Abstract
S100 proteins are a family of highly acidic calcium-binding proteins involved in calcium handling in many tissues and organs. Some of these proteins are highly expressed in cardiac tissue, and an impairment of some specific S100 proteins has been related to heart failure. To check this hypothesis, we decided to review the literature since 2008 until May 2015. According to the studies collected, recovering S100A1 levels may enhance contractile/relaxing performance in heart failure, reverse negative force–frequency relationship, improve contractile reserve, reverse diastolic dysfunction and protect against pro-arrhythmic reductions of sarcoplasmic reticulum calcium. The safety profile of gene therapy was also confirmed. Increased S100B protein levels were related to a worse outcome in chronic heart failure. S100A8/A9 complex plasma levels, as well as other inflammatory biomarkers, were significantly higher in chronic heart failure patients. S100A2 seems to increase both contractile and relaxation performance in animal cardiomyocytes. Otherwise, S100A6 cardiac expression seems to have no effects on contractility. S100A4 KO mice showed reduced cardiac interstitial fibrosis. Data collected encourage a potential prospective application in human. These proteins could be exploited as biomarkers in stadiation and prognosis of chronic heart failure, as well as therapeutic target to rescue failing heart.
Registration details The study protocol has been registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/) under registration number CRD42015027932.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the ACCF/AHA statement, they define heart failure as “a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood” [1]. It has been estimated that 5.7 million Americans aged over 18 years have heart failure (NHLBI tabulation). Heidenreich et al. in their study drew the conclusion that HF rate will increase by 46 % over a time stretch from 2012 to 2030; thus, talking numbers, more than 8 million people with age greater than 18 years will incur in HF [2, 3]. Following these percentages in a survey run in 2009 in the USA, the risk of developing HF for people over 40 years of age is 20 % [4]. Two years later, according to what NCHS and NHLBI declared in 2011, one death out nine has heart failure mentioned on the death certificate. Furthermore, despite the outstanding progresses achieved in HF treatment, the morbidity, the mortality and the medical costs still remain elevated; thus, researchers are even more motivated to further dig into the subject in order to lower the overall expense for HF treatments. In 2012, total cost for HF has been estimated to be $30.7 billion. Out of this, 68 % was attributable to direct medical costs; projections show that by 2030, the total cost of HF will increase almost of 127 % to $69.7 billion from 2012. This equals ≈$244 for each US adult [3].
These provisions tell us that during the next 15 years we will not encounter diminishing costs related to heart failure treatments as we would expect (technological advance and new discoveries).
Different factors could induce HF; life style, food and geographical areas are demonstrated to influence the development of this disease [5].
HF severely worsens patient life quality by recurring to frequent hospitalizations. This condition is the end stage of many compensative mechanisms due to the inability of the heart itself to maintain the necessary blood pressure for organ perfusion. These mechanisms lead to a vicious cycle resulting in maladaptive structural and functional modifies in failing hearts [1, 6, 7].
S100 proteins appear to be involved in a large number of cellular activities including signal transduction, cell differentiation, regulation of cell motility, transcription and cell cycle progression [8]. Apart from these intracellular functions, some S100 proteins can be secreted from cells and exhibit cytokine-like extracellular functions. These include chemotactic activities related to inflammation [9, 10].
The incoming of contractility dysfunction and the presence of a pro-inflammatory state lead to a weakened cardiac performance. These are the basis of the impairment of failing heart. Since S100 proteins play a crucial role in these processes, these alarmins have been object of several studies to assess their prognostic and therapeutic value in heart failure.
The aim of this systematic review is to examine latest advances on S100 proteins related to heart failure. On the basis of last years’ findings which were a combination of tests conducted on both animals and humans, our group focused on a pending potential clinical application of this family of proteins. For this purpose, we reviewed studies published during a time period which starts in 2008 until May 2015 involving S100 family proteins in animals and humans failing hearts.
Many serum markers were identified as risk factors for incident in heart failure: BNP, urinary albumin-to-creatinine ratio, elevated serum γ-glutamyltransferase and higher levels of haematocrit [11–13]. Also increased circulating concentrations of resistin were associated with incident HF independent of prevalent coronary disease, obesity, insulin resistance and inflammation [14]. What Djousse et al. [5] found was that lifestyle factors (normal weight, not smoking, regular exercise, moderate alcohol intake, consumption of breakfast cereals and consumption of fruits and vegetables) were related to a lower risk of HF. On the other hand, inflammatory markers such as IL-6 and TNF-alpha, serum albumin levels and cigarette smoking exposure were also associated with HF risk [15–17].
Our purpose is to lower those costs, of course assuming that our first priority is helping people.
In last advances, the attention focused on the sarcoplasmatic reticulum and on the Ca handling process; this resulted in giving a main role to this intracellular organelle in cardiomyocytes [18].
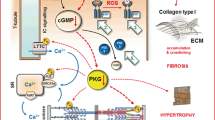
The Ca2+ sensor protein S100A1 has emerged as an attractive target for genetically targeted HF therapy in various in vivo HF models because of its molecular profile [19]. As shown in Fig. 1, S100A1 regulates a network in cardiomyocytes controlling sarcoplasmic reticulum Ca2+ cycling and mitochondrial function through the interaction with the ryanodine receptor (RyR2), sarcoplasmic reticulum Ca2+-ATPase (SERCA2) and mitochondrial F1-ATPase activity, causing anti-hypertrophic, positive inotrope and anti-arrhythmic effects and reducing energy depletion in HF [20–26]. In addition, S100A1 in myofilaments facilitates diastolic Ca2+ dissociation in order to ameliorate relaxation during diastole [27]. Importantly, the S100A1 protein is significantly down-regulated in human end-stage HF, rendering S100A1 an appropriate target for cardiac gene therapy [28, 29].
S100A1 action sites in cardiomyocytes. a Interaction of S100A1 with RyR2 and SERCA. These interactions modulate calcium flux in sarcoplasmic reticulum. b S100A1 in myofilaments facilitates diastolic Ca2+ dissociation in order to ameliorate relaxation during diastole. c S100A1 increases mitochondrial ATP retrieval during augmented energy request
In cardiac muscles, S100A1 is the predominant S100 family member, while S100A4, S100A6 and S100B are also expressed with much less abundance [30]. These other S100 proteins family’s members have been studied during last decades either for prognostic or for therapy purpose.
We describe here the protocol of a systematic review to analyse latest advances on S100 Proteins role, utilization and potential application in the heart failure disease. For this purpose, we reviewed experimental studies that investigated these proteins as markers as well as potential treatments in failing hearts. This systematic review has been registered with PROSPERO (the NIHR International Prospective Register of Systematic Reviews) under registration number CRD42015027932.
Experimental design
Research strategy
This systematic review has been conducted according to PRISMA guidelines [31] employing PubMed and ScienceDirect databases. On these websites, we searched for articles from January 2008 through May 2015 using two key terms related to heart diseases, “heart” or “heart failure” and two key terms related to alarmins, “S100 proteins” and “S100A1”. The electronic search strategy for PubMed is given in Table 1.
Articles have been selected by title and abstract; the entire article was read if title/abstract indicated that the association between heart and S100 proteins was evaluated and that the article potentially met the inclusion criteria. Lastly, we reviewed and searched references of the selected articles and the ones whose titles suggested that could have researched the association between heart and S100 proteins in order to identify additional studies that met the inclusion criteria.
Study selection
Articles were included in the present review according to the following inclusion criteria: English language, publication in peer reviewed journals, year of publication at least 2008. Articles were excluded by title, abstract or full text for irrelevance to the topic in question. Further exclusion criteria were: review articles, editorial comments. Furthermore, we arbitrarily decided to start our research from 2008 to give a more recent view of heart failure findings.
Data extraction
Two authors (MC, SQ) performed the initial search and independently reviewed and selected the references based on the inclusion and exclusion criteria. The data derived from our research of articles include: study author names, publication dates, study designs (i.e. case–control, cross-sectional, longitudinal), groups studied, clinical and biological variables and outcome of interest of the study. Principal outcome of interest included studies in advanced gene therapies on animals and clinical practice prognostic advances in human models. Given the considerable heterogeneity in the study designs and subjects of the selected studies (in terms of biological and clinical variables and targets), the characteristics of the studied populations and protocols were summarized and the study outcome was reported using descriptive statistics without conducting any meta-analyses.
Results
Although S100A1 has a well-known and probably main role in HF, recent findings also suggest the involvement of other S100 proteins in this disease. Consistently, we decided to focus our systematic review on S100 family in its ensemble.
In Fig. 2, the flow of articles retrieved for the review is reported. As given in Table 2, a total of 14 studies assessing the association between S100 proteins (S100) and heart failure (HF) were identified. Out of these 14, 4 evaluated S100 studies in human patients with HF and 10 evaluated S100 studies in animals affected by HF. The search on PubMed and ScienceDirect provided a total of 46 citations (S100 e Heart). An extra amount of three articles was added by matching S100A1 and heart. Eight studies were excluded because they did not meet the criteria as they were reviews or editorial comments. Some articles (n = 27) were excluded by reading the full text because they were clearly not relevant to key purpose of this review. The remaining 14 citations were examined in a more detailed way. All these articles met the inclusion criteria and were included in the systematic review. No additional studies that met the criteria for inclusion were identified by checking the references of selected papers.
Finally, 14 studies have been acknowledged as assessing the association between S100 proteins (S100) and heart failure (HF). Of these, three studies explored the potential employment of S100 as marker in HF while the other studies evaluated the potential therapeutic effects of S100 in both human and animal failing hearts. Table 1 summarizes the studies selected with a particular emphasis on the type of S100 protein in examination, the type of subjects, in vivo or in vitro dosage, the principal outcomes and potential application of the protein. The table shows literature results of the association between S100 family proteins and HF. The search gave a total of 14 results including S100 A1, A2, A4, A6, A8–A9, B protein. These studies had either a prognostic or a therapeutic purpose and were conducted either on animals or on humans.
S100 proteins in animal models
Table 3 shows a total of nine studies about S100 proteins in small and large size animals.
According to the evidence reached during the period before 2008, during last years the attention of the authors focused on a potential therapeutic effect of S100 in failing hearts.
Only one study focused on the confirmation of S100A1 as biomarker. Gupta and his collaborators evaluated the restoration of S100A1 proteins together with other HF markers in dogs with failing heart after chronic therapy with cardiac contractile modulation (CCM). The elements observed in this study confirm the role of S100A1 as a marker of therapy efficacy in HF [32].
Six studies evaluated S100A1 as a treatment by using gene therapy.
Pleger et al. in 2008 demonstrated that S100A1 could potentially improve endothelial function by the generation of NO. This result led to a potential therapeutic strategy by restoring S100A1 levels which are lessened in HF [33].
Both Most and Pleger et al. made a study aimed to determine long-term therapeutic efficacy, safety and feasibility of S100A1 gene therapy with the usage of adeno-associated viral-9 as vector in pigs affected with HF condition. After 12 weeks, animals showed improved cardiac performance and no alteration in blood levels and life parameters. Thanks to these results, they incited a human trial [34, 35].
Yamaguchi et al. proved that S100A1 prevented the development and the progression of HF in small animals. They were led to this conclusion by studying mice with RyR2 mutation that cancelled the inhibitory effect of S100A1 on the ryanodine receptor. Mice with this mutation developed heart failure more quickly than normal mice [36].
Weber and his collaborators compared vector specificity in S100A1 gene therapy. In a study comparing AAV6-S100A1 versus AAV9-S100A1 proteins treating failing pig hearts, they found that S100A1 was significantly over-expressed in pigs treated with AAV6. After these results, Weber et al. focused on treatment efficacy confirming the rescuing ability of this treatment on failing hearts. Their last target was answering authors’ concern on the safety of this kind of therapy. Data obtained confirmed the absence of side-effects such as ventricular tachyarrhythmia or impairment of contractile function. This last therapeutic study confirms once more the efficacy of S100A1 gene therapy on animals encouraging the beginning of human trials [37].
Ritteroff et al. unveiled novel mechanisms explaining the therapeutic effect of this S100 protein by an in vitro/in vivo study on rats analysing S100A1 interaction with RyR2. According to their findings, sarcoplasmic reticulum calcium leak could be recovered in failing cardiomyocytes by restoring the S100A1-RyR2 link (by gene therapy). S100A1, in fact, improved RyR2 closure during diastole allowing a better relaxing and avoiding futile SERCA2a activity compensating for the arrhythmic sarcoplasmic reticulum calcium leak [38].
Based on previous studies involving a RAGE-mediated inflammation damage in HF [39, 40], Volz et al. speculated a S100A8/A9-RAGE driven feed-forward loop. In fact, they treated both mice RAGE-knockout and normal mice with recombinant S100A8/A9. This resulted in a worsening of cardiac performance only in normal mice. According to their findings, inhibiting the ligation of S100A8/A9 with RAGE could be a potential therapeutic target [41].
Another S100 protein, the A4 was analysed by Tamaki’s group basing their study on the known role in cell proliferation and collagen expression through p53. They agreed on the fact that S100A4 modulates p53 function in cardiac fibroblasts and myocardial interstitial fibrosis through cell proliferation control and collagen production. Tamaki et al. did not explain how S100A4 modulates p53. According to them, the inhibition of p53 by blocking S100A4 may have a potential therapeutic role in heart failure by controlling cardiac fibrosis [42].
Wang and his group focused for the first time on S100A2 and A6 on rodent. Data obtained revealed that S100A2 (but not S100A6) enhances Ca2+ cycling and improves contraction and relaxation performance in normal and failing cardiomyocytes. They obtained ectopic expression of these proteins by using gene transfer [43].
S100 proteins in humans
Data obtained in animal studies during last years encouraged studies on humans. Only four studies given in Table 4 were conducted during the period analysed. Three of them aimed to find a biomarker of HF. Only one study aimed to confirm animal results of gene therapy in human cardiomyocytes.
Ma et al. speculated that S100A8 and A9 could be additional markers in elderly patient with failing hearts. A8/A9 complex, as well as IL-6 and IL-8, was found to be increased in enrolled patients (54-year-old patients with chronic HF). These findings suggested a potential use of S100A8 and A9 as pro-inflammatory biomarkers adding sensitivity to the already existing markers [44].
S100B was evaluated as a potential biomarker by Li et al. by assessing S100B protein levels in patients affected by chronic HF. They reported not only increased levels of the alarmin in patients with failing heart, but also that S100B was even more increased if they had chronic kidney disease. By analysing other disease markers such as hsCRP, TNF-alpha, NT-proBNP levels together with echocardiographic assessment, they concluded that S100B is an independent risk factor for chronic HF and for major cardiac events. Increased levels of this protein were correlated with poorer prognosis [45].
In a study published in 2014, Bennett et al. aimed to evaluate whether unloading human failing hearts by applying LVAD (left ventricular assist device) also S100A1 levels would have been recovered. This hypothesis was tested in order to have a better comprehension of the mechanism in rescuing failing hearts by this protein. Indeed, after the support therapy, S100A1 levels were not restored in these patients. According to this study, in fact, the improvement in heart function obtained by unloading the heart by application of LVAD does not correspond to an augmentation of S100A1 levels [46].
Only one study with therapeutic target was conducted on humans in vitro (ventricular cardiomyocytes obtained by failing hearts). Brinks et al. decided to evaluate the potential of gene target therapy of S100A1 in ventricular myocardium of 27 patients with chronic HF [29]. They observed that even modest changes in S100A1 protein by genetically targeted gene addition are actually therapeutic in failing human myocardium in vitro. These findings are consistent with those obtained in animal models [34, 35, 37, 47].
S100 proteins as biomarkers
Since S100A1 is highly expressed in cardiomyocytes, this protein is hypothesized to have a pivotal role in the sarcoplasmic reticulum function of the cardiomyocyte, also due to its ability to interact with the ryanodine receptor and with the SERCA/phospholamban complex [48]. Several years ago, a significant down-regulation of S100A1 protein in heart failure has been already shown [28]. According to these results, many studies aimed to focus on the level of proteins of S100 family in animals and patients with failing hearts. As given in Table 5, four studies evaluated S100 proteins as biomarkers from 2008.
Gupta and his collaborators evaluated S100A1 as HF marker in animals with failing hearts due to its well-known down-regulated expression in this heart disease. Elements observed in Gupta study confirm the role of S100A1 as a marker of therapy’s efficacy in HF [32]. The remaining three studies focused on S100 proteins as biomarkers of HF in humans. Ma et al. speculated that S100A8 and A9 could be additional markers in elderly patient with failing hearts. They confirmed a potential use of S100A8 and A9 as pro-inflammatory biomarkers adding sensitivity to the already existing markers [44]. Li et al. [45] noticed an increased level of S100B not only in patients with failing hearts but that it was even more increased if they had comorbidity. Bennett et al. demonstrated that LVAD therapy in patients with heart failure did not restore S100A1 levels [44]. Tamaki’s group observed that, as S100A4 modulates p53 function in myocardial interstitial fibrosis, the inhibition of p53 by blocking S100A4 could have a potential therapeutic role in heart failure by controlling cardiac fibrosis [42].
S100 proteins as potential therapy
The finding of low levels of S100A1 in animals and humans affected by heart failure encouraged to focus on potential therapies aimed to restore S100A1 levels in failing hearts. As given in Table 6, 10 studies are focused on the therapeutic role of S100 proteins in HF. Six studies evaluated S100A1 as a treatment by using gene therapy in animals. Most, Yamaguchi, Weber and Pleger in two different studies confirmed the efficacy of S100A1 gene therapy in rescuing failing hearts on animal models [33–38].
Other authors decided to focus on potential treatment based on different S100 proteins. Volz et al. [41] found that inhibiting the ligation of S100A8/A9 with RAGE could improve cardiac performance. Wang et al. [43] revealed that S100A2 (but not S100A6) enhances Ca2+ cycling and improves contraction and relaxation performance in normal and failing cardiomyocytes.
Only one study with therapeutic target was conducted on humans in vitro. Brinks’ group observed that even modest increase in S100A1 by target gene therapy in failing myocardiocytes was therapeutic in vitro, consistently with data already obtained in previous animal studies [33, 34, 37, 47].
Discussion
In this review, 14 studies examining latest advances in S100 proteins related to heart failure were identified and systematically reviewed. Different S100 proteins were evaluated in different reviewed articles, due to the enormousness of the S100 proteins family. S100 proteins, in fact, constitute a sizeable family of Ca2+-binding proteins characterized by the EF-hand structural motif [9, 10]. These proteins modulate a multitude of processes, including enzyme activities, calcium handling, energy metabolism, contractility, secretion, genotype modifications [9, 49–51].
Among S100 family’s members, S100A1 is mainly situated in cardiomyocytes, while S100A4, S100A6 and S100B are less represented, but, however, reported in heart tissue [30]. On the other hand, S100A8/A9 complex has been acknowledged as a useful biomarker of inflammation [42].
S100A1 modulates cardiac Ca2+ cycling both by releasing and retaking, and it is also fundamental for the cardiac muscle contractility and in regulating mitochondrial metabolism [19, 27].
Several studies suggested that diminished levels of S100A1 correlate with heart failure severity [24, 35, 52–54], and an accelerated progression to heart failure in mice missing of S100A1 proteins was also confirmed [24, 55]; we found nine studies focused on the therapeutic role of S100A1; seven out of these evaluated S100A1 gene therapy. Six of these studies were conducted on animals, while one only on human models [29]. Four out of these studies reported that restoring S100A1 levels in animals HF models by cardiac-targeted S100A1 gene transfer improved cardiac function in the long term [24, 35, 52, 54]. Brinks et al. demonstrated that S100A1 gene therapy recovered the protein levels in human cardiomyocytes obtained from patients with severe HF undergoing heart transplantation. Obviously, the study was conducted in vitro evaluating only the cardiomyocyte microenvironment itself. Actually, there is no evidence of this kind of gene therapy in vivo on humans [29].
Bennett et al. [46] investigated S100A1 tissue levels in failing left ventricular assist device (LVAD)-supported hearts, with the aim to evaluate whether the rescuing of cardiac performance is accompanied to an increase in S100; the findings appeared to be controversial, since although several indices suggested an improvement of left ventricle performance, the expected normalization of S100A1 levels was not confirmed. This mismatch was probably due to the use of a different LVAD technology from the one used in previous studies [56].
The remaining five studies evaluated other S100 family proteins. Two of them (one in animals and the other in humans) focused on the S100A8–A9 complex [41, 44]; based on these studies, a prognostic role was given to the complex levels. As seen in many other studies, these proteins could be also increased in many inflammatory diseases [57–61].
S100B was closely associated with HF stadiation and with the severity of coexisting kidney failure by Jin Ping Li and his group. According to these data, S100B over-expression could be considered a negative prognostic marker [45].
Only two studies evaluated the therapeutic role of different S100 proteins in animals [42, 43]. S100A2-6 and S100A4 in fact appear to have a potential in failing heart treatment.
During the last decades, the attention in HF mechanisms moved from the organic and functional to the molecular approach. The review of these 14 studies over the last 7 years gave us a better comprehension of advanced HF physiopathology. Data are quite consistent. Together with macroscopic alterations, including cardiac remodelling and fibrosis, that may result in a worse cardiac performance, a microscopic damage coexists that may lead to impaired cardiac function.
According to the evidence gathered, a main role in HF is attributed to calcium handling and to sarcoplasmic reticulum, and S100A1 protein has a key role in calcium homoeostasis through this organelle [62–66]. Advanced heart failure modifies cardiomyocyte expression of S100A1, resulting in lowering S100A1 tissue levels [67]. Data from animal studies are consistent: S100A1 gene therapy recovers tissue S100A1 levels and improves cardiac performance [33, 34, 37, 47]. This suggestion has been also confirmed by in vitro study on human cardiomyocytes [29].
S100B and S100A8–A9 complex appear to have a minor clinical impact, but their levels could add more specificity to the prognosis and stadiation in HF. Moreover, study results suggest that S100 proteins family has a main role also in local damage and inflammation.
An interesting aspect concerns the potential ability of S100 proteins as biomarkers in HF. C-reactive protein (CRP) has been already widely investigated in HF, already evaluating a potential pathophysiological role; several studies reported similar findings: patients affected by HF with higher levels of CRP have a worse prognosis [68–71]. However, CRP is not a cardiac-specific biomarker, and its levels are conditioned by many causes, since it is the most clinical-adapt marker of active inflammation.
S100B protein on the contrary is a tissue-specific protein (chondrocytes, adipocytes, skeletal myofibers, cardiomyocytes, dendritic cells, etc.); it is released after a damage (and the consequent remodelling) involving these tissues, increasing its plasma levels. These findings suggest S100B to be a more specific marker of heart disease [72, 73].
Limitations
Given the considerable heterogeneity in the study designs and subjects (sample sizes, proteins analysed, animal/human studies) of the selected studies, the characteristics of study populations, the objectives and the study results are reported using descriptive statistics without conducting meta-analyses.
Tables redundancy may appear as a limitation, but is voluntarily obtained to focus reader attention on the topic in examination.
Conclusions
New insight into molecular mechanisms in heart failure is a current challenge for a modern medicine approach. More evidence on efficacy and safety of gene therapy has to be collected in humans. Also, S100A1 safety profile has to be further investigated. Other kinds of target therapy (i.e. monoclonal antibodies versus S100A1 ligand-site like RyR2, SERCA and phospholamban) could be considered as an alternative. S100A8–A9 and S100B have a good potential and should be evaluated on a larger cohort of patients. In a near future, these proteins could be exploited as biomarkers in stadiation and prognosis of chronic heart failure, as well as therapeutic target to rescue failing heart.
References
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 accf/aha guideline for the management of heart failure: a report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol 62:e147–e239
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2013) Heart disease and stroke statistics—2013 update: a report from the american heart association. Circulation 127:e6–e245
Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG (2013) Forecasting the impact of heart failure in the united states: a policy statement from the american heart association. Circ Heart Fail 6:606–619
Djousse L, Driver JA, Gaziano JM (2009) Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA 302:394–400
Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A (2013) Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol 168:1186–1194
Mehra MR, Uber PA, Francis GS (2003) Heart failure therapy at a crossroad: are there limits to the neurohormonal model? J Am Coll Cardiol 41:1606–1610
Nieminen MS, Dickstein K, Fonseca C, Serrano JM, Parissis J, Fedele F, Wikstrom G, Agostoni P, Atar S, Baholli L, Brito D, Colet JC, Edes I, Gomez Mesa JE, Gorjup V, Garza EH, Gonzalez Juanatey JR, Karanovic N, Karavidas A, Katsytadze I, Kivikko M, Matskeplishvili S, Merkely B, Morandi F, Novoa A, Oliva F, Ostadal P, Pereira-Barretto A, Pollesello P, Rudiger A, Schwinger RH, Wieser M, Yavelov I, Zymlinski R (2015) The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 191:256–264
Schafer BW, Heizmann CW (1996) The s100 family of ef-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci 21:134–140
Heizmann CW (2002) The multifunctional s100 protein family. Methods Mol Biol 172:69–80
Heizmann CW, Fritz G, Schafer BW (2002) S100 proteins: structure, functions and pathology. Front Biosci 7:d1356–d1368
Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, Selhub J, Jacques PF, Meigs JB, Tofler GH, Vasan RS (2010) Multimarker approach for the prediction of heart failure incidence in the community. Circulation 122:1700–1706
Dhingra R, Vasan RS (2012) Diabetes and the risk of heart failure. Heart Fail Clin 8:125–133
Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL (2012) Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham heart study. Circulation 126:1596–1604
Frankel DS, Vasan RS, D’Agostino RB Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB (2009) Resistin, adiponectin, and risk of heart failure the framingham offspring study. J Am Coll Cardiol 53:754–762
Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J (2010) Inflammatory markers and incident heart failure risk in older adults: the health abc (health, aging, and body composition) study. J Am Coll Cardiol 55:2129–2137
Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins-Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB, Butler J (2012) Cigarette smoking exposure and heart failure risk in older adults: the health, aging, and body composition study. Am Heart J 164:236–242
Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Tang WW, Methvin A, Smith AL, Bauer DC, Newman AB, Kim L, Harris TB, Kritchevsky SB, Butler J (2010) Serum albumin concentration and heart failure risk the health, aging, and body composition study. Am Heart J 160:279–285
Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ (2011) Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (cupid): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-atpase in patients with advanced heart failure. Circulation 124:304–313
Kraus C, Rohde D, Weidenhammer C, Qiu G, Pleger ST, Voelkers M, Boerries M, Remppis A, Katus HA, Most P (2009) S100a1 in cardiovascular health and disease: closing the gap between basic science and clinical therapy. J Mol Cell Cardiol 47:445–455
Boerries M, Most P, Gledhill JR, Walker JE, Katus HA, Koch WJ, Aebi U, Schoenenberger CA (2007) Ca2+-dependent interaction of s100a1 with f1-atpase leads to an increased atp content in cardiomyocytes. Mol Cell Biol 27:4365–4373
Gusev K, Ackermann GE, Heizmann CW, Niggli E (2009) Ca2+ signaling in mouse cardiomyocytes with ablated s100a1 protein. Gen Physiol Biophys 28:371–383
Most P, Bernotat J, Ehlermann P, Pleger ST, Reppel M, Borries M, Niroomand F, Pieske B, Janssen PM, Eschenhagen T, Karczewski P, Smith GL, Koch WJ, Katus HA, Remppis A (2001) S100a1: a regulator of myocardial contractility. Proc Natl Acad Sci USA 98:13889–13894
Most P, Remppis A, Pleger ST, Loffler E, Ehlermann P, Bernotat J, Kleuss C, Heierhorst J, Ruiz P, Witt H, Karczewski P, Mao L, Rockman HA, Duncan SJ, Katus HA, Koch WJ (2003) Transgenic overexpression of the Ca2+-binding protein s100a1 in the heart leads to increased in vivo myocardial contractile performance. J Biol Chem 278:33809–33817
Most P, Seifert H, Gao E, Funakoshi H, Volkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR Jr, Eckhart AD, Feldman AM, Koch WJ (2006) Cardiac s100a1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation 114:1258–1268
Tsoporis JN, Marks A, Zimmer DB, McMahon C, Parker TG (2003) The myocardial protein s100a1 plays a role in the maintenance of normal gene expression in the adult heart. Mol Cell Biochem 242:27–33
Volkers M, Loughrey CM, Macquaide N, Remppis A, DeGeorge BR Jr, Wegner FV, Friedrich O, Fink RH, Koch WJ, Smith GL, Most P (2007) S100a1 decreases calcium spark frequency and alters their spatial characteristics in permeabilized adult ventricular cardiomyocytes. Cell Calcium 41:135–143
Volkers M, Rohde D, Goodman C, Most P (2010) S100a1: a regulator of striated muscle sarcoplasmic reticulum Ca2+ handling, sarcomeric, and mitochondrial function. J Biomed Biotechnol 2010:178614
Remppis A, Greten T, Schafer BW, Hunziker P, Erne P, Katus HA, Heizmann CW (1996) Altered expression of the Ca(2+)-binding protein s100a1 in human cardiomyopathy. Biochim Biophys Acta 1313:253–257
Brinks H, Rohde D, Voelkers M, Qiu G, Pleger ST, Herzog N, Rabinowitz J, Ruhparwar A, Silvestry S, Lerchenmuller C, Mather PJ, Eckhart AD, Katus HA, Carrel T, Koch WJ, Most P (2011) S100a1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol 58:966–973
Davis J, Westfall MV, Townsend D, Blankinship M, Herron TJ, Guerrero-Serna G, Wang W, Devaney E, Metzger JM (2008) Designing heart performance by gene transfer. Physiol Rev 88:1567–1651
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Gupta RC, Mishra S, Rastogi S, Wang M, Rousso B, Mika Y, Remppis A, Sabbah HN (2009) Ca(2+)-binding proteins in dogs with heart failure: effects of cardiac contractility modulation electrical signals. Clin Transl Sci. 2:211–215
Pleger ST, Harris DM, Shan C, Vinge LE, Chuprun JK, Berzins B, Pleger W, Druckman C, Volkers M, Heierhorst J, Oie E, Remppis A, Katus HA, Scalia R, Eckhart AD, Koch WJ, Most P (2008) Endothelial s100a1 modulates vascular function via nitric oxide. Circ Res 102:786–794
Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, Most P (2011) Cardiac aav9-s100a1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med 3:92ra64
Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ (2007) Stable myocardial-specific aav6-s100a1 gene therapy results in chronic functional heart failure rescue. Circulation 115:2506–2515
Yamaguchi N, Chakraborty A, Huang TQ, Xu L, Gomez AC, Pasek DA, Meissner G (2013) Cardiac hypertrophy associated with impaired regulation of cardiac ryanodine receptor by calmodulin and s100a1. Am J Physiol Heart Circ Physiol 305:H86–H94
Weber C, Neacsu I, Krautz B, Schlegel P, Sauer S, Raake P, Ritterhoff J, Jungmann A, Remppis AB, Stangassinger M, Koch WJ, Katus HA, Muller OJ, Most P, Pleger ST (2014) Therapeutic safety of high myocardial expression levels of the molecular inotrope s100a1 in a preclinical heart failure model. Gene Ther 21:131–138
Ritterhoff J, Volkers M, Seitz A, Spaich K, Gao E, Peppel K, Pleger ST, Zimmermann WH, Friedrich O, Fink RH, Koch WJ, Katus HA, Most P (2015) S100a1 DNA-based inotropic therapy protects against proarrhythmogenic ryanodine receptor 2 dysfunction. Mol Ther 23:1320–1330
Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM (1999) Rage mediates a novel proinflammatory axis: a central cell surface receptor for s100/calgranulin polypeptides. Cell 97:889–901
Vogl T, Gharibyan AL, Morozova-Roche LA (2012) Pro-inflammatory s100a8 and s100a9 proteins: self-assembly into multifunctional native and amyloid complexes. Int J Mol Sci 13:2893–2917
Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, Andrassy M (2012) S100a8/a9 aggravates post-ischemic heart failure through activation of rage-dependent nf-kappab signaling. Basic Res Cardiol 107:250
Tamaki Y, Iwanaga Y, Niizuma S, Kawashima T, Kato T, Inuzuka Y, Horie T, Morooka H, Takase T, Akahashi Y, Kobuke K, Ono K, Shioi T, Sheikh SP, Ambartsumian N, Lukanidin E, Koshimizu TA, Miyazaki S, Kimura T (2013) Metastasis-associated protein, s100a4 mediates cardiac fibrosis potentially through the modulation of p53 in cardiac fibroblasts. J Mol Cell Cardiol 57:72–81
Wang W, Asp ML, Guerrero-Serna G, Metzger JM (2014) Differential effects of s100 proteins a2 and a6 on cardiac Ca(2+) cycling and contractile performance. J Mol Cell Cardiol 72:117–125
Ma LP, Haugen E, Ikemoto M, Fujita M, Terasaki F, Fu M (2012) S100a8/a9 complex as a new biomarker in prediction of mortality in elderly patients with severe heart failure. Int J Cardiol 155:26–32
Li JP, Lu L, Wang LJ, Zhang FR, Shen WF (2011) Increased serum levels of s100b are related to the severity of cardiac dysfunction, renal insufficiency and major cardiac events in patients with chronic heart failure. Clin Biochem 44:984–988
Bennett MK, Sweet WE, Baicker-McKee S, Looney E, Karohl K, Mountis M, Tang WH, Starling RC, Moravec CS (2014) S100a1 in human heart failure: lack of recovery following left ventricular assist device support. Circ Heart Fail 7:612–618
Most P, Raake P, Weber C, Katus HA, Pleger ST (2013) S100a1 gene therapy in small and large animals. Methods Mol Biol 963:407–420
Ehlermann P, Remppis A, Guddat O, Weimann J, Schnabel PA, Motsch J, Heizmann CW, Katus HA (2000) Right ventricular upregulation of the Ca(2+) binding protein s100a1 in chronic pulmonary hypertension. Biochim Biophys Acta 1500:249–255
Salama I, Malone PS, Mihaimeed F, Jones JL (2008) A review of the s100 proteins in cancer. Eur J Surg Oncol 34:357–364
Chan WY, Xia CL, Dong DC, Heizmann CW, Yew DT (2003) Differential expression of s100 proteins in the developing human hippocampus and temporal cortex. Microsc Res Tech 60:600–613
Buckiova D, Syka J (2009) Calbindin and s100 protein expression in the developing inner ear in mice. J Comp Neurol 513:469–482
Most P, Pleger ST, Volkers M, Heidt B, Boerries M, Weichenhan D, Loffler E, Janssen PM, Eckhart AD, Martini J, Williams ML, Katus HA, Remppis A, Koch WJ (2004) Cardiac adenoviral s100a1 gene delivery rescues failing myocardium. J Clin Invest 114:1550–1563
Pleger ST, Most P, Heidt B, Voelkers M, Hata JA, Katus HA, Remppis A, Koch WJ (2006) S100a1 gene transfer in myocardium. Eur J Med Res 11:418–422
Pleger ST, Remppis A, Heidt B, Volkers M, Chuprun JK, Kuhn M, Zhou RH, Gao E, Szabo G, Weichenhan D, Muller OJ, Eckhart AD, Katus HA, Koch WJ, Most P (2005) S100a1 gene therapy preserves in vivo cardiac function after myocardial infarction. Mol Ther 12:1120–1129
Desjardins JF, Pourdjabbar A, Quan A, Leong-Poi H, Teichert-Kuliszewska K, Verma S, Parker TG (2009) Lack of s100a1 in mice confers a gender-dependent hypertensive phenotype and increased mortality after myocardial infarction. Am J Physiol Heart Circ Physiol 296:H1457–H1465
Ogletree ML, Sweet WE, Talerico C, Klecka ME, Young JB, Smedira NG, Starling RC, Moravec CS (2010) Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transpl 29:554–561
Altwegg LA, Neidhart M, Hersberger M, Muller S, Eberli FR, Corti R, Roffi M, Sutsch G, Gay S, von Eckardstein A, Wischnewsky MB, Luscher TF, Maier W (2007) Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J 28:941–948
Frosch M, Vogl T, Seeliger S, Wulffraat N, Kuis W, Viemann D, Foell D, Sorg C, Sunderkotter C, Roth J (2003) Expression of myeloid-related proteins 8 and 14 in systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum 48:2622–2626
Hermani A, Hess J, De Servi B, Medunjanin S, Grobholz R, Trojan L, Angel P, Mayer D (2005) Calcium-binding proteins s100a8 and s100a9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res 11:5146–5152
Ikemoto M, Tanaka T, Takai Y, Murayama H, Tanaka K, Fujita M (2003) New elisa system for myeloid-related protein complex (mrp8/14) and its clinical significance as a sensitive marker for inflammatory responses associated with transplant rejection. Clin Chem 49:594–600
Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI (2006) Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 113:2278–2284
Hasenfuss G, Pieske B (2002) Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34:951–969
Lompre AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR (2010) Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 121:822–830
Piacentino V 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR (2003) Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res 92:651–658
Tomaselli GF, Marban E (1999) Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 42:270–283
Yano M, Yamamoto T, Kobayashi S, Ikeda Y, Matsuzaki M (2008) Defective Ca2+ cycling as a key pathogenic mechanism of heart failure. Circ J 72(Suppl A):A22–A30
Rohde D, Brinks H, Ritterhoff J, Qui G, Ren S, Most P (2011) S100a1 gene therapy for heart failure: a novel strategy on the verge of clinical trials. J Mol Cell Cardiol 50:777–784
Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M (2003) Inflammatory markers and onset of cardiovascular events: results from the health abc study. Circulation 108:2317–2322
Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BH, Witteman JC (2006) C-reactive protein and risk of heart failure. The Rotterdam study. Am Heart J 152:514–520
Williams ES, Shah SJ, Ali S, Na BY, Schiller NB, Whooley MA (2008) C-reactive protein, diastolic dysfunction, and risk of heart failure in patients with coronary disease: heart and soul study. Eur J Heart Fail 10:63–69
Araujo JP, Lourenco P, Azevedo A, Frioes F, Rocha-Goncalves F, Ferreira A, Bettencourt P (2009) Prognostic value of high-sensitivity c-reactive protein in heart failure: a systematic review. J Card Fail 15:256–266
Tsoporis JN, Marks A, Kahn HJ, Butany JW, Liu PP, O’Hanlon D, Parker TG (1997) S100beta inhibits alpha1-adrenergic induction of the hypertrophic phenotype in cardiac myocytes. J Biol Chem 272:31915–31921
Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I (2009) S100b’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793:1008–1022
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Imbalzano, E., Mandraffino, G., Casciaro, M. et al. Pathophysiological mechanism and therapeutic role of S100 proteins in cardiac failure: a systematic review. Heart Fail Rev 21, 463–473 (2016). https://doi.org/10.1007/s10741-016-9529-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9529-8