of species vulnerability to these impacts (Arantes et al. 2019). For instance, Oliveira et al. (2018) found that damming of the Upper Paraná River (Brazil) reduced fish functional diversity, with fish assemblage biomass shifting from dominance by large migratory fishes that are trophic specialists to smaller non-migratory species that tend to be trophic generalists. A seven-year fish monitoring program in the lower Mekong River Basin concluded that alteration of natural flow regimes by upstream hydropower dams affected fish life cycles and spatial and temporal distributions of fish species (Ngor et al. 2018). For instance, surveys conducted during 2007–2010 showed that fish assemblages in the Sekong and Srepok rivers included large, migratory species that spawn within the river channel (e.g., carps such as Hypsibarbus lagleri and H. malcolmi, and shark catfishes such as Helicophagus waandersii and Pangasius conchophilus), and surveys conducted after 2011 were dominated by small minnows and carps that are habitat generalists. In contrast, the fish fauna of the Sesan River changed relatively little during the same time period, and was dominated by small minnows and carps, presumably a reflection of impacts from upstream dams constructed prior to 2007. Ngor et al. (2018) stated that flow alteration caused by dams likely was responsible for not only the low diversity observed in the Sesan River, but for the overall trend of low species diversity and abundance throughout the Lower Mekong after 2010 when there was an increase in hydropower dam construction in the 3S system. In this regard, dams may function as environmental filters by altering flow regimes and fluvial geomorphology that reduce migratory fish stocks and benefit more sedentary species, a trend observed in other systems worldwide (Liermann et al. 2012; Li et al. 2013; Santos et al. 2017; Oliveira et al. 2018). The impacts of dams on fisheries of the Mekong-3S system will require long-term monitoring, and analysis of functional diversity could strengthen inferences about cause and effect.
Here, we analyze fish morphology and isotopic ratios of carbon and nitrogen to evaluate functional diversity of species from the orders Cypriniformes, Perciformes and Siluriformes, species-rich clades within the Lower Mekong-3S system. Species within these orders span a great variety of life history strategies, morphologies and ecological niches (Baran et al. 2011, 2013; Ou and Winemiller 2016; Ou et al. 2017). Field surveys were conducted during the dry season (January 2010) when river discharge is low, aquatic habitat is reduced, and fish densities are relatively high (Ou and Winemiller 2016). Multiple metrics were evaluated to make inferences about the size and structure of morphological and isotopic spaces occupied in the four rivers. We anticipated that assemblage morphological and isotopic spaces would vary among rivers in relation to environmental differences. More specifically, we predicted that fish assemblage in the Sesan River, which is more severely impacted by dams, would have low functional diversity and lack large migratory fishes compared to other rivers in the region.
Methods
Study area
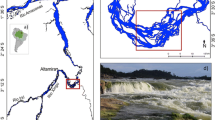
The Mekong-3S river system (Fig. 1) is located in the northeastern Cambodia and was designated a Ramsar wetland of international significance owing to its high value for conservation of biodiversity, including migratory fishes (Try and Chambers 2006) [see Ou and Winemiller (2016) and Ou et al. 2017 for more descriptions of these rivers]. The 3S rivers (Sekong, Sesan, Srepok) are Mekong tributaries that drain Southern Laos, Vietnam’s central highlands, and north-eastern Cambodia. The rivers join the Mekong near Stung Treng, a provincial capital in Cambodia. The 3S rivers contribute ca. 25% of the Mekong’s annual discharge and influence the hydrology of the Tonle Sap, the largest lake in southeast Asia with fringing wetlands that supports one of the world’s major inland fisheries (MRC 2005).
Fish surveys were conducted at locations within the lower Mekong River mainstream at Stung Treng (13.579383°N, 105.994366°E), the Sekong River at Siem Pang (14.11434°N, 106.39104°E), the Sesan River at Veurn Sai (13.94585°N, 106.79701°E) and the Srepok River at Lomphat (13.47508°N, 106.99683°E) during January (dry season) of 2010 (Fig. 1). Local habitat conditions differed primarily in substrate composition, hydrology, and water depth (Table 1), and local environmental conditions, including climate, land cover and land use, were similar. Construction of hydropower dams in the Lower Mekong Basin has already altered flow regimes (MRC 2015; Sithirith 2016), causing shifts in the fish composition (Baran 2006, 2013; Ngor et al. 2018). Among the four rivers, the Mekong River at Stung Treng appeared to be the least altered by hydropower dams and retained a relatively natural seasonal flow pattern during the period of our field study (MCR 2010). The 3S rivers had flow regimes that were less predictable due to effects from dams operating upstream (MRC 2010), and this especially was the case for the Sesan River with hundreds of small hydropower dams already operating by the time of our field survey in 2010 (Baird and Meach 2005; Baran et al. 2011, 2013; MRC 2015). When compared to the Sekong and Srepok rivers, the Sesan River had lower flows and shallower water during the dry season (Baran et al. 2011). The largest dam on the Sesan, Yali Falls Dam (720 MW), is located in Vietnam approximately 80 km upstream from the Cambodian border and blocks fish migration between upper and lower reaches. At the time of our field study (2010), the Yali Falls Dam had a major influence on hydrology of the lower Sesan River. In more recent years, construction was initiated on the Sesan 2 hydroelectric dam near the Sesan’s confluence with the Srepok River. This new dam will not only submerge an extensive reach of the Lower Sesan and riparian areas, but also will block fish migration between the Lower Mekong and Sesan basins.
Sample collections
Samples of important basal production sources (common riparian plants, benthic filamentous algae, seston [phytoplankton and other suspended organic matter]) and fishes were collected from a reach of approximately 3 km in each of the four rivers [see Ou and Winemiller 2016 and Ou et al. 2017 for more details; Fig. 1]. In each survey reach, fishes were collected from all available habitat types (e.g., sandbanks, pools, riffles) using multiple gears including seines, cast nets, and dip nets. Additional fish specimens were obtained from local fishers who primarily fished with gillnets and baited hooks (deep areas in main channels). Given that multiple gear types were used to survey all habitat types with similar effort in each reach, samples were assumed to reflect the composition of the local communities during the dry season. Specifically, we assumed that all common species were represented in samples, and some of the rare species were not collected. After collection, fishes that were alive were anesthetized with clove oil and, except for 3–5 adult specimens of each species that from which tissue samples were later obtained for stable isotopic analysis (see protocol in the Stable Isotope Analysis section), preserved in 15% formalin.
Morphological analysis
Morphological measurements were made on 328 fish specimens (Mekong: 100, Sekong: 99, Sesan: 56, and Srepok: 73, Appendix 1). Only species belonging to three species-rich orders (Cypriniformes, Perciformes and Siluriformes) were included for analysis. Our surveys yielded 163 species within these three orders, which represents almost half of the total number of species reported by Ngor et al. (2018) from a 7-year fish monitoring program in the Lower Mekong Basin. As expected, the Sesan River yielded the lowest number of species, and this is not attributed to lower sampling effort (which was similar at all four study sites), but instead seems to be related to severe flow regulation from dozens of hydropower dams operating upstream in the Sesan Basin, including the Yali Falls Dam. Whenever possible, we measured up to five adult specimens per species. Twenty-five morphological characters (Appendix 2) with well-known functions related to locomotion, feeding, and/or habitat use were measured to the nearest 0.01 mm using a digital caliper following methods described in Montaña and Winemiller (2013). Among the 28 trait measurements, 21 that were descriptors of body shape and fin size were converted to proportions (Trait measurement/Standard length) to remove the effect of body size differences (Montaña and Winemiller 2013). Use of ratios or proportions top removal the influence of body size on shape can introduce allometric bias; however, allometric influences should be negligible for interspecific comparisons based on samples with limited intraspecific size variation (Winemiller 1991; Montaña and Winemiller 2013). Ratios as descriptors of fish body and fin shape components have robust functional interpretations with regard to locomotion, habitat use, and feeding (Gatz 1979a, b; Webb 1984).
Stable isotope analysis
Fish muscle tissue for stable isotope analysis was taken from three to five adult specimens of each species used for the morphological analysis. Muscle tissue was taken from the flank near the base of the dorsal fin. We also obtained three to five tissue samples for each major basal production source in each river. Fish tissues and basal sources were preserved in salt and processed as described by Arrington and Winemiller (2002). In the laboratory, the samples were soaked in distilled water for 5 h, rinsed, and dried in an oven at 60 °C for 48 h. After drying, samples were ground to a fine powder with a mortar and pestle, and then stored in clean glass vials. Subsamples of 1.5 to 3 mg for each ground sample were weighed and packaged into Ultra-Pure tin capsules (Costech) and sent to the Stable Isotope Ecology Laboratory, University of Georgia, for measurement of stable isotope ratios of carbon (13C/12C) and nitrogen (15N/14N). Stable isotope ratios were expressed in standard delta (δ) notation and reported in parts per thousand (‰) standardized in relation to reference material (Pee Dee Belemnite for C, and atmospheric nitrogen for N) and reported as δX = [(Rsample/Rstandard) – 1)]×103, where R = 13C/12C or 15N/14N (the ratio of heavy and light stable isotopes of carbon and nitrogen) [see Ou and Winemiller (2016) for more details about stable isotope analysis].
Statistical analysis
Principal components analysis of mixed data (PCAmix; Chavent et al. 2014) was performed on the morphological data to describe morphological spaces occupied by fish assemblages and to examine patterns of functional diversity across rivers and taxonomic orders. PCAmix was used rather than principal components analysis (PCA) because one of the morphological variables, mouth orientation, was categorical. A bi-plot of δ13C and δ15N values of fish species was used to compare trophic spaces of assemblages in the four rivers. Here it is assumed that δ13C reflects how material from various production sources is assimilated into consumer tissue, and δ15N reflects the vertical trophic position of the consumer as well as assimilation of production sources [for more details see Ou and Winemiller 2016]. Interpretation of animal isotopic patterns as representations of trophic niche variation (Layman et al. 2007) can be confounded if there is large isotopic variation among basal production sources supporting individuals collected from different places and/or time periods (Hoeinghaus and Zeug 2008). For our analysis, the use of stable isotope ratios to infer trophic diversity is justified because the mean isotopic ratios for major basal sources were highly consistent among the four survey locations during the dry season (see Fig. 5).
Species averages were calculated for morphological measurements and isotopic data, and these mean values were used to analyze the assemblage structure based on patterns of interspecific variation. Given the low number of specimens examined for each species (N = 3 to 5), our analysis does not account for intraspecific variation. Six metrics were used to describe species dispersion within assemblage morphological space expressed in two dimensions (Dim 1 and Dim 2 represented by the first two PCAmix axes) and isotopic space (δ13C and δ15N) (Layman et al. 2007) of assemblages from each river: 1) dY range (Morphology: Dim2 range; Isotopes: δ15N range [NR], a representation of the vertical structure within a community or food web, i.e. larger values of NR suggest a greater degree of trophic diversity); 2) dX (Morphology: Dim1 range; Isotopes: δ13C range [CR], a representation of basal resources diversity, i.e. increased CR would be expected in food webs in which there are multiple basal resources with varying δ13C values); 3) total area (TA), a measure of the total amount of morphological or isotopic niche space occupied (i.e., the magnitude of the morphological or isotopic diversity); 4) mean centroid distance (CD), an estimate of average degree of morphologic/isotopic diversity; 5) mean of nearest neighbor distance (MNND), a measure of the relative density of species packing within the assemblage space (i.e., smaller MNND values indicate greater morphologic/trophic similarity); and 6) standard deviation of nearest neighbor distance (SDNND), an estimate of evenness, with lower SDNND values indicating a more even distribution of species within the morphologic/isotopic space. We used scree plots to select the most relevant PCAmix axes that explain variation in the morphological dataset. Dominant PCAmix axes for morphological data do not represent the same functional gradients as the dominant axes derived independently based on δ13C and δ15N data. Given differences in sample size for species and sampling effort between rivers, we compared these six metrics using rarefaction curves based on 10,000 random draws without replacement. This bootstrapping procedure was conducted without replacement to avoid the possibility that rarefactions with replacement would generate communities with species duplication.
Morphological and isotopic spaces were estimated using standard ellipses areas (SEA) based on Bayesian inference (Jackson et al. 2011), an approach similar to bootstrapping techniques. The Bayesian approach generates a SEA distribution (the posterior) that reflects the uncertainty (larger for smaller sample sizes) associated with the sampling process, and that may affect ellipse size, location, and shape. The posterior distribution generated was then used to compare statistically the differences between SEA of different rivers (Jackson et al. 2011). To avoid potential bias caused by different sample sizes (i.e., numbers of species in local taxonomic assemblages), the correction proposed by Jackson et al. (2011) was applied to estimates of standard ellipse area (SEAc).
Permutational multivariate analysis of variance (PERMANOVA) was performed to test whether fish assemblages from different rivers occupied significantly different morphospaces and isospaces. If the PERMANOVA result was significant, pairwise tests were conducted. In addition, the SEA overlap index was computed to estimate the percentage of morphological space and isotopic space that overlapped between pairs of river assemblages. The SEA overlap index was calculated as the ratio between overlap area and non-overlap area (i.e., SEA12 overlap / [(SEA1 + SEA2)-SEA12 overlap] of the ellipses. A SEA index <1 indicates that the area of overlap is less than the area of non-overlap, a value >1 indicates that the overlap area is larger than the non-overlap area, and a value = 1 indicates complete overlap between the ellipses. For interpretations of these analyses, we assumed that isotopic signatures of basal resources did not differ greatly between rivers during the dry season, which is confirmed by findings presented in Ou and Winemiller (2016). We also assumed that between-river intraspecific variation in morphology was negligible in relation to interspecific variation within rivers. All analyses were conducted in R (R Core Team 2018). PCAmix was computed in the package PCAmixdata (Chavent et al. 2017), and SEAs and community metrics proposed by Layman et al. (2007) were computed using the SIBER package (Jackson et al. 2011). The packages vegan (Oksanen et al. 2017) and pairwiseAdonis were used to compute PERMANOVA and pairwise comparisons, respectively.
Results
Relationships of morphological space among rivers
Results from the PCAmix revealed that local assemblages from the Mekong and Sekong rivers were more similar in morphospace than those from the Sesan and Srepok rivers (Fig. 2). Mekong and Sekong assemblages had more overlap in morphospace (82%) than each had with the Sesan and Srepok assemblages (Mekong vs Sesan = 48%, Mekong vs Srepok = 73%, Sekong vs Sesan = 43%, Sekong vs Srepok = 62%; Fig. 2). However, results from the PERMANOVA did not find statistically significant differences in the morphological spaces occupied by the four assemblages (F3 = 1.30, p = 0.28).
Results of principal components analysis of mixed data with 25 morphological traits related to habitat use and trophic ecology of fishes from the Lower Mekong River and 3S rivers. Symbols represent fish families and colors represent orders. Each symbol represents the average value of a species within a family and order. Text below axes show morphological traits with highest loadings
The two first PCAmix axes explained 40.47% of the total morphological variation of fish species, independently of sampling location. The first axis (Dim1 = 27.39% of variation) described a gradient contrasting species with elongated bodies, long caudal peduncles, long anal fins and superior mouths, with species having deeper bodies, shorter caudal peduncles, longer pectoral and dorsal fins, and terminal mouths. Species of the family Siluridae (Siluriformes), which were common in the Mekong and Sekong Rivers, have elongate bodies, dorso-ventrally flat bodies, and superior mouths, and had low scores on Dim1 (< −2); whereas cyprinids (Cypriniformes), many of which have deep bodies and terminal mouths, were common in all rivers and had high scores on Dim1 (> 2). The second axis (Dim2 = 13.08%) described a gradient contrasting fishes with deeper caudal peduncles, longer heads, deeper bodies, superior mouths and larger eyes, with species having narrower bodies, smaller heads, smaller eyes and subterminal mouths. Species of the families Cyprinidae and Osphronemidae (Perciformes) were differentiated from Cobitidae (Cypriniformes) and Sisoridae (Siluriformes) along the gradient defined by Dim2. Cobitids and sisorids are characterized by small bodies, small heads and mouths, and their bodies are relatively elongate compared to cyprinids and osphronemids. Overall pattern suggests that species dispersion within morphological space differed among rivers (Fig. 2).
Analysis based on standard ellipse area indicated that the Mekong and Sekong assemblages had greatest morphological dispersion (SEAc = 17.75‰ and 20.70‰, respectively) and the Sesan assemblage had the lowest dispersion (SEAc = 12.97‰) (Figs. 2 and 3). Rarefaction curves indicated an increase of dY range, dX range, and TA with increasing number of species (Fig. 4). dY range was similar among the four rivers independent on sample size. Conversely, the Sekong assemblage had the highest dX range, the Sesan assemblage had the lowest values of dX range, and Mekong and Srepok assemblages had a greater overlap of dX range, which were slightly lower than the Sekong and higher than the Sesan assemblage. A smaller dX range for the Sesan assemblage, when compared to those from other rivers, could be attributed to the lack of species in the family Siluridae, as shown in Fig. 2 (Dim 1). According to the rarefaction curves, the Mekong and Sekong assemblages had greater TA values than those of the Sesan and Srepok, and this difference is greater as the difference in the number of species in samples increases. CD did not appear to vary with sample size. However, despite some uncertainty in CD estimates (e.g., large coefficient intervals, CI), there was a trend for the CD metric in which Sekong > Mekong > Srepok > Sesan. Both MNND and SDNND decreased with increasing numbers of species. MNND values tended to be similar, with the exception of the Sekong assemblage that had higher MNND than those of the Srepok and Mekong. The Sesan assemblage had higher SDNND than the other assemblages (Fig. 4).
Morphologic (a) and isotopic spaces occupied by fish assemblages from the Mekong and 3S rivers. Box plots represent 50, 75, and 95% Bayesian credibility intervals. Solid black dots represent mean standard ellipse areas (SEA) and asterisks represent SEAs calculated using the correction for sample size
Comparison of isotopic spaces and dispersion among rivers
Overall, most fishes had carbon isotopic signature values that reflected assimilation of material from a combination of C3 plants, seston and benthic algae (Fig. 5). The Mekong fish assemblage occupied a distinct isotopic space (both δ13C and δ15N) compared with the other assemblages (Fig. 5), and this was further supported by the PERMANOVA (F3 = 3.04, p < 0.01). Results from PERMANOVA post-hoc pairwise comparisons revealed significant differences in isotopic spaces occupied by assemblages of the Mekong and Sesan (F3 = 4.60, p < 0.02; overlap = 0.43), Sekong and Sesan (F3 = 7.60, p < 0.03; overlap = 0.42), and Srepok and Sesan (F3 = 3.86, p < 0.005; overlap = 0.30), but no significant differences were observed between assemblages of the Mekong and Sekong (F3 = 1.65, p = 0.20; overlap = 0.43) or Mekong and Srepok rivers (F3 = 0.54, p = 0.54; overlap = 0.74). The analysis of assemblage isotopic space based on SEAc confirmed that assemblages in the Mekong River had greatest dispersion (SEAc =10.57‰; Fig. 3), and assemblages in Sekong had the lowest dispersion (SEAc = 6.69‰; Fig. 3).
Rarefaction curves for isotopic data revealed a pattern similar to that obtained for morphological data, in which CR, NR, and TA metrics increased with number of species, MNND and SDNND decreased with number of species, and CD was relatively constant in relation to number of species in the assemblage (Fig. 6). Nitrogen isotopic range (NR) was greater for Mekong and Srepok assemblages (Fig. 6), and although the NR of the Sekong and Sesan assemblages indicated some overlap, the vertical trophic structure (indicated by NR) of the Sekong assemblage was smaller compared to the others, perhaps indicating shorter average food chain length. Carbon isotopic range (CR) was greatest for the Mekong River, and CR values were similar for the 3S-river assemblages (Fig. 6), suggesting fishes in those rivers had assimilated carbon from a less diverse array of basal resources. The isotopic TA of assemblages in the Mekong and Srepok rivers was large compared to the Sekong and Sesan rivers, whereas isotopic CD values were similar among the four rivers (Fig. 6). Assemblages in the Mekong River had higher isotopic MNND and SDNND values compared to the 3S assemblages. The Sekong assemblage had slightly lower MNND and SDNND than the Srepok assemblage, indicating that assemblages in the Sekong were more evenly packed within isotopic space.
Discussion
Fish assemblages of the Lower Mekong and 3S rivers shared many fish species, although a notable exception was the absence of migratory catfishes (e.g., Pangasidae) and carps (e.g., Cyprinidae) (Dugan et al. 2010; Baran et al. 2013) in our Sesan sample. Also, endangered species were completely absent in the samples of all rivers. Despite considerable compositional similarity, our findings for species from three dominant fish orders in the region revealed some morphological and isotopic differences in assemblage structure during the dry season. Our prediction that the Sesan River, which is the most strongly altered by dams, would have lower species dispersion in morphological space when compared to the Mekong, Sekong and Srepok rivers was supported by our comparison of SEAs. Although species packing within morphospace was similar among river fish assemblages, species in the Sesan assemblages were less evenly distributed. Our survey reach of the Sesan lacked deep pools and instead was uniformly shallow water with deep sand substrate. Dam development in the Mekong and 3-S river system has reduced flows during the wet season, with a decline in flow pulses that maintain deep pools by scouring, suspending and transporting sediments (Conlan et al. 2008). Dams trap sediments, which can lead to impacts, such as bank erosion and altered water quality and nutrient dynamics (Kondolf 1997). Therefore, dams reduce fish abundance and diversity not only by disruption of river connectivity affecting fish migration, but also by altering sediment and nutrient dynamics. The Sesan River’s shallow and relatively homogeneous in-stream habitat appears to act as an environmental filter that excludes several functional groups from the fish assemblage.
The most common fishes in the Sesan were small and medium-sized, non-migratory or short-distance migrants. Notably, large carps (Cyprinidae) and catfishes (Pangasidae, Siluridae) were absent from our Sesan sample. Some species that commonly inhabit floodplains and tolerate warm temperatures and aquatic hypoxia, such as airbreathing snakeheads (Channa spp.), climbing perch (Anabas testudineus) and walking catfishes (Clarias spp.) were captured from marginal habitats of the Sesan during the dry season. Two recent investigations (Ou and Winemiller 2016; Ngor et al. 2018) also inferred that hydropower impacts to the Sesan River filters fish community assembly in such as way that small cypriniforms (minnows and carps) are more common while large migratory fishes that are dependent on seasonal flood pulses are rare or are absent. Many small minnows and carps are habitat and trophic generalists that are less dependent on flow conditions to complete their life cycles than are large migratory carps and catfishes. Also, reservoirs provide expanded habitat for certain species adapted for lentic conditions. It is noteworthy that no exotic lentic species were found, which is usually common in other dammed basins worldwide (Johnson et al. 2008).
Species ordination in morphological space (PCAmix) revealed functional differences among assemblages in the four rivers. Differences were associated with morphological gradients inferred to be associated with swimming mode/performance and habitat use (e.g., body depth, mouth and eye position, caudal peduncle and fin dimensions). The Sesan River’s regulated flows apparently have not excluded many of the small benthic cypriniforms and siluriforms that are native in that system. Fish traits that were common in the Sesan assemblage were small body with streamlined shape, relatively short fins and caudal peduncles, and small eyes. The Sekong and Mekong assemblages, in particular, had more large carps (e.g., Hypsibarbus malcolmi, Labeo chrisophekadion, Cyclochilichthys mekongensis) and catfishes (e.g., Pangasius larnaudii, Pangasius conchophilus, Wallago attu) that are considered migratory (Appendix 1) and species of high conservation value (Baran et al. 2013). Many of the migratory catfishes within the families Siluridae and Pangasidae have morphological adaptations to lotic conditions (e.g., relatively elongated bodies and long caudal fins with high aspect ratios). Some of the large migratory carps have streamlined bodies, but some have relatively deep, muscular bodies and broad, forked caudal fins (e.g., L. chrysophekadion), traits that facilitate sustained powerful swimming as well as lateral maneuverability (Webb 1984). Perciform fishes were more common in the Mekong River and tended to have intermediate positions along the dominant morphological gradient. Perciforms encompass diverse body forms, ranging from fusiform (e.g., climbing perch, Anabas testudineus) to relatively deep-bodied (e.g., Mekong tiger perch, Datnioides undecimradiatus). This diversity in fish body shapes likely reflects use of diverse habitats and food resources in the Lower Mekong River. The most common perciform fishes in the Sekong and Sesan were the climbing perch and snakeheads, these species generally were captured from habitats near the channel margins where currents are slower (Baran 2006; Baran et al. 2013; Ngor et al. 2018).
The Sesan assemblage had MNND values for morphology that were similar to values for assemblages from other rivers, but its SDNND was higher, indicating a less even distribution of species. Lack of evenness in nearest neighbor distance could indicate lower importance of biological interactions (and/or ecological release) relative to abiotic factors in structuring the fish assemblage in this impacted river (Montaña et al. 2014), however, this is merely speculation given that species interactions were not examined directly in this study.
Isotopic data revealed less distinct between-assemblage differences than morphological data. Variation in isotopic space of fish assemblages did not appear correlated with flow alteration, and SEAs was greater for the Sesan and Srepok rivers compared to the Mekong River. As expected, the Mekong assemblage occupied a larger isotope space, with species being more evenly spaced, when compared to other rivers. Nonetheless, there was considerable overlap in isotopic space among all assemblages. This finding is consistent with an earlier study’s conclusion that similar production sources support fish biomass in the Lower Mekong and 3S rivers during the dry season (Ou and Winemiller 2016). Importantly, all three of the fish orders included in the present study have species within the four assemblages that span a broad spectrum of trophic niches (Ou et al. 2017).
Although the Sesan assemblage had comparatively low species diversity and many small fishes, it included several cyprinids and catfishes with low trophic positions (indicated by low δ15N) and cobitids with high trophic positions (high δ15N). The range of carbon isotopic values in fishes from the Sesan was not significantly different when compared to those in the Sekong and Srepok rivers. If fishes in these 3S rivers assimilate carbon from the same basal sources during the dry season (i.e., seston and algae primarily; Ou and Winemiller 2016), then it is possible that their assemblage carbon isotopic distributions would not differ significantly regardless of assemblage composition. For example, common piscivorous fishes, such as Hampala spp. and Channa spp., had δ13C values that were similar (~ −23‰) in all four rivers. The detritivorous/algivorous cyprinid Labeo chrysophekadion also had similar δ13C (~ −26‰) across all rivers. Small, insectivorous cyprinids (e.g., Rasbora spp.), which are important prey for piscivores, also had 13δC values that were similar across rivers (~ 23‰). The range for assemblage 13δC was greater for the Mekong assemblage compared to those of the other rivers, which suggests that the Mekong had greater basal resource diversity, more isotopic variation among basal sources associated with higher habitat heterogeneity, or greater trophic diversity within its more species-rich fish assemblage. Fish assemblages in the Sesan contained fewer piscivorous species than the other rivers, yet the distribution of δ15N values was similar to the other river fish assemblages. While other studies (Ou and Winemiller 2016) also suggest that most fishes inhabiting the Sesan River channel during the dry season are strongly supported by trophic pathways originating from seston and benthic algae, more extensive surveys of sources and consumers over time and space could reveal greater variation and potential differentiation of trophic diversity.
Despite having relatively high taxonomic and morphological diversity, the Sekong fish assemblage did not have high isotopic diversity, as indicated by its low value for SEAc. This mismatch between morphological and isotopic results could be caused by several factors, including: 1) certain species with divergent morphologies are functionally equivalent (many-to-one hypothesis; Wainwright et al. 2005); 2) a pulse in the availability of one or more profitable food resources results in their exploitation by multiple species despite interspecific differences in their functional traits (Liem 1990; Robinson and Wilson 1998); and 3) isotopic similarity of various resources supporting fish biomass within and among rivers. When compared to the other assemblages, the Sekong assemblage had a relatively short nearest neighbor distance and less even spacing of species within isotopic space, which suggests greater trophic functional redundancy (i.e., many species with similar trophic niches) in this assemblage.
During the dry season, certain aspects of functional diversity of the Sesan River fish assemblage were found to be divergent when compared to assemblages of the other three rivers that were less impacted by dams. Our findings are consistent with those from prior studies (Baran et al. 2013; Ngor et al. 2018) that inferred dams and flow regulation have reduced fish taxonomic diversity in the Sesan River. Our analysis additionally reveals that this comparatively low taxonomic diversity also is associated with lower functional diversity. Findings from the current study contribute to our understanding of how fish functional and isotopic diversity change in response to flow regulation. Future research should evaluate assemblage trait distributions during the wet season when fish densities are lower and availability of most habitats and certain resources is greater. Importantly, more research is needed within the Lower Mekong and its sub-basins as they continue to undergo hydropower development. A major challenge for future research is to identify and analyze sets of traits with strongest responses to environmental variation and interpretability with regard to niche differentiation and community assembly (Fitzgerald et al. 2017). Given the rapid pace of hydropower development in the Mekong Basin, there is an urgent need for field research to aid assessments and improve knowledge that can help stem the loss of biodiversity and associated ecosystem functions.
References
Agostinho AA, Pelicice FM, Petry AC, Gomes LC, Júlio HF (2007) Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquatic Ecosystem Health & Management 10 (2):174–186
Agostinho AA, Gomez LC, Santos NC, Ortega JC, Pelicice FM (2016) Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fish Res 173:26–36. https://doi.org/10.1016/j.fishres.2015.04.006
Arantes CC, Fitzgerald DB, Hoeinghaus DJ, Winemiller KO (2019) Impacts of hydroelectric dams on fish and fisheries in tropical rivers through the lens of functional traits. Curr Opin Environ Sustain 37:28–40. https://doi.org/10.1016/j.cosust.2019.04.009
Arrington DA, Winemiller KO (2002) Preservation effects on stable isotope analysis of fish muscle. T Am Fish Soc 131:337–342. https://doi.org/10.1577/1548-8659(2002)131<0337:PEOSIA>2.0.CO;2
Baird IG, Meach M (2005) Sesan River fisheries monitoring in Ratanakiri province, northeast Cambodia: before and after construction of the Yali falls dam in the central highlands of Vietnam. 3S Rivers Protection Network and the Global Association for the People and the Environment, Ratanakiri
Baird I, Baird M, Chum MC, Kim S, Nuon M, Phat S, Phouy BN, Prom S, Ros S, Rushton H, Sia P (2002) A community-based study of the downstream impacts of the Yali falls dam along the se san, Sre Pok and Sekong rivers in Stung Treng Province, Northeast Cambodia. Fisheries and Forestry Office, Stung Treng
Baran E (2006) Fish migration triggers and cues in the lower Mekong Basin and other freshwater tropical systems. Mekong River Commission no 14, Vientiane
Baran E, Saray S, Teoh SJ, Tran TC (2011) Fish and fisheries in the Sesan River basin-catchment baseline, fisheries section. The WorldFish Center, Phnom Penh
Baran E, Samadee S, Jiau TS, Tran TC (2013) Fish and fisheries in the Sesan, Sekong and Srepok River basins (Mekong watershed). ICEM – International Centre for Environmental Management, Hanoi
Chavent M, Kuentz-Simonet V, Labenne A, Saracco J (2014) Multivariate analysis of mixed data: the PCAmixdata R package. arXiv [stat.CO] 1411.4911
Chavent M, Kuentz V, Labenne A, Liquet B, Saracco J (2017) PCAmixdata: multivariate analysis of mixed data. R package version 3.1. https://CRAN.R-project.org/package=PCAmixdata. Accessed 29 Jan 2019
Chea R, Lek S, Ngor P, Grenouillet G (2017) Large-scale patterns of fish diversity and assemblage structure in the longest tropical river in Asia. Ecol Freshw Fish 26:575–585. https://doi.org/10.1111/eff.12301
Conlan I, Rutherfurd I, Finlayson B, Western A (2008) The geomorphology of deep pools on the lower-Mekong River: controls on pool spacing and dimensions, processes of pool maintenance and potential future changes to pool morphology. Final report submitted to the Mekong River Commission Secretariat. Vientiane, Lao
Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Dugan PJ, Barlow C, Agostinho AA, Baran E, Cada GF, Daqing C, Cowx IG, Ferguson JW, Jutagate T, Mallen-Cooper M, Marmulla G, Nestler JM, Petrere M, Welcomme RL, Winemiller KO (2010) Fish migration, dams, and loss of ecosystem services in the Mekong Basin. Ambio 39:344–348. https://doi.org/10.1007/s13280-010-0036-1
Fitzgerald DB, Winemiller KO, Sabaj-Pérez MH, Sousa LM (2017) Using trophic structure to reveal patterns of trait-based community assembly across niche dimensions. Funct Ecol 31:1135–1144. https://doi.org/10.1111/1365-2435.12838
Gatz AJ Jr (1979a) Ecological morphology of freshwater stream fishes. Tulane Studies Zool Bot 21:91–124
Gatz AJ Jr (1979b) Community organization in fishes as indicated by morphological features. Ecology 60:711–718
Hap N, Un S, Nasielski J (2016) A review of socioeconomic studies in the fisheries sector in Cambodia. Inland Fisheries Research and Development Institute (Fisheries Administration) and WorldFish, Phnom Penh
Hirsch P, Wyatt A (2004) Negotiating local livelihoods: scales of conflict in the Se San River basin. Asia Pac Viewp 45:51–68. https://doi.org/10.1111/j.1467-8376.2004.00227.x
Hoeinghaus DJ, Zeug S (2008) Can stable isotope ratios provide for community-wide measures of tropic structure? Comment. Ecology 89:2353–2357 https://www.jstor.org/stable/27650761
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER – stable isotope Bayesian ellipses in R. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Johnson PTJ, Olden JD, Zanden MJV (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6:357–363. https://doi.org/10.1890/070156
Kondolf GM (1997) Hungry water: effects of dams and gravel mining on river channels. Environ Manag 21:533–552. https://doi.org/10.1007/s002679900048
Layman CA, Arrington DA, Montaña CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 86:2530–2535. https://doi.org/10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO;2
Lévêque C, Oberdorff T, Paugy D, Stiassny MLJ, Tedesco PA (2008) Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595:545–567. https://doi.org/10.1007/978-1-4020-8259-7_53
Li J, Dong S, Peng M, Yang Z, Liu S, Li X, Zhao C (2013) Effects of damming on the biological integrity of fish assemblages in the middle Lancang-Mekong River basin. Ecol Indic 34:94–102. https://doi.org/10.1016/j.ecolind.2013.04.016
Liem KF (1990) Aquatic versus terrestrial feeding modes: possible impacts on the trophic ecology of vertebrates. Am Zool 30:209–221. https://doi.org/10.1093/icb/30.1.209
Liermann CR, Nilsson C, Robertson J, Ng RY (2012) Implications of dam obstruction for global freshwater fish diversity. Bioscience 62:539–548. https://doi.org/10.1525/bio.2012.62.6.5
Lima AC, Sayanda D, Agostinho CS, Machado AL, Soares AMVM, Monaghan KA (2018) Using a trait-based approach to measure the impact of dam closure in fish communities of a Neotropical River. Ecol Freshw Fish 27:408–420. https://doi.org/10.1111/eff.12356
Mekong River Commission (2005) Work program report. Mekong River Commission, Vientiane
Mekong River Commission (2010) State of the basin report 2010. Mekong River Commission, Vientiane
Mekong River Commission (2015) Strategic plan 2010–2015. Mekong River Commission, Vientiane
Mims MC, Olden JD (2013) Fish assemblages respond to altered flow regimes via ecological filtering of life history strategies. Freshw Biol 58:50–62. https://doi.org/10.1111/fwb.12037
Montaña CG, Winemiller KO (2013) Evolutionary convergence in Neotropical cichlids and Nearctic centrarchids: evidence from morphology, diet and stable isotope analysis. Biol J Linn Soc 109:146–164. https://doi.org/10.1111/bij.12021
Montaña CG, Winemiller KO, Sutton A (2014) Intercontinental comparison of fish ecomorphology: null model tests of community assembly at the patch scale in rivers. Ecol Monogr 84:91–107. https://doi.org/10.1890/13-0708.1
Ngor PB, Legendre P, Oberdorff T, Lek S (2018) Flow alterations by dams shaped fish assemblage dynamics in the complex Mekong-3S river system. Ecol Indic 88:103–114. https://doi.org/10.1016/j.ecolind.2018.01.023
Nilsson C (2005) Fragmentation and Flow Regulation of the World's Large River Systems. Science 308 (5720):405–408
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2017) vegan: Community Ecology Package. R package version 2.4–3. https://CRAN.R-project.org/package=vegan. Accessed 29 Jan 2019
Oliveira AG, Baumgartner MT, Gomes LC, Dias RM, Agostinho AA (2018) Long-term effects of flow regulation by dams simplify fish functional diversity. Freshw Biol 63:293–305. https://doi.org/10.1111/fwb.13064
Ou C, Winemiller KO (2016) Seasonal hydrology drives shifts in production sources supporting fishes in the lower Mekong River basin. Can J Fish Aquat Sci 73:1342–1362. https://doi.org/10.1139/cjfas-2015-0214
Ou C, Montaña CG, Winemiller KO (2017) Body size and trophic position relationships among fishes of the lower Mekong Basin. R Soc Open Sci 4:160645. https://doi.org/10.1098/rsos.160645
Pelicice FM, Azevedo-Santos VM, Vitule JRS, Orsi ML, Lima DP, ALB M Jr, Pompeu PS, Petrere M Jr, Agostinho AA (2017) Neotropical freshwater fishes imperiled by unsustainable policies. Fish Fish 10:1–15. https://doi.org/10.1111/faf.12228
Petrere M Jr (1996) Fisheries in large tropical reservoirs in South America. Lakes Reserv Res Manag 2(1–2):111–133. https://doi.org/10.1111/j.1440-1770.1996.tb00054.x
Piman T, Cochrane TA, Arias MA, Green A, Dat ND (2013) Assessment of flow changes from hydropower ddevelopment and operations in Sekong, Sesan, and Srepok River of the Mekong Basin. J Water Res Plan Man 139:723–732. https://doi.org/10.1061/(ASCE)WR.1943-5452.0000286
Poff NL, Olden JD, Merritt DM, Pepin DM (2007) Homogenization of regional river dynamics by dams and global biodiversity implications. Proc Natl Acad Sci USA 104:5732–5737. https://doi.org/10.1073/pnas.0609812104
Pokhrel Y, Shin S, Lin Z, Yamazaki D, Qi J (2018) Potential disruption dynamics in the lower Mekong River basin due to upstream flow regulation. Sci Rep 8:17767. https://doi.org/10.1038/s41598-018-35823-4
Post DM (2002) Using stable isotope to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Robinson BW, Wilson DS (1998) Optimal foraging, specialization, and a solution to Liem’s paradox. Am Nat 151:223–235 https://www.jstor.org/stable/10.1086/286113
Santos NL, Santana HS, Ortega JC, Diaz RM, Stegmann LF, Araujo IMS, Severi W, Bini LM, Gomes LC, Agostinho AA (2017) Environmental filters predict the trait composition of fish communities in reservoir cascades. Hydrobiologia 802:245–253. https://doi.org/10.1007/s10750-017-3274-4
Sithirith M (2016) Dams and state security: damming the 3S rivers as a threat to Cambodian state security. Asia Pac Viewp 57:60–75. https://doi.org/10.1111/apv.12108
Toussaint A, Charpin N, Brosse S, Villéger S (2016) Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci Rep 6:22125. https://doi.org/10.1038/srep22125
Try T, Chambers M (2006) Situation analysis: Stung Treng Province, Cambodia. Mekong Wetlands Biodiversity Conservation and Sustainable Use Programme, Vientiane
Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD (2005) Many-to-one mapping of form to function: a general principle in organismal design? Integr Comp Biol 45:256–262. https://doi.org/10.1093/icb/45.2.256
Webb PW (1984) Body form, locomotion and foraging in aquatic vertebrates. Integr Comp Biol 24:107–120. https://doi.org/10.1093/icb/24.1.107
Whyatt A, Baird IG (2007) Transboundary impact assessment in the Sesan river basin: the case of the Yali falls dam. Water Res Devel Man 23:427–442. https://doi.org/10.1080/07900620701400443
Winemiller KO (1991) Ecomorphological diversification of freshwater fish assemblages from five biotic regions. Ecol Monogr 61:343–365. https://doi.org/10.2307/2937046
Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S, Baird IG, Darwall W, Lujan NK, Harrison I, Stiassny ML, Silvano RA, Fitzgerald DB, Pelicice FM, Agostinho AA, Gomes LC, Albert JS, Baran E, Petrere M Jr, Zarfl C, Mulligan M, Sullivan JP, Arantes CC, Sousa LM, Koning AA, Hoeinghaus DJ, Sabaj M, Lundberg JG, Armbruster J, Thieme ML, Petry P, Zuanon J, Torrente Vilara G, Snoeks J, Ou C, Rainboth W, Pavanelli CS, Akama A, van Soesbergen A, Sáenz L (2016) Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong: basin-scale planning is needed to minimize impacts in mega-diverse rivers. Science 351:128–129. https://doi.org/10.1126/science.aac7082
Ziv G, Baran E, Nam S, Rodríguez-Iturbe I, Levin SA (2012) Trading-off fish biodiversity, food security, and hydropower in the Mekong River basin. Proc Natl Acad Sci USA 109:5609–5614. https://doi.org/10.1073/pnas.1201423109
Acknowledgments
We thank the citizens of Stung Treng, Siem Pang, Veurn Sai, and Lomphat in Cambodia for their help with fieldwork. We also thank So Nam and the Inland Fisheries Research and Development Institute of Cambodia for logistic support and providing a scientific permit, and Putrea Solyda for help with fieldwork, logistics, and relations with the local communities. Funding for this project was provided by the Estate of Carolyn and George Kelso via the International Sport Fish Fund at Texas A&M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Fish specimen collection and export permits for this study were granted to Chouly Ou by the Inland Fisheries Research and Development Institute (IFReDI) of Cambodia. IFReDI personnel supervised and assisted sampling. During our field study, IFReDI had not yet established animal use protocols; therefore, guidelines established by the American Fisheries Society (UFR Committee, 2004. Guidelines for the use of fishes in research. American Fisheries Society, Bethesda, MD) were adopted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Montaña, C.G., Ou, C., Keppeler, F.W. et al. Functional and trophic diversity of fishes in the Mekong-3S river system: comparison of morphological and isotopic patterns. Environ Biol Fish 103, 185–200 (2020). https://doi.org/10.1007/s10641-020-00947-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-00947-y