Abstract
Studies showing that tagged reef fish connect different habitat types are crucial for effective ecosystem management on a seascape-level, but are rare. Therefore we analysed movement of juvenile Haemulon flavolineatum and Haemulon sciurus among seagrass beds, mangroves and fossilised eroded coral shoreline. Fishes were tagged individually with external, short-term bead-tags (both species) or with internal, long-term coded wire tags (H. flavolineatum only). We also tested the hypothesis that in spatially continuous habitat types with many seemingly suitable resting sites, these fishes show high fidelity to only a small number of sites. The linear distribution range of daytime sites was 4–171 m for H. flavolineatum and 4–152 m for H. sciurus, but in agreement with our hypothesis, externally tagged fishes showed high fidelity to small spatial areas within this range: the percentage of resightings within a 10 m radius of the core area of presence (i.e. the site used most intensively) was 69% for bead-tagged H. flavolineatum, and 62% for H. sciurus during the 47-day study-period. Site fidelity was also present over a longer time span: of the 1114 coded wire tagged H. flavolineatum 51 were recaptured and 49 of them were still present at the tagging location after 163–425 days at liberty. Median linear movement within a day was small (5 m for H. flavolineatum and 8 m for H. sciurus), nonetheless, part of the bead-tagged Haemulidae moved from shoreline shelter habitats (mangroves and rocky shoreline) to adjacent seagrass beds (mean ± SD distance moved 23 ± 10 m) in the afternoon, likely to start feeding there during daylight. When comparing the habitat type occupied during the late afternoon (15:30–17:30 h) and morning (8:00–10:30 h) on two subsequent days, most movement occurred from seagrass beds back to shoreline habitats (mean distance moved 23 ± 10 m), indicating that in the morning these fishes had returned to shelter sites at the shoreline. The current study thus shows existence of connectivity between back-reef habitats through fish movement on a relatively small spatial scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological interactions between mangroves, seagrass beds and coral reefs are of great importance for coral reef fishes, their movement being one of the processes connecting these habitat types (Ogden & Gladfelter, 1983; Ogden & Quinn, 1984; Moberg & Rönnbäck, 2003). Although effective ecosystem management and restoration requires extensive knowledge of space use by fishes (Kramer & Chapman, 1999) and habitat connectivity (Moberg & Rönnbäck, 2003), remarkably few studies have provided direct evidence that reef fish movements connect different habitat types (Beets et al., 2003).
Many studies of coral reef fish movement have been limited to the reef habitat itself. They have described a wide range of home ranges of various species, as well as the general tendency of fishes to intensively use a small number of sites within their home range (i.e. high site fidelity) (Zeller, 1997; Bell & Kramer, 2000; Marnane, 2000; Jones, 2005). However, various coral reef fish species do not only use the coral reef habitat, and studies that focus on a single habitat may be insufficient for fishes that use a mosaic of habitat types (Pittman & McAlpine, 2003). Back-reef habitats such as mangroves and seagrass beds are putative nurseries for certain coral reef fish species (e.g. Parrish, 1989; Nagelkerken et al., 2000a). If they are true nurseries, these habitats are connected to coral reefs through long-term life history migrations by sub-adult reef fish, but direct evidence of these migrations is scarce (Beck et al., 2001; Gillanders et al., 2003; Chittaro et al., 2004).
In contrast, short-term daily (e.g. feeding) migrations between shallow-water habitat types of the coastal seascape have been studied to a greater extent. In areas with large tidal differences, such as many locations in the Indo-Pacific, tidal fish migrations connect different habitat types. Certain fish species visit shoreline habitats, such as mangroves and eroded fossil reef when these are inundated during high tide, to seek shelter and/or food, and return to sub-tidal habitat types, such as seagrass beds, at low tide (Vance et al., 1996; Sheaves & Molony, 2000; Dorenbosch et al., 2004; Krumme et al., 2004). In areas with smaller tidal differences, such as many Caribbean regions, mangrove roots and other shoreline habitat types are continuously inundated. Therefore the use of mangroves and seagrass beds by fishes is not dependent on the tidal regime (Pinto & Punchihewa, 1996). No tidal migrations occur here, but fish movements during dawn and dusk (twilight migrations) connect various Caribbean habitat types: diurnally active herbivores forage in seagrass beds during the daytime, and migrate to shelter habitats (e.g. rocks and corals) at night, while nocturnally active zoobenthivores move from daytime resting habitats (e.g. mangroves and patch reefs) to seagrass beds and sand flats to feed at night. These movement patterns have mostly been inferred from studies comparing daytime and night time fish abundances (e.g. Ogden & Zieman, 1977; Robblee & Zieman, 1984; Rooker & Dennis, 1991; Nagelkerken et al., 2000b). Studies that showed direct evidence of movement by tagged fishes between certain types of reef and seagrass/sand/coral rubble habitats are those on twilight feeding migrations of Mullidae (goatfish; Holland et al., 1993; Meyer et al., 2000), and Haemulidae (grunts; Ogden & Ehrlich, 1977; Tulevech & Recksiek, 1994; Burke, 1995; Beets et al., 2003). Besides these twilight movements, no detailed knowledge exists on whether Caribbean fishes show additional between-habitat movement during daytime, so the degree of habitat connectivity may yet be underestimated.
Haemulidae display high fidelity to daytime resting sites on small patch reefs within sandy areas and seagrass beds across periods of several weeks (McFarland & Hillis, 1982), and this site fidelity appeared to persist for up to 3 years (Helfman et al., 1982; Helfman & Schultz, 1984). However, whether nocturnally active zoobenthivores (such as Haemulidae and Lutjanidae) also show this high resting site fidelity to other, spatially less confined back-reef habitats, such as large and continuous areas of seagrass beds and shoreline habitats, has rarely been shown. Juvenile snappers showed high site fidelity in extensive Caribbean seagrass beds (Ocyurus chrysurus: Watson et al., 2002) and Indo-Pacific rocky shoreline structures (Lutjanus fulviflamma: Dorenbosch et al., 2004), although fishes were studied only over very short periods of <2 weeks. Based on this evidence of short-term site fidelity, and on the evolutionary advantages of familiarity with certain locations, including reduced predation risk and increased foraging efficiency (see references in Zeller, 1997; Jones, 2005), we hypothesised that even in spatially continuous habitat types, Haemulidae will show long-term fidelity to specific sites and thus have the ability to home to the same sites after nocturnal foraging elsewhere.
The goal of our study was to determine the degree of fish movement between different habitat types and to test our site fidelity hypothesis. We investigated movement patterns of tagged juvenile Haemulon flavolineatum (Desmarest 1823) and Haemulon sciurus (Shaw 1803) collected in various back-reef habitat types (mangroves, seagrass beds and rocky shoreline) in a shallow Caribbean embayment. We used two tagging-methods: (1) external tags that lasted up to 6 weeks, and (2) internal, coded wire tags that are known to be retraceable up to 3 years (e.g. Munro et al., 2003; Brennan et al., 2005). Movement patterns were examined at four time-scales: (1) within single days, (2) between two subsequent days, (3) across days over a period of up to 47 days and (4) across months over a period of up to 425 days.
Materials and methods
Study area
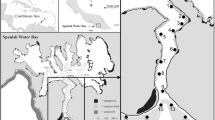
The present study was carried out in Spanish Water Bay (surface area approx. 3 km2) at the south-western coast of Curaçao, Netherlands Antilles (Fig. 1). This non-estuarine embayment has a narrow entrance (70–100 m wide) and a relatively long (1.1 km) and deep (11–18 m) channel that connects the inland bay to the sea and an adjacent continuous fringing reef. Other parts of the bay are relatively shallow (<6 m). The shoreline of the channel part of the bay (Fig. 1) consists of a fossil coral reef terrace up to 3 m high, which partly extends into the water. Biochemical degradation has led to the formation of submarine shaded notches and crevices cutting approx. 0.8 m into the reef terrace (de Buisonjé & Zonneveld, 1960). Rocks and boulders that have broken off from the terrace are found in front of the reef terrace. In this article, the notches, crevices, rocks and boulders will be referred to as ‘rocky shoreline’. The bay shoreline is partly fringed by isolated mangrove stands, Rhizophora mangle, of which the roots are permanently inundated (mean daily tidal range ±30 cm; de Haan & Zaneveld, 1959). The bay floor is covered by seagrasses, predominantly Thalassia testudinum, and macro-algal flats (Kuenen & Debrot, 1995).
Map of Spanish Water Bay on Curaçao (‘C’) indicating coded wire tag sites, and a detailed map of the bead-tag study area. Fishes used for the tagging mortality experiment were captured just outside Piscadera Bay. ‘mg’ = mangroves, ‘sg’ = seagrass beds, ‘rsl’ = rocky shoreline, ‘cdo’ = channel drop-off. The rocky shoreline and mangrove stands were separated from the adjacent seagrass bed by a muddy substratum of ca. 2 m wide. The arrow in the lower panel indicates movement of the recaptured coded wire tagged fish that had moved farthest (1,350 m) of all fish inside the bay. Bold lines in the inset map represent survey and transect routes for bead-tagged fishes
Short-term tagging (external bead-tags)
The short-term tagging experiment took place in an area that included a rocky shoreline, three fringing R. mangle mangrove stands (9, 12 and 74 m long and all 3–4 m wide), and a T. testudinum seagrass bed (inset Fig. 1). Mangrove stands and the rocky shoreline were marked by a nylon twine line attached to the substratum and following the contours of the shoreline at a distance of approx. 3 m from the high tide waterline. This transect was 300 m long, 4 m wide, and was divided into 2-m long sections. Externally tagged fishes were never observed in the seagrass beds during the daytime, except in one specific part of the seagrass bed adjacent to the two small mangrove stands (Fig. 1). Therefore, only this seagrass area was marked using a grid of nylon lines, enclosing an 18 × 10 m rectangular area (Fig. 1), divided into recognisable 2 m2 plots by using coloured flags. In all other seagrass areas, the only Haemulidae that were observed during daytime were very small, non-tagged H. flavolineatum (<6.5 cm fork length, FL).
H. flavolineatum (N = 83) and H. sciurus (N = 21) were caught in the marked seagrass area, the three mangrove stands, and at one boulder along the rocky shoreline (Fig. 1), using hook and line during the daytime, and baited Antillean fish traps during the daytime and night time. Freshly caught fishes were kept briefly in an underwater net. Before tagging, fish FL was determined, and the fishes were wrapped in a wet cloth. Fishes were tagged using monofilament line (∅ 0.18–0.25 mm) and coloured beads (∅ 1.5–2.6 mm). Tags were inserted into the muscle tissue just under the anterior or the central part of the dorsal fin base, using a hollow injection needle (∅ 0.50 mm). The skin was pierced at a downward angle of about 60° to the fishes’ body, so that scales were not torn off. On the thread protruding from the other side of the fish, the mirror image of the series of beads (maximum of two on either side) was attached. The number and combination of coloured beads was unique for each individual and was recognisable underwater. The tag was fastened at the end with a knot, and fishes were released at the same place where they had been caught. The tagging procedure took <1 min. and needles were cleaned with diluted ethanol before tagging the next fish.
The short-term tag study lasted from 15 January to 2 March 2004 (48 days). Tagging took place on 7 different days between day 1 and day 9, while surveys took place on 28 days between day 2 and day 48. Surveys were conducted during 8:00–10:30 h (T1), 11:45–14:15 h (T2), and 15:30–17:30 h (T3). On 10 of the 28 survey days, surveys were conducted during two or three of the selected time intervals, by two or three snorkelling observers. On the remaining 18 days, the selected sites were observed during at least one of the three time intervals, by one observer. When a tagged individual was spotted (i.e. ‘resighted’), the tag code, time of day and location were recorded. Transects were surveyed once or twice per time interval. If an individual was resighted more than once during the same time interval, only the first resighting was used in data analysis. During some surveys, unmarked areas outside the line transects were also searched, including unmarked seagrass beds adjacent to the shoreline transect and 100 m extensions of the rocky shoreline transect at both ends. To see whether fishes had moved away from their daytime sites at night, surveys were conducted by three snorkelling observers holding flashlights on two nights between 18:00 and 19:30 h. These surveys were carried out in all marked and unmarked areas described above.
Long-term tagging (internal tags)
For the long-term tagging experiment, only the most abundant species, H. flavolineatum, was selected. Fishes were caught and tagged during 10 May 2005–11 October 2005. A total of 1114 H. flavolineatum were caught in 8 mangrove stands, 11 seagrass bed sites, 19 rocky shoreline sites and at 2 sites at the channel drop-off (Fig. 1, Table 2), using hook and line during the daytime, and baited Antillean fish traps during the daytime and night time. These fishes were provided with an individually coded wire tag (CWT, Northwest Marine Technology) using a single-shot tag injector. The tags consisted of magnetised stainless steel wire (diameter 0.25 mm and length 2.2 mm). CWTs were inserted between the skin and muscle layer at the base of the anal fin. Tagged fishes were released at their catch location.
During 6 February 2006–19 July 2006, H. flavolineatum (N = 1195; Table 2) were caught at all tagging sites of 2005 and at an additional 65 sites at the drop-off of the tidal channel, measured (FL) and scanned for the presence of a tag, visible as a magnetic anomaly. Recaptured tagged fishes were killed rapidly by cutting through the spinal cord above the gill slits, after which the tag was removed and the individual code was read under magnification.
Tagging mortality
In order to assess tagging mortality, 12 bead-tagged (FL = 8.5–16.0 cm), 12 coded wire-tagged (FL = 8.0–12.4 cm) and 12 non-tagged (FL = 8.3–16.8 cm) H. flavolineatum (viz. three treatments) were kept in captivity for 14 days. Fish were captured with hook and line at the sandy substratum and shallow coral boulders (1–2 m deep) just outside Piscadera Bay (Fig. 1). They were transported underwater in a net and released into outdoor tanks containing small boulders as a shelter habitat. The non-transparent tanks measured 1 m3 and had a continuous seawater flow of approx. 5.8 l min−1. The top of each tank was covered by shading cloth. The number of fish per tank was four, and three tanks were assigned per treatment, adding up to 12 fishes per treatment. Control fish were not tagged, but were measured and wrapped in a wet cloth, to experience a similar handling. Tagged fish were measured and tagged as described above and bead-tagged fish were given one bead on either side of the body. Fish were fed equal amounts of squid around dusk and checked daily. Dead fish were removed, and unusual behaviour and infections were recorded.
Data analysis
Coordinates of catch locations, line transects and recapture sites were measured by GPS and plotted on a map of the bay using ArcView 3.1. Linear distances between locations were calculated using ArcView 3.1 and Bersoft 4.03.
Fish movement was characterised in terms of (1) daytime activity radius (DAR), (2) between-habitat movement, and (3) site fidelity. Because many bead-tagged fishes moved between the habitat types during the entire study period (see % of resightings in the various habitat types, Table 1), movement variables were calculated for pooled catch habitat types, per species.
We refrained from using the term ‘home range’ because we only examined daytime space use and in our opinion ‘home range’ encompasses both daytime and night time activity spaces. The DAR was expressed as the shortest linear distance between the two most extreme resighting locations (following Zeller, 1997; Chapman & Kramer, 2000). This depicts the minimal distance a fish has traversed. Between-habitat movement was expressed as the number of times a fish was resighted in a different habitat type compared to the habitat type of the previous resighting, and compared to where it was caught and tagged. Site fidelity was calculated by determining the transect section where an individual was resighted most frequently, i.e. the ‘core area of presence’ (CAP). The linear distance between all resightings and the CAP was then calculated.
DAR and between-habitat movement were calculated for four time-scales: (1) within days, for fishes resighted during at least two different time intervals on the same day, (2) between the late-afternoon (15:30–17:30 h) resighting of one day and the first resighting (8:00–10:30 h) of the following day (referred to below as ‘between two subsequent days’, which assesses overnight return and fidelity to daytime resting sites), (3) ‘across days’ over periods of 15–47 days, using all resightings of the entire short-term tag study period (which assesses the linear distribution of all daytime resting sites) and (4) ‘across months’ for recaptured coded wire-tagged fishes of the long-term study period, by comparing the initial catch location at the time of tagging with that of recapture, which covered time spans of 163–425 days. Because site fidelity is a process covering relatively long time periods, it was only calculated for the time scales ‘across days’ and ‘across months’.
Because movement variables may be influenced by the number of resightings (e.g. Laundré & Keller, 1984; Odum & Kuenzler, 1995; Samietz & Berger, 1997), we calculated DAR and site fidelity across days only for those fishes that showed stabilising DAR with increasing number of resightings (e.g. following Zeller, 1997). To find out which fishes met this criterion, we created area observation curves for each individual. We calculated the linear distance between all previous and every consecutive resighting and plotted this against the number of resightings. Fishes reached stabilising DAR at a number of 11 resightings, so we calculated DAR and site fidelity across days only for individuals resighted >10 times. Simple linear regressions (SPSS 14.0) showed that the number of resightings did not significantly affect movement variables across days calculated for fishes with >10 resightings (DAR across days: R 2 < 0.025, P > 0.420; number of resightings at CAP (site fidelity): R 2 < 0.251, P > 0.081).
Differences in daytime activity radii for bead-tagged fishes were tested using a mixed General Linear Model (SPSS 14.0) with ‘time scale’ as a within-subjects factor (repeated measure) and ‘species’ as a between-subjects factor. Sphericity conditions were not violated (following Field, 2005).
Results
Tagging mortality
After 2 weeks, survival of captive fishes was 83% for control fishes (10 out of 12), 92% for bead-tagged fishes (11 out of 12), and 100% for coded wire tagged fishes (12 out of 12). After 2 weeks, 17% (2 out of 12) of bead-tagged fishes had lost their external tag.
Resightings and recaptures
Because maturation sizes of H. flavolineatum and H. sciurus approximate 15.5 and 22.0 cm, respectively (Munro, 1983), all fishes in this study were juveniles (see size range in Tables 1 and 2).
A minimum of 57% of bead-tagged individuals of the two species in all catch habitat types were resighted at least once (Table 1). The number of individuals resighted decreased over time. The maximum number of resightings per individual ranged from 12 to 37 for both species and all catch habitat types (Table 1). After 4–6 weeks, most fishes had either visibly lost the external tag or the tag colours were no longer recognisable due to algal fouling.
Of the 1114 coded wire tagged H. flavolineatum, 51 fishes (4.6%) were recaptured after 163–425 days at liberty (Table 2). Over this period, these recaptured fishes showed an average growth of 0.05 ± 0.03 mm day−1.
Daytime activity radii and between-habitat movement
Linear distances moved were not significantly different between species (F <1, P = 0.579), and no interaction effect between species and time-scale was found (F <1, P = 0.960). There was a significant main effect of time-scale on distances moved (F = 33.469, P < 0.001). For both species, distances moved within days were significantly smaller (medians: H. flavolineatum 5 m, and H. sciurus 8 m) than DAR across days (medians: H. flavolineatum 50 m, and H. sciurus 30 m) (Fig. 2). Additionally, for H. flavolineatum DAR between two subsequent days (median 15 m) was also significantly smaller than DAR across days (Fig. 2).
Boxplots of linear distances between the two most extreme resightings (DAR) for bead-tagged H. flavolineatum and H. sciurus calculated for the time scales within days (‘within’, for fishes resighted during two different time intervals on the same day), between two subsequent days (‘between’, for fishes resighted during the late-afternoon survey on one day and during the first morning survey of the following day), and across days (‘across’, over periods of 15–47 days: for fishes resighted more than ten times). A boxplot indicates the median (horizontal line in box), the interquartile range (box height), 10 and 90 percentiles (whiskers), and outliers (dots). Different letters above boxplots indicate significant differences (mixed GLM). N is the number of individual fish (sample size) and differs between time scales, within species, as a result of the different time scales and criteria
For fishes resighted during two time intervals within one day, 17 out of 41 (41%) H. flavolineatum, and 9 out of 14 (64%) H. sciurus moved between two different habitat types (Fig. 3a, b). The percentage of their within-day movements leading to a habitat shift was 40% for H. flavolineatum and 59% for H. sciurus. Most of these movements were towards seagrass beds: H. flavolineatum showed most frequent movement from the mangroves to the seagrass bed, whereas H. sciurus showed most frequent movement from the rocky shoreline to the seagrass bed (Fig. 3a, b). Movement to seagrass beds occurred most often after T1 (Fig. 3a, b). Both species showed very little movement from the seagrass beds back to the rocky shoreline or mangrove stands during the day, but they did show some movement back and forth between the mangroves and the rocky shoreline (Fig. 3a, b). For fishes that did not move between different habitats within days, the mean DAR (±SE) within days was 3.4 ± 0.6 m for H. flavolineatum and 3.5 ± 1.0 m for H. scirurus, which were significantly shorter distances than those for fishes that did move between habitats (14–35 m; Fig. 3a, b), (t-test, t >8.438, df >12, P < 0.001).
Between-habitat movement for bead-tagged fishes: (a) within days (viz., between different time-intervals within the same day) for H. flavolineatum, (b) within days for H. sciurus and (c) between two subsequent days (viz., between the late-afternoon resighting on a day and the first morning resighting on the following day) for both species (black bars = H. flavolineatum, grey bars = H. sciurus). (a) and (b) show the exact time interval at which the habitat shift was recorded. ‘sg’ = seagrass beds, ‘mg’ = mangroves, ‘rsl’ = rocky shoreline. X-axes show all possible changes between habitat types, with the first habitat type noted being the habitat departed from, the second that arrived in. For example, ‘sg–mg’ means movement from seagrass beds to mangroves. Numbers above the bars indicate mean ± SE distances moved in metres
For fishes resighted between subsequent days (i.e. at T3 on one day and T1 on the next day), 19 out of 33 (58%) H. flavolineatum, and 6 out of 11 (55%) H. sciurus moved between different habitat types (Fig. 3c). The percentage of their between-day movements leading to a habitat shift was 57% for H. flavolineatum and 93% for H. sciurus. Both species were most likely to move from seagrass beds to mangroves or rocky shoreline (Fig. 3c). Overnight movements from shoreline habitats to seagrass beds were rarely observed (Fig. 3c), in contrast to the regular occurrences of such movements within days (Fig. 3a, b). For fishes that did not move between different habitats when comparing subsequent days, the mean linear distance (±SE) moved was 3.2 ± 0.7 m for H. flavolineatum and 27.8 ± 24.3 m for H. scirurus. For H. flavolineatum, these distances were significantly shorter than those for fishes that did move between habitats (19–75 m; Fig. 3c) (t-test, t = 5.032, df = 31, P < 0.001).
Compared to within and between-day movement, even more fishes showed between-habitat movement across days: the number of bead-tagged fishes resighted in at least one other habitat type than their catch habitat was 45 out of 56 (80%) for H. flavolineatum and 15 out of 19 (79%) for H. sciurus that were resighted at least once. The patterns of within and between-day habitat shifts described above were also visible for the diurnal variation among habitats across all days: the mean percentages of resightings in seagrass beds increased with increasing time of day (T1, T2 and T3, respectively), and were 11, 23 and 28% for H. flavolineatum and 36, 44 and 60% for H. sciurus. This means that in the morning, most resightings (89% for H. flavolineatum and 64% for H. sciurus) were in non-seagrass habitats (i.e. the rocky shoreline and the mangroves).
For long-term movement ‘across months’, 49 of the 51 recaptured coded wire tagged fishes were recaptured at the same location (with an accuracy of 10–20 m) as they had been tagged 163–425 days earlier, while two fishes were recaptured in a different habitat type from where they were caught and tagged. The first individual had moved 1,350 m (see Fig. 1) after 316 days at liberty. It was tagged at a size of 8.2 cm in a seagrass bed and was recaptured at a length of 9.2 cm at the part of the rocky bay shoreline that was the nearest to the bay mouth and the coral reef. The second individual had moved 60 m after 311 days at liberty. It was tagged at a size of 8.0 cm at the rocky shoreline and was recaptured at a length of 11.1 cm, at the drop-off of the tidal channel (3 m deep).
During night time observations seven H. flavolineatum and six H. sciurus were resighted. Three of them were resighted at the rocky shoreline and in the mangroves, while the other 10 were resighted in seagrass beds. The median distance that these fishes had moved away from the location of their late-afternoon resighting on the same day was 9 m (range 2–170 m) for H. flavolineatum and 6 m (range 4–7 m) for H. sciurus, while the median distance to their daytime CAP was 16 m for both species (range 2–170 m).
Site fidelity
The numbers of CAPs located in seagrasses, mangroves and rocky shoreline were 6, 13 and 9, respectively, for H. flavolineatum, and 4, 2 and 7 for H. sciurus. The mean percentage of resightings within the CAP was 40% for H. flavolineatum and 27% for H. sciurus (Fig. 4). More than half of all resightings were within a 10 m radius around the CAP (69% for H. flavolineatum and 62% for H. sciurus). Both species also showed a peak of resightings at distances of 11–30 m from the CAP. These peaks were mainly caused by fish movement between the marked seagrass area and the adjacent rocky shoreline with two small mangrove stands (see Fig. 1), a distance of approx. 20 m. Another small peak was found for H. flavolineatum at distances of 41–60 m from their CAP (Fig. 4). This was due to movement between the large mangrove stand and a particularly large shoreline boulder (also a tagging site: Fig. 1).
Site fidelity across days for bead-tagged H. flavolineatum and H. sciurus expressed as the mean percentage of resightings (‘rs’) at various linear distances away from the CAP. Note the increasing scale for the distance classes on the x-axis. The N given is the number of individuals resighted more than 10 times
Discussion
Survival of tagged H. flavolineatum kept in captivity for 14 days did not differ from that of untagged fish, suggesting that fish resightings and recaptures were not influenced by mortality due to the tagging procedure, at least not in the first period after tagging. The reason why 2 out of 12 control fishes died is unknown, since they did not behave differently nor showed external infections.
During daytime, Haemulidae generally rest and shelter in or near structurally complex habitat types (Ogden & Ehrlich, 1977; Valdés-Muñoz & Mochek, 2001; Verweij et al., 2006a). In the present study, their passive daytime behaviour was obvious from the relatively small DAR within days (median <10 m for both species). Nonetheless, some fishes (41% for H. flavolineatum and 64% for H. sciurus) did move between habitat types within days: they mostly moved from mangroves and rocky shoreline to the seagrass bed during the afternoon, but there was little movement from seagrass beds back to the rocky shoreline or mangrove stands, meaning that once in the seagrass beds, fishes stayed there for the remaining part of the day. This suggests that part of the population of these species already move to the seagrass beds during T2 and T3 (11:45–17:30 h, i.e. during daylight). This contrasts observations and the general belief that grunts resting in structurally complex habitat types during the daytime move to feeding grounds around or just after sunset (e.g. Ogden & Ehrlich, 1977; McFarland et al., 1979; Rooker & Dennis, 1991). However, because we did not count all Haemulidae (tagged and untagged), we cannot rule out the possibility that the bulk of migration occurs around sunset. For H. flavolineatum in the present study (size range 6–11 cm) the movements to the seagrass bed in the afternoon are probably for the purpose of feeding, because seagrass beds are used as daytime feeding habitats by 5–10 cm sized H. flavolineatum (Verweij et al., 2006b). The same may be true for small individuals of H. sciurus. In agreement with our results, another study in the same bay showed that 5–10 cm sized H. flavolineatum leave the fringing mangroves after 15:30 and start foraging actively on the adjacent muddy substratum (Verweij et al., 2006b); the same phenomenon has been observed on the Caribbean island of Aruba (Verweij et al., unpublished data). The timing of grunt migrations is strongly influenced by predator presence, as was shown experimentally on patch reefs surrounded by seagrass beds (Helfman, 1986). Low numbers of piscivorous predators in shallow embayments of Curacao and Aruba might facilitate grunt movement to seagrass beds during daylight. The shift between habitat types between the late afternoon and morning resightings on two subsequent days for Haemulidae was most often from seagrass beds to mangroves or rocky shoreline. This suggests that the fishes residing in the seagrass beds during the afternoon may stay overnight (to feed), after which they move back to the shelter of mangroves and rocky shoreline in the morning of the following day.
Another type of between-habitat movement involved movement directed towards the coral reef, which is the presumed adult habitat of H. flavolineatum. One recaptured coded wire tagged H. flavolineatum moved 1,350 m towards the bay mouth, thus in the direction of the coral reef. Moreover, in a follow-up experiment, during which 345 H. flavolineatum were caught on the coral reef up to 350 m away from the bay mouth, 1 fish tagged with CTW at a length of 9.8 cm at the bay rocky shoreline was recaptured at a length of 14.2 cm on the coral reef 2,000 m away from the tagging site. Even though reef-directed movements were only observed for two fishes, they do indicate that some H. flavolineatum may grow up in bay nurseries and move towards the reef at later life-stages.
The spatial distribution of daytime sites (DAR across days: H. flavolineatum range 4–171 m, H. sciurus 4–152 m) showed that individuals do not always use the exact same sites every day, and that used sites are sometimes located at some distance from one another. However, daytime site use across days was relatively small in comparison to the available continuous structurally complex habitat (kilometres), and only two recaptured coded wire tagged fishes showed movement in this order of magnitude (1,350 and 2,000 m). Moreover, both species used one specific core area within their DAR most intensively and, in agreement with our hypothesis, in continuous seagrass beds and rocky shoreline with mangrove stands, where fishes theoretically have a choice from a wide range of apparently suitable resting sites, H. flavolineatum and H. sciurus showed high fidelity to specific locations over periods up to 47 days (short-term tag data). H. flavolineatum were even recaptured at their tagging locations after 163–425 days (long-term tag data). However, had long-term site fidelity been high, recapture numbers of coded-wire tagged H. flavolineatum would have been expected to be higher than 4.6%. Nonetheless, for fishes showing site fidelity, our findings implicate that in the morning, fishes mostly return to the same familiar sites after their nocturnal foraging elsewhere, for at night most tagged fishes were not present at their daytime sites, and Haemulidae sized 4–12 cm are known to move 100–300 m to nocturnal foraging grounds during twilight (Ogden & Ehrlich, 1977). Return to familiar daytime sites is also reflected by the relatively small linear distances between late afternoon and morning resightings on two subsequent days (median 15 m for H. flavolineatum and 18 m for H. sciurus). A preference for familiar daytime resting sites in a continuous reef habitat type has been shown experimentally for planktivorous Apogonidae, which chose to return to original resting sites after being displaced distances of 1–2 km, rather than choosing apparently suitable resting sites nearer to the release site (Marnane, 2000).
The present study clearly shows that Haemulidae move between different back-reef habitats. These may form a mosaic of essential habitat types for grunts, and an increasing number of studies address the importance of ecological processes that function on a landscape (or seascape) scale and influence populations (e.g. Layman et al., 2004; Boström et al., 2006). Since coastal development can lead to habitat fragmentation, which may negatively affect habitat connectivity, our finding of a high degree of habitat connectivity has implications for managing these habitats. Habitat fragmentation has also occurred in our bead-tag study area (Fig. 1), where, after finishing the current study, an estimated 75% of the largest mangrove stand was cut away to build a house. The destruction of intensively used sites (core areas of presence) of fishes may have detrimental effects on the fish population because of the strong site fidelity of fishes.
Conclusions
The present study shows that juvenile H. flavolineatum and H. sciurus show fidelity to daytime sites across periods between 47 and 425 days, even though these sites were located in extensive and spatially continuous back-reef habitats. The data furthermore show that part of the H. flavolineatum and H. sciurus population sheltering in shoreline shelter habitats (mangroves and rocky shoreline) during daytime move to adjacent seagrass beds in the afternoon (after 11:45 h), likely to start feeding in seagrasses during daylight. They probably continue feeding here during night time, away from the daytime shelter sites, but in the morning of the next day, most fishes return to exactly the same shoreline shelter sites as those of the previous day. The current study thus provides direct proof of connectivity between back-reef habitats through fish movement, although on a relatively small spatial scale.
References
Beck, M. W., K. L. Heck, K. W. Able, D. L. Childers, D. B. Eggleston, B. M. Gillanders, B. Halpern, C. G. Hays, K. Hoshino, T. J. Minello, R. J. Orth, P. F. Sheridan & M. P. Weinstein, 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51: 633–641.
Beets, J., L. Muehlstein, K. Haught & H. Schmitges, 2003. Habitat connectivity in coastal environments: patterns and movement of Caribbean coral reef fishes with emphasis on bluestriped grunt, Haemulon sciurus. Gulf and Caribbean Research 14: 29–42.
Bell, T. & D. L. Kramer, 2000. Territoriality and habitat use by juvenile blue tangs, Acanthurus coeruleus. Environmental Biology of Fishes 58: 401–409.
Boström, C., E. M. Jackson & C. A. Simenstad, 2006. Seagrass landscapes and their effects on associated fauna: A review. Estuarine, Coastal and Shelf Science 68: 383–403.
Brennan, N. P., K. M. Leber, H. L. Blankenship, J. M. Ransier, & R. DeBruler, 2005. An evaluation of coded wire and elastomer tag performance in juvenile common snook under field and laboratory conditions. North American Journal of Fisheries Management 25: 437–445.
Burke, N. C., 1995. Nocturnal foraging habitats of French and bluestriped grunts, Haemulon flavolineatum and H. sciurus, at Tobacco Caye, Belize. Environmental Biology of Fishes 42: 365–374.
Chapman, M. R. & D. L. Kramer, 2000. Movements of fishes within and among fringing coral reefs in Barbados. Environmental Biology of Fishes 57: 11–24.
Chittaro, P. M., B. J. Fryer & P. F. Sale, 2004. Discrimination of French grunts (Haemulon flavolineatum, Desmarest, 1823) from mangrove and coral reef habitats using otolith microchemistry. Journal of Experimental Marine Biology and Ecology 308: 169–183.
de Buisonjé, P. H. & J. I. S. Zonneveld, 1960. De kustvormen van Curaçao, Aruba en Bonaire. Natural Science Study Group Netherlands Antilles 11. Martinus Nijhoff, ‘s-Gravenhage: 1–24 + 7 plates.
de Haan, D. & J. S. Zaneveld, 1959. Some notes on tides in Annabaai harbour, Curaçao, Netherlands Antilles. Bulletin of Marine Science of the Gulf and Caribbean 9: 224–236.
Dorenbosch, M., M. C. Verweij, I. Nagelkerken, N. Jiddawi & G. van der Velde, 2004. Homing and daytime tidal movements of juvenile snappers (Lutjanidae) between shallow-water nursery habitats in Zanzibar, western Indian Ocean. Environmental Biology of Fishes 70: 203–209.
Field, A., 2005. Discovering Statistics Using SPSS (Second Edition). Sage Publications, London.
Gillanders, B. M., K. W. Able, J. A. Brown, D. B. Eggleston & P. F. Sheridan, 2003. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Marine Ecology Progress Series 247: 281–295.
Helfman, G. S., J. L. Meyer & W. N. McFarland, 1982. The ontogeny of twilight migration patterns in grunts (Pisces: Haemulidae). Animal Behaviour 30: 317–326.
Helfman, G. S. & E. T. Schultz, 1984. Social transmission of behavioural traditions in a coral reef fish. Animal Behaviour 32: 379–384.
Helfman, G. S., 1986. Behavioral responses of prey fishes during predator-prey interactions. In Feder M. E., & G. V. Lauder (eds) Predator–prey relationships. The University of Chicago Press, Chicago and London: 135–156.
Holland, K. N., J. D. Peterson, C. G. Lowe & B. M. Wetherbee, 1993. Movements, distribution and growth rates of the white goatfish Mulloides flavolineatus in a fisheries conservation zone. Bulletin of Marine Science 52: 982–992.
Jones, K. M. M., 2005. Home range areas and activity centres in six species of Caribbean wrasses (Labridae). Journal of Fish Biology 66: 150–166.
Kramer, D. L. & M. R. Chapman, 1999. Implications of fish home range size and relocation for marine reserves. Environmental Biology of Fishes 55: 65–79.
Krumme, U., U. Saint-Paul & H. Rosenthal, 2004. Tidal and diel changes in the structure of a nekton assemblage in small intertidal mangrove creeks in northern Brazil. Aquatic Living Resources 17: 215–229.
Kuenen, M. M. C. E. & A. O. Debrot, 1995. A quantitative study of the seagrass and algal meadows of the Spaanse Water, Curaçao, The Netherlands Antilles. Aquatic Botany 51: 291–310.
Laundré, J. W. & B. L. Keller, 1984. Home-range size of coyotes: a critical review. Journal of Wildlife Management 48: 127–139.
Layman, C. A., D. A. Arrington, R. B. Langerhans & B. R. Silliman, 2004. Degree of fragmentation affects fish assemblage structure in Andros Island (Bahamas) estuaries. Caribbean Journal of Science 40: 232–244.
Marnane, M. J., 2000. Site fidelity and homing behaviour in coral reef cardinalfishes. Journal of Fish Biology 57: 1590–1600.
McFarland, W. N., J. C. Ogden & J. N. Lythgoe, 1979. The influence of light on the twilight migrations of grunts. Environmental Biology of Fishes 4: 9–22.
McFarland, W. N. & Z. Hillis, 1982. Observations on agonistic behavior between members of juvenile French and white grunts - Family Haemulidae. Bulletin of Marine Science 32: 255–268.
Meyer, C. G., K. N. Holland, B. M. Wetherbee & C. G. Lowe, 2000. Movement patterns, habitat utilization, home range size and site fidelity of whitesaddle goatfish, Parupeneus porphyreus, in a marine reserve. Environmental Biology of Fishes 59: 235–242.
Moberg, F. & P. Rönnbäck, 2003. Ecosystem services of the tropical seascape: interactions, substitutions and restoration. Ocean & Coastal Management 46: 27–46.
Munro, J. L., 1983. Caribbean Coral Reef Fishery Resources, p. 276. Philippines: ICLARM.
Munro, A. R., T. E. McMahon, S. A. Leathe & G. Liknes, 2003. Evaluation of batch marking small rainbow trout with coded wire tags. North American Journal of Fisheries Management 23: 600–604.
Nagelkerken, I., M. Dorenbosch, W. C. E. P. Verberk, E. Cocheret de la Morinière & G. van der Velde, 2000a. Importance of shallow-water biotopes of a Caribbean bay for juvenile coral reef fishes: patterns in biotope association, community structure and spatial distribution. Marine Ecology Progress Series 202: 175–192.
Nagelkerken, I., M. Dorenbosch, W. C. E. P. Verberk, E. Cocheret de la Morinière & G. van der Velde, 2000b. Day-night shifts of fishes between shallow-water biotopes of a Caribbean bay, with emphasis on the nocturnal feeding of Haemulidae and Lutjanidae. Marine Ecology Progress Series 194: 55–64.
Odum, E. P. & E. J. Kuenzler, 1995. Measurement of territory and home range size in birds. Auk 72: 128–137.
Ogden, J. C. & P. R. Ehrlich, 1977. The behavior of heterotypic resting schools of juvenile grunts (Pomadasyidae). Marine Biology 42: 273–280.
Ogden, J. C. & J. C. Zieman, 1977. Ecological aspects of coral reef-seagrass bed contacts in the Caribbean. Proceedings of the 3rd International Coral Reef Symposium 1: 377–382.
Ogden, J. C. & E. H. Gladfelter, 1983. Coral reefs, seagrass beds and mangroves: their interaction in the coastal zones of the Caribbean. UNESCO Reports in Marine Science 23: 6–16.
Ogden, J. C. & T. P. Quinn, 1984. Migration in coral reef fishes: ecological significance and orientation mechanisms. In McCleave J. D., G. P. Arnold, J. J. Dodson, & W. H. Neill (eds), Mechanisms of Migration in Fishes. Plenum Press, New York: 293–308.
Parrish, J. D. 1989. Fish communities of interacting shallow-water habitats in tropical oceanic regions. Marine Ecology Progress Series 58: 143–160.
Pinto, L. & N. N. Punchihewa, 1996. Utilisation of mangroves and seagrasses by fishes in the Negombo Estuary, Sri lanka. Marine Biology 126: 333–345.
Pittman, S. J. & C. A. McAlpine, 2003. Movements of marine fish and Decapod crustaceans: process, theory and application. Advances in Marine Biology 44: 205–294.
Robblee, M. B. & J. C. Zieman, 1984. Diel variation in the fish fauna of a tropical seagrass feeding ground. Bulletin of Marine Science 34: 335–345.
Rooker, J. R. & G. D. Dennis, 1991. Diel, lunar and seasonal changes in a mangrove fish assemblage off southwestern Puerto Rico. Bulletin of Marine Science 49: 684–698.
Samietz, J. & U. Berger, 1997. Evaluation of movement parameters in insects – bias and robustness with regard to resight numbers. Oecologia 110: 40–49.
Sheaves, M. & B. Molony, 2000. Short-circuit in the mangrove food chain. Marine Ecology Progress Series 199: 97–109.
Tulevech, S. M. & C. W. Recksiek, 1994. Acoustic tracking of adult white grunt, Haemulon plumieri, in Puerto Rico and Florida. Fisheries Research 19: 301–319.
Valdés-Muñoz, E. & A. D. Mochek, 2001. Behavior of marine fishes of the Cuban shelf. In Claro R., K. C. Lindeman, & L. R. Parenti (eds), Ecology of the Marine Fishes of Cuba. Washington and London: Smithsonian Institution Press: 58–72.
Vance, D. J., M. D. E. Haywood, D. S. Heales, R. A. Kenyon, N. R. Loneragan & R. C. Pendrey, 1996. How far do prawns and fish move into mangroves? Distribution of juvenile banana prawns Penaeus merguiensis and fish in a tropical mangrove forest in northern Australia. Marine Ecology Progress Series 131: 115–124.
Verweij, M. C., I. Nagelkerken, D. de Graaff, M. Peeters, E. J. Bakker & G. van der Velde, 2006a. Structure, food and shade attract juvenile coral reef fish to mangrove and seagrass habitats: a field experiment. Marine Ecology Progress Series 306: 257–268.
Verweij, M. C., I. Nagelkerken, S. L. J. Wartenbergh, I. R. Pen & G. van der Velde, 2006b. Caribbean mangroves and seagrass beds as diurnal feeding habitats for juvenile French grunts, Haemulon flavolineatum. Marine Biology 149: 1291–1299.
Watson, M., J. L. Munro & F. R. Gell, 2002. Settlement, movement and early juvenile mortality of the yellowtail snapper Ocyurus chrysurus. Marine Ecology Progress Series 237: 247–256.
Zeller, D. C., 1997. Home range and activity patterns of the coral trout Plectropomus leopardus (Serranidae). Marine Ecology Progress Series 154: 65–77.
Acknowledgements
This study was financially supported by a VIDI-grant from the Netherlands Organisation of Scientific Research (NWO) rewarded to IN and the Schure-Beijerinck-Popping Fund. The following people are thanked for help with catching, tagging and observing fishes: Antony van den Beld, Bas Budel, Dafne de Graaff, Ingmar Hans, Karianne Hol, Astrid Hoogstraten, Chantal Huijbers, Mischa Peeters, Susanne Ruseler, and Suzanne Wartenbergh. We thank the Carmabi Foundation on Curaçao for their hospitality and provision of research materials. Asiento Marina kindly provided docking space for our research boat. The Department of Animal Behaviour of the University of Groningen provided office space for writing an early version of the manuscript (ms). This is Centre for Wetland Ecology Publication nr. 460.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. Martens
Rights and permissions
About this article
Cite this article
Verweij, M.C., Nagelkerken, I. Short and long-term movement and site fidelity of juvenile Haemulidae in back-reef habitats of a Caribbean embayment . Hydrobiologia 592, 257–270 (2007). https://doi.org/10.1007/s10750-007-0772-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-0772-9