Abstract
Bonefish (Albula spp.) have ecological, economic, and cultural importance throughout their tropical and subtropical range. These fish reside primarily in shallow, nearshore habitats, and their movement patterns are largely dominated by tidal flows, thermal regime, and seasonal spawning migrations. Previous studies of their spatial ecology show that bonefish exhibit moderate site fidelity to specific tidal creeks and flats; however, to date, limited research has looked at movement patterns of bonefish that reside in small fringing reef flats, such as those found associated with some islands in the Caribbean. This study used fixed station acoustic telemetry to quantify the movement patterns of bonefish inhabiting small fringing reef flats in the nearshore waters of Culebra, Puerto Rico, for nearly 3 years. Bonefish inhabiting these flats exhibited high site fidelity and small home ranges, with limited movements to flats that were no further than 3 km away. Network analyses revealed distinct groups of bonefish that were associated with the specific reef flats where they were tagged. This high site fidelity has considerable implications for the risk of disturbance to bonefish inhabiting reef flats. These small, isolated groups of fish are likely vulnerable to localized impacts such as habitat degradation or harvest and highly dependent on these specific locations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bonefish (Albula spp.) are a group of marine fishes that inhabit shallow, nearshore tropical and subtropical waters around the globe (Adams and Cooke 2015). Bonefish are benthivores and often reside and feed in large schools, contributing to their ecological role as a vector for nutrient flow and as key components within food webs of nearshore flats ecosystems (Murchie et al. 2013; Brownscombe et al. 2017). Their occupancy of very shallow nearshore marine habitats, wary behavior, and powerful swimming abilities also make several species of bonefish (A. vulpes in the Atlantic, and A. glossodonta in the Indo-Pacific) the focus of highly popular catch-and-release recreational fisheries that support the economy of local communities and entire regions (Fedler 2010, 2013). Some species of bonefish also play an important role as a subsistence food item for local communities (Filous et al. 2019), contributing to food security as well as cultural heritage (Filous et al. 2021).
Given the biological and socioeconomic importance of several bonefish species, their conservation and management are highly relevant for maintaining healthy coastal environments, sustainable fisheries, and resilient communities and economies (Wood et al. 2013; Adams and Cooke 2015). However, the shallow, nearshore flats they inhabit have come under considerable pressure during the Anthropocene due to a range of human-induced stressors (Brownscombe et al. 2019a). For example, A. vulpes, now considered Near Threatened on the IUCN Red List (Adams et al. 2014), has experienced major declines in abundance in South Florida (Frezza and Clem 2015; Rehage et al. 2019), potentially linked to habitat loss (Adams et al. 2014), historical harvest (Barbieri et al. 2008), and increased susceptibility to disease and infection because of the proximity to urbanization (Dunn et al. 2020). Also, tropical and subtropical habitats that bonefish rely on, such as mangrove forests and seagrass beds, have been in decline for decades because of direct physical disturbance and destruction tied to coastline development (Orth et al. 2006; Waycott et al. 2009; Polidoro et al. 2010). To better manage bonefish populations, considerable efforts are being directed towards understanding their biology and ecology to shape conservation strategies and management actions (Adams 2017).
A sound understanding of animal behavior, including their movements and spatial use in the environment, is central to effective conservation planning (Berger-Tal et al. 2011; Cooke et al. 2022). Quantifying when and where individuals spend their time can lead to deeper insights on essential habitats, how organisms make a living throughout their life history, and what influences population dynamics across a range of spatial and temporal scales (Nathan et al. 2008; Morales et al. 2010). As such, the spatial ecology and movement patterns of bonefish have been the focus of considerable attention over the past few decades to identify important habitats across life history stages (Adams et al. 2019, 2021; Haak et al. 2019). For large juveniles and adults, this work has been conducted primarily through the use of mark-recapture (Boucek et al. 2019; Perez et al. 2019b) and acoustic telemetry (Humston et al. 2005; Murchie et al. 2013, 2015; Brownscombe et al. 2017, 2019b). Much of this research has shown that movement patterns of bonefish to and from coastal flats are linked to tidal cycles and thermal tolerances (Humston et al. 2005; Murchie et al. 2011a; Brownscombe et al. 2017, 2019b; Perez et al. 2019a). Studies from The Bahamas, Mexico, and Belize for A. vulpes (Danylchuk et al. 2011; Boucek et al. 2019; Perez et al. 2019b; Adams et al. 2021) and French Polynesia for A. glossodonta (Filous et al. 2020) have shown that, although they live primarily in shallow water with relatively small home ranges, they make seasonal migrations to deeper nearshore habitats such as channels for prespawning aggregations, prior to movements offshore into deep-water drop offs to spawn (Danylchuk et al. 2011, 2019; Lombardo et al. 2020).
Although past studies on the movement patterns of bonefish have provided important insights related to habitat use and connectivity among nearshore coastal seagrass and sand flats, to date there has been limited research on the spatial ecology of bonefish that use smaller and more isolated fringing reef flats that are common to the islands of the Caribbean (but see Finn et al. 2014; Brownscombe et al. 2017, 2019c). These fringing reef flats are often physically much smaller than large sand flats and tidal creeks in other regions, such as The Bahamas and Florida, and spatially disconnected from the coastline, bounded by deeper lagoonal waters. Fringing reef flats are directly adjacent to productive reefs and largely consist of heterogeneous and compacted habitats, whereas most bonefish habitats in The Bahamas and Florida are many kilometers from coral reefs and consist of more homogeneous soft-substrate habitats. Nevertheless, these areas of the coastline remain threatened by anthropogenic disturbances. As such, understanding how bonefish use fringing reef flats is fundamental for developing conservation strategies and management plans that can help maintain the ecological and economic services they provide.
The purpose of this study was to characterize the spatial ecology of bonefish inhabiting coastal waters of Culebra, Puerto Rico. We selected this study location because it supports an existing recreational fishery that targets bonefish inhabiting shallow fringing reef flats that are largely decoupled from adjoining landmasses via deeper, back-reef lagoons. For this work, we used fixed station acoustic telemetry and network analyses to quantify bonefish movement patterns. Given the results of studies elsewhere, we hypothesized that bonefish in Culebra would exhibit some degree of site fidelity to specific flats (their “home flats”). But because of the proximity (< 3 km) of reef flats along the coast of Culebra, we anticipated considerable rates of mixing among individuals from different home flats, and that the timing of movements between reef flats would be associated with potential spawning related movements and the lunar cycle, a well-established spawning cue for bonefish in The Bahamas (Danylchuk et al. 2011, 2019; Lombardo et al. 2020).
Methods
Study sites
The study took place from July 2012 to June 2015 on Culebra, Puerto Rico (18.31578°N, –65.28763°W). Culebra is a relatively small island (28 km2) situated in between the main island of Puerto Rico and St. Thomas, United States Virgin Islands, and is a popular tourist destination.
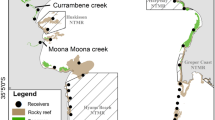
Recreational angling for bonefish in Culebra occurs principally on shallow reef flats (< 2 m deep) separated by deeper channels (8–10 m deep) and we concentrated our efforts on two main flats and their adjacent waters. We heretofore refer to these flats only in terms of their general localities, Dakity or Manglar Bay (Fig. 1a). This approach represents best practice for ensuring that potentially sensitive data on the space use of vulnerable animals is protected (Lennox et al. 2020). These shallow fringing reef flats are comprised of a several benthic habitat types, including sand and coral rubble flats, seagrass beds (Thalassia testudinum, Syringodium filiforme, Halodule wrightii), macroalgae (e.g., Halimeda spp., Penicillus spp.), sponges, hard bottom, and small patches of live coral. Deeper lagoons are inshore of the reef flats, consisting mostly of sand and seagrass, while the seaward slopes are dominated by fringing reefs and reef patches (Brownscombe et al. 2017).
a Culebra Island, Puerto Rico, with Dakity and Manglar Bay labeled. Receiver locations (including aggregated receivers from Vemco Positioning System) and habitat types are indicated by colors. Habitat mapping was provided by the National Oceanic and Atmospheric Administration (Kågesten et al. 2015); b capture locations for tagged bonefish
Acoustic tracking system
The telemetry array included 59 fixed acoustic receivers (VR2W receivers, Innovasea, Amirix Inc., Bedford, NS, Canada) deployed on the focal reef flats, as well as locations along the perimeter of Culebra, the neighboring island of Culebrita, or deployed as part of a Vemco Positioning Systems (VPS) sub-array within Manglar Bay (n = 25) to quantify the fine-scale movement patterns of bonefish (e.g., Brownscombe et al. 2017). The receiver positions outside of the VPS were selected to monitor the overall space use of bonefish and green turtles (Chelonia mydas) (Griffin et al. 2019a) with the highest concentration of receivers around Dakity or Manglar Bay. For the purposes of this paper, only broad-scale movement patterns of bonefish were analyzed, with receivers in the VPS being collapsed into aggregate receiver groups. Specifically, VPS receivers that were in the lagoon were aggregated (n = 17) as a single node and those predominantly around the reef (n = 8) were aggregated together. The mean latitude and longitude for these receivers were derived for each group, respectively. Thus, for the final analyses, position data were available for 36 distinct locations (Fig. 1a) with an approximate area (including land) of 40 km2.
Individual receivers were secured to a 60-cm piece of rebar anchored into a cement base (50 × 30 × 10 cm, approx. 30 kg) and were situated vertically when depths were greater than 1 m (at low tide), and oriented either horizontally or 5–10° above horizontal when the water depth at low tide was less than 1 m (as per Murchie et al. 2011b). Detection probability, as assessed and described in Griffin et al. (2019a), indicated our shallow water flats receivers had 50% detection efficiency at a radius of 80 m.
Tagging
Tags were deployed over two time intervals, occurring between 25 June and 10 Aug 2012 and between 23 April and 25 May 2013. A total of 43 bonefish (n = 20, Dakity; n = 23, Manglar Bay, Fig. 1b) were surgically implanted with V13-1L coded acoustic transmitter tags (13 mm diameter, 36 mm long, 6.0 g in air, min and max delay times 45 to 135 s, 880-day battery life, Innovasea, Amirix Inc., Bedford, NS, Canada) or V13AP accelerometer tags (12.2 g, 45–135 s transmission delay, ± 3.43 g acceleration range, 323-day battery life, 5 Hz sampling frequency, Innovasea, Amirix Inc., Bedford, NS, Canada).
All bonefish were caught using rod and reel, either with fly-fishing gear (8–9 weight rods, 6 kg break strength leaders with no. 4–8 flies) or spinning gear (medium-heavy strength rods, 6 kg break strength monofilament line with 1/0 circle hooks with small live crabs ~ 2 cm width). Based on 50% size at maturity estimates in Florida (418 mm fork length [FL] for males; 488 mm FL for females, Crabtree et al. 1996) and in an attempt to tag only large subadults or adults, only bonefish that were greater than or equal to 410 mm were tagged. Once landed, bonefish were anesthetized with MS-222 (approx. 100 ppm) in a cooler (45 L), and then placed on a portable surgery table in a small skiff. While on the surgery table, a recirculating pump was used to irrigate the gills of the bonefish and supply a maintenance dose of MS 222 (approx. 50 ppm) in fresh seawater. Transmitters and surgical tools were cleaned with Betadine and the surgeon always wore surgical gloves. To implant transmitters, a small incision (2–3 cm) was made through the body wall into the coelomic cavity to the right side of the ventral midline and posterior to the pectoral fin girdle using a #10 disposable scalpel. The transmitter was gently inserted through the incision and slid towards the pectoral fins. The incision was closed with 2–4 interrupted sutures (Ethicon 3–0 PDS II, monofilament absorbable suture material, Johnson and Johnson, New Jersey), and the length of the fish measured (total length [TL] and FL, to nearest mm). The entire surgical procedure took less than 5 min and was conducted by trained surgeons. Fish were then held for up to 30 min in a floating net pen (1.2L × 1.2 W × 1.2H m) in situ prior to release to allow for recovery and minimize the risk of post-release predation. All procedures were approved under the University of Massachusetts Amherst IACUC protocol #2010–004.
Data analyses
All statistical analyses were conducted using R 3.6.2 (R Core Team 2019) and R studio 1.4.953 (RStudio Team 2020) and, unless otherwise indicated, means are presented with one standard deviation. Raw detection records were inspected for overall individual detection patterns and anomalies (e.g., expelled tag sitting near a receiver), and then sorted by transmitter identity and location so that individual broad scale movement patterns could be elucidated. A Student’s t-test was used to identify any differences in the size of individuals between the two tagging areas. In addition, multiple data filters identified potential false detections from code mutations or collisions (Simpfendorfer et al. 2015) using three criteria (1) consecutive detections that were less than the minimum tag delay (45 s), (2) unrealistic movements between receivers (> 3 m per second), and (3) singular detections occurring within a specific time frame (120 min).
Using the VTrack package (Campbell et al. 2012), summary statistics for each fish were extracted including number of detections, number of stations visited (including the aggregated VPS receivers), days detected, tracking duration (time difference from the first detection to the last), residency (days detected divided by tracking duration), and dispersal distances. Subsequently, we converted the detections into short-term centers of activity (COAs) using the mean position algorithm (Simpfendorfer et al. 2002), again, using the VTrack package (Campbell et al. 2012). This method provides an estimated xy position for a specified time window (here, 60 min) based on the weighted means of the number of detections among each receiver (Simpfendorfer et al. 2002). Using the constructed COAs and the VTrack package (Campbell et al. 2012), overall and cumulative monthly space use estimates were derived from minimum convex polygons (MCPs). Here, an MCP encapsulates the extent of the distribution of locations of a given animal and provides a simplified and approximate home range estimate. Furthermore, for bonefish with fewer than six unique locations, MCPs were unable to be estimated. For bonefish with greater than six unique locations, it should be noted that MCPs could span over small areas of land that separate the two regions. Lastly, considering space use MCP estimates of 0 km2 are unrealistic, we added 0.02 km2 (via π * 802) to each MCP estimate based on the approximate 50% detection efficiency at 80 m distance from receivers (Griffin et al. 2019a).
Linear regressions were implemented to test (with an alpha level set to 0.05) if fish size influenced residency, dispersal distance, and MCP size. Model assumptions were evaluated for normality and homogeneity of variance by simulating the residuals 10,000 times using the DHARMa package (Hartig 2017), and if violated, the respective dependent variable was log10 transformed.
To understand connectivity across the region, we examined if and how frequently bonefish moved between Dakity and Manglar Bay. Subsequently, we examined if individuals demonstrated coordinated and directed movements across these areas that may indicate potential pre-spawning/spawning related movements. Furthermore, we evaluated the relationship between the number of bonefish movements between the areas (dependent variable) and lunar cycle (independent variable), classified as new, waxing, full, or waning. This model was implemented with a generalized linear mixed model (Poisson), via the glmmTMB package (Brooks et al. 2017), using an alpha level of 0.05 and with tag ID included as a random effect to control for dependency at the level of individuals. The model was evaluated again using the DHARMa package (Hartig 2017) and checked for overdispersion using the performance package (Lüdecke et al. 2019).
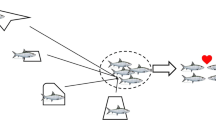
Network analysis, a useful acoustic telemetry analysis technique based in graph theory (Jacoby et al. 2012, Jacoby & Freeman 2016), was also used to explore bonefish space use across Culebra via the igraph package (Csardi and Nepusz 2006). This approach links the movements of each tagged individual to each visited area (Dakity or Manglar Bay) by an edge (arrow) and is weighted by the number of movements/detections, thus providing an indicator of the use of each region. When the connections between individuals and the areas they visited are plotted, they produce bipartite graphs (Dale and Fortin 2010) and can be arranged/positioned by numerous layout algorithms. To qualitatively determine homogeneous or heterogeneous space use of bonefish, we applied the Fruchterman-Reingold force-directed layout algorithm (Fruchterman and Reingold 1991). This algorithm, commonly used with acoustic telemetry data (Finn et al. 2014; Ledee et al. 2016), balances repulsive forces among all nodes with attractive forces between adjacent nodes, and the attractive force being proportional to the weight of the edges connecting adjacent nodes (Tamassia 2013).
To explicitly determine if bonefish displayed similar or dissimilar space use patterns to one another, especially respective to where they were captured, we applied a community detection algorithm that clustered groups of nodes based on their connections. This acoustic telemetry network community approach was first applied as an exploratory analysis on a subset of bonefish detection data from this study by Finn et al. (2014) but has since been implemented in multiple studies using acoustic telemetry (e.g., Griffin et al. 2018, 2019a; Becker et al. 2020; Casselberry et al. 2020). Specifically, Finn et al. (2014) demonstrated that the Fast-Greedy algorithm (Clauset et al. 2004; Newman and Girvan 2004) produced partitions that isolated reef flats where bonefish tend to spend much of their time and was the best algorithm to test our hypothesis regarding site fidelity. Using this algorithm, we identified groups of highly connected nodes that represented potential “communities.” Subsequently, we used a Wilcoxon rank-sum test to confirm or reject community designations (Song and Singh 2013; Finn et al. 2014). If a potential community was significant and the nodes were linked to more nodes within the group (in-degree) than other groups (out-degree), it was deemed a community.
Results
A total of 43 bonefish (522 ± 56 mm) were tagged for this study and a Student’s t-test indicated bonefish tagged at Manglar Bay (540 ± 57 mm) were significantly larger than those tagged at Dakity (501 ± 48 mm) (Supplementary Information Table S1). Across all bonefish, mean tracking duration was 412 days (± 237 days), for fish tagged with transmitters that had 880-day battery lives, mean tracking duration was 457 days (± 246 days), and for fish tagged with transmitters that had 323-day battery lives, mean tracking duration was 243 days (± 61 days). Overall residency was 0.78 (± 0.25), and overall dispersal distances from respective tagging locations ranged from 0 to 3.52 km (1.42 ± 1.08 km) (Supplementary Information Table S1). For tagged individuals with enough locations for MCP calculations (n = 32), MCP estimates ranged from 0.02 to 2.82 km2 (0.65 ± 0.77 km2) with fish on average generally reaching their full MCP estimates 6 months after their first detection (Fig. 2). Fish size had no effect on residency (linear regression, r2 = 0.05, F1,41 = 2.19, P = 0.15), dispersal distance (linear regression, r2 = 0.03, F1,41 = 1.70, P = 0.29), or MCP size (linear regression, r2 = 0.05, F1,30 = 1.60, P = 0.22).
No bonefish was ever detected outside the Dakity or Manglar Bay regions. While only 11 of the generated MCPs (constructed from COAs) spanned across Dakity and Manglar Bay (Fig. 3), examining raw detections highlighted that a total of 15 bonefish actually traveled between the two areas, ranging from 1 to 32 times (7.13 ± 7.98) (Fig. 4, Supplementary Information Figure S1). Bonefish detection histories were widely consistent across time, indicating the majority of time was spent within their respective region; however, occasional detection gaps occurred across multiple days or even months for multiple fish (Fig. 4a). Examining the movements across the two areas by individuals did not highlight any distinctive directed movements. However, between December 2013 and December 2014, when most tracking data occurred, movements across the two areas were generally lowest between May and September (Fig. 4b). While the results from the generalized linear mixed model indicated there was no effect of lunar cycle on the number of trips between Dakity and Manglar Bay (marginal r2 = 0.08, conditional r2 = 0.38, intraclass correlation coefficient = 0.33, Supplementary Information Table S2), the full moon stage had a P value of 0.07.
Network analysis revealed that bonefish showed high site fidelity to their respective capture areas (Fig. 5). Furthermore, community analysis identified two statistically significant regional communities associated with Dakity (Wilcoxon rank-sum test, W = 341, P < 0.001; in-degree = 40, out-degree = 15), and Manglar Bay (Wilcoxon rank-sum test, W = 472.5, P < 0.001; in-degree = 46, out-degree = 15). Although 15 bonefish crossed between these two communities, all fish belonged to the regional community where they were captured and tagged.
Bipartite graph of bonefish region network in Culebra, Puerto Rico, with Fruchterman-Reingold force-directed layout algorithm. The network displays the links (gray edges) between the bonefish (color nodes) and regions visited (white nodes labeled Dakity or Manglar Bay). Bonefish nodes are colored by the region they were captured within, and the width of edges is proportional to the number of detections at each region per individual. This algorithm balances attractive and repulsive forces among nodes which are proportional to the weight of edges connecting adjacent nodes
Discussion
Bonefish movements during our study showed a high degree of fidelity and residency to the fringing reef flats and general areas where individual fish were caught, tagged, and released. Although 15 of the 43 bonefish (35%) were found to move between the reef flats of Dakity and Manglar Bay, individuals spent the majority of their time on or near their respective capture location. Of the 15 fish that moved between Dakity and Manglar Bay (< 3 km), only three individuals did so with some regularity and, overall, while we found no effect of lunar cycle on inter-flats movements, movements between flats generally occurred between October and April. Moreover, no tagged bonefish were detected on receivers deployed outside of Dakity and Manglar Bay, including the west and north sides of Culebra, or on receivers in the channel between Culebra and Culebrita, a smaller island to the east.
While we were unable to identify any distinctive spawning migrations, the mean MCP of less than 1 km2 (± 0.77 km2) in-combination with bonefish rarely leaving their respective flats represents one of the most extreme examples of Albula spp. having a small home range and high site fidelity. Similarly, past studies have also documented high site fidelity to shallow water foraging habitats (e.g., Humston et al. 2005; Boucek et al. 2019); however, bonefish space use was more extensive than in Culebra. For example, across three islands within The Bahamas, excluding movements related to spawning, the mean distance between mark and recapture for bonefish was 3.5–11.1 km (Boucek et al. 2019). In another bonefish study example, within a reef atoll in the Indian Ocean, acoustic telemetry documented a mean MCP of 5.42 km2 (± 1.85 km2) (Moxham et al. 2019). Collectively, the small size of MCPs, low estimates of distance traveled, high rates of residency, and the rarity with which we observed individuals beyond their “home flats” suggest that bonefish in Culebra, Puerto Rico, exhibit strong site fidelity to specific fringing reef flats.
Given bonefish are shallow water benthivores (Colton and Alevizon 1983; Crabtree et al. 1998; Griffin et al. 2019b; Murchie et al. 2019), their energy landscape and associated movements may be influenced by the extent and availability of preferred habitats. Using stable isotope analysis, Murchie et al. (2019) suggested that seagrass-dominated habitats and food webs in The Bahamas may offer a greater availability of high energy density prey items for foraging bonefish relative to macroalgal-dominated food webs and sand habitats. Interestingly, in The Bahamas, where bonefish tend to exhibit lower site fidelity (e.g., 3.5–11.1 km; Boucek et al. 2019) and where their habitats are far from productive reefs and are comprised of predominantly soft substrates such as sand and mud, bonefish growth rates are slow relative to the Florida Keys and the main island of Puerto Rico (Crabtree et al. 1996; Adams et al. 2007). However, it should be noted that growth rates across locations with seagrass-dominated habitats are variable. For example, in Cuba, where collection of some bonefish were within/nearby seagrass habitats, their growth rates were unexpectedly lower than bonefish from Florida Keys; however, this may be linked to over harvesting of bonefish and/or their prey bases (Rennert et al. 2019). Regardless, relative to The Bahamas, Culebra’s compact reef flat habitats are more heterogeneous per unit area and are directly adjacent to both highly productive reefs and seagrasses, potentially resulting in an increase in prey availability. Indeed, within a single flat in Manglar Bay, Brownscombe et al. (2017), using energy expenditure estimates and acoustic telemetry data, demonstrated bonefish foraging behaviors were most concentrated in these seagrass and reef crest areas. Furthermore, magnifying these differences in habitat configuration, tides within Culebra (mean variation = 0.21 m, max = 0.52 m) are relatively weaker than in The Bahamas (e.g., max = 0.8) and have less of an effect on bonefish movements (Murchie et al. 2013; Brownscombe et al. 2019c), likely enabling bonefish to remain longer within not only their preferred depth but also in or near optimal foraging areas. Thus, Culebra’s relatively stable environment and its arrangement and spatial extent of fringing reef flats habitats adjacent to productive reefs may support smaller home ranges with restricted movements.
The observed small home range and high site fidelity to reef flats for bonefish may also be in part motivated by predation risk associated with longer-distance movements (Lima and Dill 1990; Sih 2005), as bonefish may avoid deeper waters where refugia are limited and predator encounters may be higher (Rypel et al. 2007; Guttridge et al. 2012). In this study, deeper water is directly adjacent to the reef flats that bonefish occupy, thus may help to constrain bonefish space use. In contrast, predation risk may be lower for bonefish that occupy contiguous shallow water flats, like that in The Bahamas where deep-water areas are many kilometers away and bonefish space use is higher (Boucek et al. 2019). In Puerto Rico, the most common predator for bonefish is the great barracuda (Sphyraena barracuda). In fact, using acoustic telemetry data from two barracuda tagged within this study site, Finn et al. (2014) demonstrated that barracuda space use aligned with deeper coastal habitats that bonefish would have to traverse to reach other nearby reef flats. Similarly, Humston et al. (2005) noted that the limited use of deeper channel habitats by bonefish in the Florida Keys may be attributed to predator avoidance.
Ultimately, whether due to foraging or predation risk, the high affinity of bonefish to specific shallow fringing reef flats brings to question population resilience in the face of disturbance (Adams et al. 2014). Should future disturbance (e.g., hurricanes or netting) alter habitats or reduce one of these bonefish groups, then the recovery time on a given flat may be longer due to limited inflow of individuals. Furthermore, considering the suggested bonefish reliance on isolated fringing reef flats in Culebra (Brownscombe et al. 2017, 2019b), protecting these productive habitats should also be prioritized. Since Culebra is largely protected through the Culebra National Wildlife Reserve and by its classification of Resource Category I critical habitat for the green turtle (Chelonia mydas) (63 FR 46,693, September 2, 1998), its coastal habitats are comparatively undamaged, providing a means into examining unaltered bonefish behaviors. However, sewage wastewater contamination and sediment runoff have been observed within both Dakity and Manglar Bay (Griffin et al. 2019a; Mclaughlin 2019), potentially threatening adjacent bonefish habitats (e.g., seagrass), as it has in other regions such as in South Florida (Brownscombe et al. 2019a).
The region-wide population connectivity of bonefish across the Caribbean (Wallace and Tringali 2016) is largely mediated through source-sink dynamics via larval dispersal (Zeng et al. 2019; Douglas et al. 2021). Thus, isolation through physical, individual behavior, or other ecological processes, can increase the risk of population declines, especially if these factors lead to the loss of large/older individual bonefish from the population (Filous et al. 2019), or if they affect recruitment (Zeng et al. 2019). Although we did not detect any spawning-related movement patterns similar to those observed in the Florida Keys (Larkin et al. 2007), The Bahamas (A. vulpes, Danylchuk et al. 2011; Murchie et al. 2013, 2015; Adams et al. 2021), and French Polynesia (A. glossodonta, Filous et al. 2020b), if larger/older individuals are lost from these isolated flats, their contribution to the spawning potential of the population could be impacted. This may be increasingly important in this region since larvae recruitment is believed to originate from local spawning populations (Zeng et al. 2019).
As with all studies with acoustic telemetry, our conclusions could be influenced by the distribution of the receivers, temporal coverage/consistency in detection histories, as well as where bonefish were tagged (Heupel et al. 2006; Brownscombe et al. 2019c). While no clear space use patterns were observed that suggested spawning-related movements, movements across the two areas appeared to occur more often between October and April, the general period when other studies have noted bonefish spawning is likely to occur (Danylchuk et al. 2011; Adams et al. 2019, 2021; Perez et al. 2019b). Furthermore, congruent with Brownscombe et al. (2019b), we found lunar cycle to have no effect on bonefish space use. This was a surprising result, considering Albula vulpes are well documented to form pre-spawning aggregations in The Bahamas prior to moving offshore in the night to spawn during the new and full moons (Danylchuk et al. 2011, 2019; Lombardo et al. 2020). Collectively, these results highlight unresolved questions about their spawning behaviors and future opportunities to fill important life history knowledge gaps. If we had the capacity to deploy additional receivers for our study, it may have been prudent to create small curtains to better capture movement patterns to and from fringing reef flats and to identify spawning migrations.
As the threats of anthropogenic disturbances to shallow nearshore marine environments increase during the Anthropocene (Crain et al. 2009), understanding how fish move among their key habitats is essential for adapting conservation and management plans to improve resilience (Lowerre-Barbieri et al. 2021). In the case of bonefish that have small home ranges and high site fidelity to small reef flats, impacts from disturbance can be especially high and result in long lasting population-level effects. Even if harvest is prohibited by laws and regulations, undocumented and uncontrolled illegal harvest via gillnetting, for instance, can quickly reduce bonefish numbers. Such declines may not only impact the ecological role bonefish play on reef flats, but also quickly reduce the viability of the catch-and-release recreational fishery. As such, the results of our study imply that a unified approach to conservation and management is needed to reduce the treats to bonefish that have a high affinity to shallow, nearshore fringing reef flats.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to sensitive fishery data but are available from the corresponding author on reasonable request.
References
Adams AJ (2017) Guidelines for evaluating the suitability of catch and release fisheries: lessons learned from Caribbean flats fisheries. Fish Res 186:672–680
Adams AJ, Cooke SJ (2015) Advancing the science and management of flats fisheries for bonefish, tarpon, and permit. Environ Biol Fishes 98:2123–2131
Adams AJ, Horodysky AZ, McBride RS et al (2014) Global conservation status and research needs for tarpons (Megalopidae), ladyfishes (Elopidae) and bonefishes (Albulidae). Fish Fish 15:280–311
Adams AJ, Lewis JP, Kroetz AM, Grubbs RD (2021) Bonefish (Albula vulpes) home range to spawning site linkages support a marine protected area designation. Aquat Conserv Mar Freshwat Ecosyst 31:1346–1353
Adams AJ, Shenker JM, Jud ZR et al (2019) Identifying pre-spawning aggregation sites for bonefish (Albula vulpes) in the Bahamas to inform habitat protection and species conservation. Environ Biol Fishes 102:159–173
Adams AJ, Wolfe RK, Tringali MD et al (2007) Rethinking the status of Albula spp. biology in the Caribbean and western Atlantic. In: Ault JS (Ed) Biology and management of the world tarpon and bonefish fisheries CRC Press Boca Raton, FL 203216
Barbieri LR, Ault JS, Crabtree RE (2008) Science in support of management decision making for bonefish and tarpon conservation in Florida. In: Ault JS (ed) Biology and management of the world tarpon and bonefish fisheries, first. CRC Series on Marine Biology, 399–404
Becker SL, Finn JT, Novak AJ et al (2020) Coarse-and fine-scale acoustic telemetry elucidates movement patterns and temporal variability in individual territories for a key coastal mesopredator. Environ Biol Fishes 103:13–29
Berger-Tal O, Polak T, Oron A et al (2011) Integrating animal behavior and conservation biology: a conceptual framework. Behav Ecol 22:236–239
Boucek RE, Lewis JP, Stewart BD et al (2019) Measuring site fidelity and homesite-to-pre-spawning site connectivity of bonefish (Albula vulpes): using mark-recapture to inform habitat conservation. Environ Biol Fishes 102:185–195. https://doi.org/10.1007/s10641-018-0827-y
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Brownscombe JW, Cooke SJ, Danylchuk AJ (2017) Spatiotemporal drivers of energy expenditure in a coastal marine fish. Oecologia 183:689–699
Brownscombe JW, Danylchuk AJ, Adams AJ et al (2019a) Bonefish in South Florida: status, threats and research needs. Environ Biol Fishes 102:329–348. https://doi.org/10.1007/s10641-018-0820-5
Brownscombe JW, Griffin LP, Gagne TO et al (2019b) Environmental drivers of habitat use by a marine fish on a heterogeneous and dynamic reef flat. Mar Biol 166:18. https://doi.org/10.1007/s00227-018-3464-2
Brownscombe JW, Lédée EJII, Raby GD et al (2019c) Conducting and interpreting fish telemetry studies: considerations for researchers and resource managers. Rev Fish Biol Fisheries 29:369–400. https://doi.org/10.1007/s11160-019-09560-4
Campbell HA, Watts ME, Dwyer RG, Franklin CE (2012) V-Track: Software for analysing and visualising animal movement from acoustic telemetry detections. Mar Freshw Res 63:815–820. https://doi.org/10.1071/MF12194
Casselberry GA, Danylchuk AJ, Finn JT et al (2020) Network analysis reveals multispecies spatial associations in the shark community of a Caribbean marine protected area. Mar Ecol Prog Ser 633:105–126
Clauset A, Newman MEJ, Moore C (2004) Finding community structure in very large networks. Phys Rev E 70:66111
Colton DE, Alevizon WS (1983) Feeding ecology of bonefish in Bahamian waters. Trans Am Fish Soc 112:178–184
Cook SJ, Auld, HL, Birnie-Gauvin K, Elvidge CK, Piczak ML, Twardek WM, Raby GD, Brownscombe JW, Midwood JD, Lennox RJ (2022) On the relevance of animal behavior to the management and conservation of fishes and fisheries. Environmental Biology of Fishes 1–26
Crabtree RE, Harnden CW, Snodgrass D, Stevens C (1996) Age, growth, and mortality of bonefish, Albula vulpes, from the waters of the Florida Keys. Fish Bull 94:442–451
Crabtree RE, Stevens C, Snodgrass D, Stengard FJ (1998) Feeding habits of bonefish, Albula vulpes, from the waters of the Florida Keys. Fish Bull 96:754–766
Crain CM, Halpern BS, Beck MW, Kappel CV (2009) Understanding and managing human threats to the coastal marine environment. Ann N Y Acad Sci 1162:39–62
Crossin GT, Heupel MR, Holbrook CM et al (2017) Acoustic telemetry and fisheries management. Ecol Appl 27:1031–1049
Csardi G, Nepusz T (2006) The igraph software package for complex network research. InterJournal, Complex Systems 1695:1–9
Dale MRT, Fortin M-JJ (2010) From graphs to spatial graphs. Annual Review of Ecology, Evolution, and Systematics 41
Danylchuk AJ, Cooke SJ, Goldberg TL et al (2011) Aggregations and offshore movements as indicators of spawning activity of bonefish (Albula vulpes) in The Bahamas. Mar Biol 158:1981–1999. https://doi.org/10.1007/s00227-011-1707-6
Danylchuk AJ, Lewis J, Jud Z et al (2019) Behavioral observations of bonefish (Albula vulpes) during prespawning aggregations in the Bahamas: clues to identifying spawning sites that can drive broader conservation efforts. Environ Biol Fishes 102:175–184
Douglas MR, Chafin TK, Claussen JE et al (2021) Are populations of economically important bonefish and queen conch’open’or’closed’in the northern Caribbean Basin? Mar Ecol 42:e12639
Dunn CD, Campbell LJ, Wallace EM, et al (2020) Bacterial communities on the gills of bonefish (Albula vulpes) in the Florida Keys and The Bahamas show spatial structure and differential abundance of disease - associated bacteria. Marine Biology 1–11https://doi.org/10.1007/s00227-020-03698-7
Fedler T (2010) The economic impact of flats fishing in The Bahamas. The Bahamian Flats Fishing Alliance
Fedler T (2013) Economic impact of the Florida Keys flats fishery
Filous A, Lennox RJ, Beaury JP et al (2021) Fisheries science and marine education catalyze the renaissance of traditional management (rahui) to improve an artisanal fishery in French Polynesia. Mar Policy 123:104291
Filous A, Lennox RJ, Clua EEG, Danylchuk AJ (2019) Fisheries selectivity and annual exploitation of the principal species harvested in a data-limited artisanal fishery at a remote atoll in French Polynesia. Ocean Coast Manag 178:104818
Filous A, Lennox RJ, Raveino R et al (2020) The spawning migrations of an exploited Albulid in the tropical Pacific: implications for conservation and community-based management. Environ Biol Fishes 103:1013–1031
Finn JT, Brownscombe JW, Haak CR et al (2014) Applying network methods to acoustic telemetry data: modeling the movements of tropical marine fishes. Ecol Model 293:139–149
Frezza PE, Clem SE (2015) Using local fishers’ knowledge to characterize historical trends in the Florida Bay bonefish population and fishery. Environ Biol Fishes 98:2187–2202
Fruchterman TMJ, Reingold EM (1991) Graph drawing by force-directed placement. Software Practice Exp 21:1129–1164
Gerber BD, Hooten MB, Peck CP et al (2019) Extreme site fidelity as an optimal strategy in an unpredictable and homogeneous environment. Funct Ecol 33:1695–1707
Griffin LP, Finn JT, Diez C, Danylchuk AJ (2019a) Movements, connectivity, and space use of immature green turtles within coastal habitats of the Culebra Archipelago, Puerto Rico: implications for conservation. Endangered Species Research 40:75–90. https://doi.org/10.3354/esr00976
Griffin LP, Brownscombe JW, Adams AJ et al (2018) Keeping up with the Silver King: using cooperative acoustic telemetry networks to quantify the movements of Atlantic tarpon (Megalops atlanticus) in the coastal waters of the southeastern United States. Fish Res 205:65–76. https://doi.org/10.1016/j.fishres.2018.04.008
Griffin LP, Haak CR, Brownscombe JW, et al (2019b) A comparison of juvenile bonefish diets in Eleuthera, The Bahamas, and Florida, U.S. Environmental Biology of Fishes 102:. https://doi.org/10.1007/s10641-018-0822-3
Guttridge TL, Gruber SH, Franks BR et al (2012) Deep danger: intra-specific predation risk influences habitat use and aggregation formation of juvenile lemon sharks Negaprion brevirostris. Mar Ecol Prog Ser 445:279–291
Haak CR, Cowles GW, Danylchuk AJ (2019) Wave and tide-driven flow act on multiple scales to shape the distribution of a juvenile fish (Albula vulpes) in shallow nearshore habitats. Limnol Oceanogr 64:597–615
Hartig F (2017) Package ‘DHARMa’
Heupel MR, Semmens JM, Hobday AJ (2006) Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshw Res 57:1–13
Humston R, Ault JS, Larkin MF, Luo JG (2005) Movements and site fidelity of the bonefish Albula vulpes in the northern Florida Keys determined by acoustic telemetry. Mar Ecol Prog Ser 291:237–248. https://doi.org/10.3354/meps291237
Hussey NE, Kessel ST, Aarestrup K et al (1979) (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science 348:1255642. https://doi.org/10.1126/science.1255642
Kågesten G, Sautter W, Edwards KA, Costa BM, Kracker LM, Battista TA (2015) Shallow-Water benthic habitats of Northeast Puerto Rico and Culebra Island. NOAA Technical Memorandum NOS NCCOS 200. Silver Spring, MD, pp 112. https://doi.org/10.7289/V5Z899FH
Larkin MF, Ault JS, Humston R et al (2007) Tagging of bonefish in South Florida to study population movements and stock dynamics. In: Ault JS (ed) Biology and management of the world tarpon and bonefish fisheries. CRC Press, Boca Raton, FL, p 301
Ledee EJI, Heupel MR, Tobin AJ et al (2016) Movement patterns of two carangid species in inshore habitats characterised using network analysis. Mar Ecol Prog Ser 553:219–232
Lennox RJ, Harcourt R, Bennett JR et al (2020) A novel framework to protect animal data in a world of ecosurveillance. Bioscience 70:468–476
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lombardo SM, Adams AJ, Danylchuk AJ et al (2020) Novel deep-water spawning patterns of bonefish (Albula vulpes), a shallow water fish. Mar Biol 167:1–11
Lowerre-Barbieri SK, Friess C, Griffin LP et al (2021) Movescapes and eco-evolutionary movement strategies in marine fish: assessing a connectivity hotspot. Fish Fish 22:1321–1344. https://doi.org/10.1111/faf.12589
Lüdecke D, Makowski D, Waggoner P, Patil I (2019) performance: assessment of regression models performance. R package version 0.4. 0
Mclaughlin PW (2019) Impacts of unpaved roads on runoff and sediment production in Culebra, Puerto Rico
Morales JM, Moorcroft PR, Matthiopoulos J et al (2010) Building the bridge between animal movement and population dynamics. Philos Trans R Soc Lond B Biol Sci 365:2289–2301. https://doi.org/10.1098/rstb.2010.0082
Moxham EJ, Cowley PD, Bennett RH, von Brandis RG (2019) Movement and predation: a catch-and-release study on the acoustic tracking of bonefish in the Indian Ocean. Environ Biol Fishes 102:365–381
Murchie KJ, Cooke SJ, Danylchuk AJ et al (2011a) Thermal biology of bonefish (Albula vulpes) in Bahamian coastal waters and tidal creeks: an integrated laboratory and field study. J Therm Biol 36:38–48. https://doi.org/10.1016/j.jtherbio.2010.10.005
Murchie KJ, Cooke SJ, Danylchuk AJ et al (2013) Movement patterns of bonefish (Albula vulpes) in tidal creeks and coastal waters of Eleuthera, The Bahamas. Fish Res 147:404–412
Murchie KJ, Cooke SJ, Danylchuk AJ, Suski CD (2011b) Estimates of field activity and metabolic rates of bonefish (Albula vulpes) in coastal marine habitats using acoustic tri-axial accelerometer transmitters and intermittent-flow respirometry. J Exp Mar Biol Ecol 396:147–155
Murchie KJ, Haak CR, Power M et al (2019) Ontogenetic patterns in resource use dynamics of bonefish (Albula vulpes) in the Bahamas. Environ Biol Fishes 102:117–127
Murchie KJ, Shultz AD, Stein JA et al (2015) Defining adult bonefish (Albula vulpes) movement corridors around Grand Bahama in the Bahamian Archipelago. Environ Biol Fishes 98:2203–2212. https://doi.org/10.1007/s10641-015-0422-4
Nathan R, Getz WM, Revilla E et al (2008) A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci U S A 105:19052–19059
Newman MEJ, Girvan M (2004) Finding and evaluating community structure in networks. Phys Rev E 69:26113
Orth RJ, Carruthers TJB, Dennison WC et al (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996
Perez AU, Schmitter-Soto JJ, Adams AJ, Herrera-Pavón RL (2019a) Influence of environmental variables on abundance and movement of bonefish (Albula vulpes) in the Caribbean Sea and a tropical estuary of Belize and Mexico. Environ Biol Fishes 102:1421–1434
Perez AU, Schmitter-Soto JJ, Adams AJ, Heyman WD (2019b) Connectivity mediated by seasonal bonefish (Albula vulpes) migration between the Caribbean Sea and a tropical estuary of Belize and Mexico. Environ Biol Fishes 102:197–207
Polidoro BA, Carpenter KE, Collins L et al (2010) The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS ONE 5:e10095
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Rehage JS, Santos RO, Kroloff EKN et al (2019) How has the quality of bonefishing changed over the past 40 years? Using local ecological knowledge to quantitatively inform population declines in the South Florida flats fishery. Environ Biol Fishes 102:285–298
Rennert JJ, Shenker JM, Angulo-Valdés JA, Adams AJ (2019) Age, growth, and age at maturity of bonefish (Albula species) among Cuban habitats. Environ Biol Fishes 102:253–265
RStudio Team (2020) RStudio: integrated development environment for R. RStudio,
Rypel AL, Layman CA, Arrington DA (2007) Water depth modifies relative predation risk for a motile fish taxon in Bahamian tidal creeks. Estuaries Coasts 30:518–525
Sheer MB, Steel EA (2006) Lost watersheds: barriers, aquatic habitat connectivity, and salmon persistence in the Willamette and Lower Columbia River basins. Trans Am Fish Soc 135:1654–1669
Sih A (2005) Predator-prey space use as an emergent outcome of a behavioral response race. In: Barbosa P, Castellanos I (eds) Ecology of Predator-Prey Interactions. Oxford University Press
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci 59:23–32
Simpfendorfer CA, Huveneers C, Steckenreuter A et al (2015) Ghosts in the data: false detections in VEMCO pulse position modulation acoustic telemetry monitoring equipment. Animal Biotelemetry 3:55
Song J, Singh M (2013) From hub proteins to hub modules: the relationship between essentiality and centrality in the yeast interactome at different scales of organization. PLoS Comput Biol 9:e1002910
Tamassia R (2013) Handbook of graph drawing and visualization. CRC Press
Wallace EM, Tringali MD (2016) Fishery composition and evidence of population structure and hybridization in the Atlantic bonefish species complex (Albula spp.). Mar Biol 163:1–15
Waycott M, Duarte CM, Carruthers TJ et al (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci U S A 106:12377–12381. https://doi.org/10.1073/pnas.0905620106
White GE, Brown C (2013) Site fidelity and homing behaviour in intertidal fishes. Mar Biol 160:1365–1372
Wood AL, Butler JRA, Sheaves M, Wani J (2013) Sport fisheries: opportunities and challenges for diversifying coastal livelihoods in the Pacific. Mar Policy 42:305–314. https://doi.org/10.1016/j.marpol.2013.03.005
Zeng X, Adams A, Roffer M, He R (2019) Potential connectivity among spatially distinct management zones for Bonefish (Albula vulpes) via larval dispersal. Environ Biol Fishes 102:233–252
Acknowledgements
We thank Dr. Craig Lilyestrom (Department of Natural and Environmental Resources, Commonwealth of Puerto Rico), Ricardo Colón-Merced and Ana Roman (Culebra National Wildlife Refuges, US Fish and Wildlife Service), Capt. Chris Goldmark, Todd Plaia, Walter Rieder, Sammy Hernandez, Zorida Mendez, Henry Cruz, Zaco's Tacos, and Karl Andersen for their logistical support, and Temple Fork Outfitters, RIO Products, Costa Sunglasses, Patagonia Inc., Moldy Chum, and Umpqua Feather Merchants for their support. Additional funding for transmitters was provided by Brian Bennett, Brooks Patterson, Temple Fork Outfitters, and RIO Products. Cooke was supported by NSERC and the Canada Research Chairs program.
Funding
This research was supported by the University of Puerto Rico Sea Grant Program (R21-1-12) awarded to AJ Danylchuk and J Finn. Andy Danylchuk and Steven Cooke are Guest Editors of this special issue, but they had no involvement in the peer review of this article and had no access to information regarding its peer review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures for this study were approved by the UMass Amherst IACUC (protocol #2010–0004).
Conflict of interest
The authors have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Griffin, L.P., Brownscombe, J.W., Gagné, T.O. et al. There’s no place like home: high site fidelity and small home range of bonefish (Albula vulpes) inhabiting fringing reef flats in Culebra, Puerto Rico. Environ Biol Fish 106, 433–447 (2023). https://doi.org/10.1007/s10641-022-01312-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01312-x