Abstract
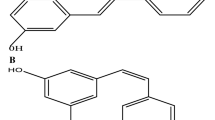
Resveratrol is a well-known antioxidant that exists in grape skin/seed, red wine, and the root of Polygonum cuspidatum, a traditional Chinese and Japanese medicinal material. Studies have found that resveratrol has many interesting properties, including anti-carcinogenic properties, anti-microbial and antiviral effects, the ability to reverse dyslipidemia and obesity, the ability to attenuate hyperglycemia and hyperinsulinemia, and the ability to protect endothelial function. Heart failure is the final consequence of the majority of cardiovascular diseases, and resveratrol has been shown to directly attenuate heart contraction. The cardiovascular protective capacities of resveratrol are associated with multiple molecular targets and may lead to the development of novel therapeutic strategies for atherosclerosis, ischemia/reperfusion, metabolic syndrome, and heart failure. This article will mainly review recently published basic researches about the protective cardiovascular effects of resveratrol because these results may lead to the development of new clinical therapeutics in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is one of the leading causes of death throughout the world. A common feature of cardiovascular risk factors, including hypertension, obesity, dyslipidemia, insulin resistance, and glucose intolerance, is the increase in oxidative stress [1, 2]. Many studies show that antioxidants such as tempol and apocynin significantly prevent organ damage induced by a variety of factors, including cuff injury, hypoxia, ischemia/reperfusion (I/R) injury, angiotensin II/high salt loading, and metabolic disorder [3–8]. However, the toxicity of tempol and apocynin limits their application in humans. Numerous clinical trials have evaluated the effects of vitamin C and vitamin E, effective antioxidants in animal experiments, on the prevention of coronary heart disease and stroke, but most of which have reached disappointing conclusions [2, 9]. Typically, HOPE and GISSI studies with antioxidative vitamin E supplement failed to improve the outcomes of cardiovascular disease patients [10–12]. The use of angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, calcium channel blockers, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, and peroxisome-proliferator-activated receptor (PPAR)-γ agonists has been reported to decrease oxidative stress in many clinical treatments [13–18], but there is not a specific, effective, and safe antioxidant that is currently used for the treatment of patients with cardiovascular disease.
On the other hand, it seems that a drug targeting multiple points may exhibit better therapeutic efficacy than that blocking or activating one target in complex conditions [19]. A possible reason is that common disorders such as cardiovascular diseases tend to result from multiple molecular abnormalities. One drug to one target and taking various tablets will cause higher cost, more interactions between drugs and lower compliance. Resveratrol (3,5,4′-trihydroxystilbene), one of the main polyphenol extracts in grape skin/seed, red wine, and the root of Polygonum cuspidatum, is found to have protective potentials to multi-targets related to cardiovascular diseases [20, 21]. Especially, resveratrol is best known for its antioxidant properties in vivo, and several protective intracellular pathways of resveratrol are associated with oxidative stress.
Antioxidant properties of resveratrol
Resveratrol acts as a radical-scavenging antioxidant via the following reaction: Res-(OH)3 + R· → Res-(OH)2O· + RH, in which Res-(OH)3 represents resveratrol and R· represents free radical [22]. The unpaired electron of Res-(OH)2O· is expected to be delocalized over aromatic rings of resveratrol, resulting in poor reactivity or nocuity [22]. However, the direct antioxidant effects of resveratrol are weak. The protective effects of resveratrol against oxidative injury both in vivo and in vitro are likely to be attributed to the upregulation of endogenous cellular antioxidant systems rather than the direct scavenging activity of reactive oxygen species (ROS) [23].
The free radical reactions are shown in Fig. 1. Resveratrol has been shown to inhibit oxygen free radical formation by suppressing pro-oxidative genes (such as nicotinamide adenine dinucleotide phosphate oxidase and myeloperoxidase) [20, 23–25] and inducing antioxidative enzymes or substrates of these enzymes including superoxide dismutase (SOD), catalase, thioredoxin, and glutathione peroxidase (GSH-Px) [23, 26–28]. Superoxide dismutase and GSH-Px are the major enzymes responsible for the inactivation of O2 − and hydrogen peroxide, respectively, and they can restore the activity of other endogenous antioxidants. The major SOD regulated by resveratrol is manganese SOD. Manganese SOD locates mainly in mitochondria and counteracts ROS production from mitochondrial electron transport chain [27]. Studies have reported that resveratrol chelates transition metallic copper, which is able to generate free radicals and cause lipid peroxidation [29, 30]. Moreover, heme oxygenase-1 (HO-1) is a stress-response protein, which may play an important role in mediating protection against oxidative injury, and resveratrol can upregulate HO-1 [26, 28].
Main endogenous oxygen radical reactions. Oxidases convert oxygen to superoxide, which is then converted to hydrogen peroxide by SOD [1]. Hydrogen peroxide can either be converted to H2O by catalase, peroxiredoxin or GSH-Px, or to a hydroxyl radical after reacting with metal ions. The substrate of GSH-Px and the substrate of peroxiredoxin are reduced glutathione and thioredoxin, which are endogenous antioxidants that play a central role in the antioxidant system of most aerobic cells. Resveratrol is also the direct scavenger of hydroxyl radical and superoxide. In addition, superoxide reacts rapidly with nitric oxide to form peroxynitrite. Peroxynitrite either is oxidized to the inactive nitrate or reacts with the –SH group of thiol and ascorbate [35, 36]. NADPH nicotinamide adenine dinucleotide phosphate, SOD superoxide dismutase, GSH-Px glutathione peroxidase
Resveratrol has been shown to enhance the expression of endothelial nitric oxide synthase (eNOS), modulate the deacetylation of eNOS, and increase plasma nitric oxide (NO) levels [31–34]. Nitric oxide [also catalyzed by inducible NO synthase (iNOS); see preconditioning] behaves as a potent antioxidant, which reacts with O2 − and generates peroxynitrate radicals (ONOO−) in vivo [35, 36]. In physiological systems such as the heart where adequate amounts of thiol and ascorbate are present, the ONOO− preferentially react with the –SH groups of thiol and ascorbate, thus losing nocuity [35]. Most importantly, the affinity of NO for O2 − is far greater than the affinity of SOD for O2 −, thus the resveratrol/NO pathway plays an important role in O2 − elimination [35].
Anti-inflammation and anti-coagulation activities
Resveratrol and inflammation
Atherosclerosis is now considered as an inflammatory disease. Inflammatory processes occur during atherogenesis, and the major events in the inflammatory process include the activated inflammatory cells with subsequent enhanced infiltration, the amplified release of chemoattractants/inflammatory cytokines, and the increased leukocyte–endothelial cell interactions. These processes can lead to a weakening of the fibrous cap that overlies the lipid core of plaque and promote the susceptibility of plaque to rupture and subsequent thrombosis. Reactive oxygen species play an important role in the development of inflammation and lead to development in metabolic dyslipidemia, impaired glucose metabolism and hypertension [37]. On the other hand, the upregulation of proinflammatory adipokines is observed to promote oxidative stress in obesity and inflammation situation. The interplay of oxidative stress, inflammation, obesity, insulin resistance, and hypertension ultimately increases atherogenic risk [37, 38].
Another characteristic of resveratrol is anti-inflammation. Resveratrol not only modulates biochemical responses of polymorphonuclear leukocytes by interfering with the release of inflammatory mediators (e.g., platelet endothelial cell adhesion molecule-1) [24, 39], but also suppresses the activity of T cells, B cells, and macrophages, which was shown by significant inhibition of their proliferation, antibody production, and lymphokine secretion [40].
Resveratrol has also been shown to mediate anti-inflammatory processes by inhibiting cyclooxygenase (COX), which catalyzes the conversion of arachidonic acid into prostanoids and thromboxane A2 (TxA2). There are two distinct membrane-anchored isoenzymes of COX: COX-1 and COX-2. Resveratrol may act similarly to aspirin, which is an effective cardioprotective agent that targets platelet-specific COX-1; however, the inhibitory efficacy of resveratrol on COX-1 may be weaker than that of other polyphenols in red wine [39, 41, 42]. Cyclooxygenase-2 is induced by proinflammatory mediators during inflammation. Resveratrol suppresses the expression and activity of COX-2 and the downstream signals like prostaglandin [43–45]. Proinflammatory mediator, particularly interleukin-1β, stimulates the production of leukotriene B4 (LTB4), which is a risk factor of atherosclerosis and produced through the lipoxidase pathway of arachidonic acid metabolism. Resveratrol has been shown to decrease LTB4. At the same time, resveratrol reduces the production of matrix metalloproteinases (MMPs) [45]. Matrix metalloproteinases not only accelerate formation and progression of plaque, but also facilitate to weaken fibrous cap. Therefore, resveratrol is a hopeful medication for plaque stabilization.
Resveratrol has also been shown to elevate proteoglycan synthesis and may be involved in the prevention and treatment of autoimmune inflammation [45]. Moreover, resveratrol inhibits nuclear factor-κB (NF-κB) and modulates the plasma concentration of NF-κB-related inflammatory and autoimmune markers in the subjects with increased risks of cardiovascular disease [46–48].
Resveratrol and platelet
As described above, resveratrol inhibits TxA2 formation via blocking COX [39, 49]. In addition, resveratrol prevents platelet activation through reducing intracellular calcium concentration ([Ca2+]i) of platelet, which plays a key role in platelet aggregation. The mechanisms are involved in the inhibition of the p38 mitogen-activated protein kinase–cytosolic phospholipase A2–arachidonic acid–TxA2–[Ca2+]i cascade and activation of NO/cyclic GMP, thus resulting in the inhibition of phospholipase C/protein kinase C and decrease in [Ca2+]i level [49, 50].
Resveratrol has also been shown to induce platelet apoptosis. Resveratrol stimulates mitochondrial membrane potential dissipation, caspase-9, caspase-3, and caspase-8 activation, human BH3 interacting domain death agonist cleavage, cytochrome C release, and phosphatidylserine exposure in washed human platelets, which promote platelet destruction and prevent pathological clotting [51].
There has been report that resveratrol prevents cigarette-smoking-induced eNOS acetylation and endothelial dysfunction via sirtuin type 1 (SIRT1), which is one of seven mammalian homologs of silent information regulator 2 (Sir2, sirtuin family of proteins in yeast), and catalyzes nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylation [34, 52]. Sirtuin type 1 is highly expressed in vascular endothelial cells. The normalization of injured endothelium will improve vascular tone, blood flow, cell adhesion, and leukocyte–endothelial cell interactions [31, 32, 53–56], which are very important contributors to platelet aggregation [57].
Anti-aging characteristics
Aging is a risk factor of cardiovascular diseases. To slower the speed of aging and prevent the occurrence of age-associated chronic diseases is a key issue in modern society. Resveratrol can activate anti-aging genes and facilitate extending lifespan in lower organisms like yeasts and worms [20, 58]. Resveratrol also increase survival of the high-calorie-diet-fed mice. However, adding resveratrol to mice fed with a normal diet did not affect the overall survival or maximum lifespan, suggesting that resveratrol might be counteracting the negative consequences of obesity and insulin resistance, thus slowing aging [58, 59]. The positive effects of resveratrol on several age-related disorders are mediated by sirtuins [21, 52, 59]. Sirtuin proteins are essential for NF-κB, p53, eNOS, and peroxisome-proliferator-activated receptor co-activator (PGC-1α) deacetylation [60–62], which shortens NF-κB response and inhibits p53 activity. A part of the response caused by resveratrol may be related to the suppression of p53, which is expressed in response to various types of stress involving DNA damage and leads to cell apoptosis. A delay in apoptosis gives the cells additional time to repair damage and prevent unnecessary cell death [60, 61]. On the other hand, the anti-proliferative effect of resveratrol on cancer cells has been extensively documented and supported by the downregulation of cell cycle proteins and an induction of apoptosis in tumorigenic cells [63].
Resveratrol activates SIRT1 and PGC-1α and contributes to mitochondrial function [59, 64], which can influence whole body metabolism. Resveratrol has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK) and the phosphorylation of its downstream indicator, acetyl-CoA carboxylase. AMPK activation can downregulate fatty acid synthase and prevent organ (liver, pancreas, heart, and aorta) damage in mice fed with high-calorie diets [59]. The changes in SIRT1, AMPK, and PGC-1α were similar to changes observed in cellular starvation, which plays an important role in the induction of a number of genes involved in anti-oxidation and cell survival.
Roles on lipid metabolism
Besides inhibiting the synthesis of fatty acid, resveratrol prevents the proliferation and differentiation of preadipocytes, promotes fat mobilization in white adipocytes, triggers lipolysis/loss of fat in differentiated fat cells, and protects mice against diet-induced-obesity by activating SIRT1 [52, 65, 66].
Resveratrol can also cause SIRT1-independent TRAIL (tumor necrosis factor-related apoptosis-inducing ligand)-mediated apoptosis in fat cells [67]. In vivo studies indicate that resveratrol lowers serum triglyceride, very-low-density lipoprotein (VLDL), and low-density lipoprotein (LDL) cholesterol [68]. These effects, along with the reduction in LDL oxidation [69], the prevention of smooth muscle cells (SMCs) proliferation (this will be discussed in the following section) [70], the inhibition of inflammatory pathways in macrophages, and the promotion of endothelial function, contribute to the anti-atherosclerotic effect of resveratrol.
In addition, a recent study demonstrated that resveratrol exerted statin-like effects by downregulating HMG-CoA reductase in a hyperlipidemia model and increased the ratio of apolipoprotein (Apo) A1 to Apo B, which is negatively related to metabolic syndrome and cardiovascular risks [71]. High-density lipoprotein (HDL) is a continuously inverse cardiovascular risk factor and the major mechanism by which HDL protects against atherosclerosis is due to reverse cholesterol transport (RCT). Reverse cholesterol transport is the process through which excess cellular cholesterol is exported from peripheral tissues and returned to the liver for excretion in the bile. Cholesteryl ester transfer protein (CETP) is a crucial enzyme of RCT. Raised CETP activity induces high LDL level, thus contributing to atherosclerosis [72, 73]. Decreased CETP activity, elevated HDL level, and a promotion in the capacity of HDL to mediate cholesterol efflux have been observed with resveratrol treatment recently [29, 71, 73, 74]. These suggest that resveratrol can reduce the transfer of cholesteryl esters from HDL to VLDL/LDL and enhance RCT (Fig. 2).
Resveratrol and cholesterol metabolism. Resveratrol reduces cholesterol synthesis by downregulating HMG-CoA reductase. Resveratrol also enhances reverse cholesterol transport/cholesterol excretion through increasing HDL levels and the capacity of HDL to mediate cholesterol efflux from macrophages in arterial walls. CETP is a plasma glycoprotein that facilitates the transfer of cholesteryl esters from the atheroprotective HDL to the proatherogenic LDL and VLDL [72] and resveratrol has been shown to inhibit the activity of CETP. Furthermore, adipocytes store cholesterol and have proatherogenic effects. Resveratrol can prevent adipogenesis and plays a role in dyslipidemia and obesity. LDL low-density lipoprotein, CE cholesteryl ester, CETP cholesteryl ester transfer protein, HDL high-density lipoprotein, VLDL very-low-density lipoprotein
Peroxisome-proliferator-activated receptors are able to provide protection against cardiovascular diseases by modifying lipid metabolism and inflammation. Resveratrol has a dual effect on PPARα activity in diethylmaleate (a glutathione-depleting agent)-treated RH7777 hepatoma cells [75]. After diethylmaleate administration, an increased PPARα activity at earlier time points and a depressed PPARα activity at later time points were observed. Resveratrol-induced SIRT1 activation may repress PPAR-γ and suppress adipogenesis or white adipocyte differentiation [65], whereas resveratrol prevented the cholesterol accumulation caused by advanced glycosylation end products (important pathogenetic mediators of almost all diabetes complications) in macrophages via PPAR-γ but not PPARα activation [76]. The relationships between resveratrol and various PPAR isoforms are still unclear. Current reports imply the difference in tissues/cells and the time course may affect the targets of resveratrol.
Values on glucose metabolism and insulin sensitivity
Insulin regulates glucose homeostasis by reducing hepatic glucose production and increasing glucose transport into the skeletal muscle. Insulin resistance happens in most of the type 2 diabetic patients. Insulin resistance is always accompanied with increased levels of the injury-inducible molecules, such as insulin-like growth factors (IGFs). People with higher blood glucose, impaired glucose tolerance, and insulin resistance are susceptible to cardiovascular disorders [59].
Resveratrol is observed to improve the changes in glucose, insulin, and IGFs through AMPK, which modulates gluconeogenesis, glucose transport, and insulin sensitivity [59, 77]. Resveratrol is first identified as an activator of SIRT1 from cell-free assays, in which AMPK was not present. Recently, chronic treatments with resveratrol have suggested that SIRT1 and AMPK are related to each other. On one hand, SIRT1 may be the upstream of AMPK. This hypothesis is supported by the findings that SIRT1 gain of function increases AMPK activity, an effect which may be mediated by deacetylation/activation of the AMPK upstream kinase, serine/threonine protein kinase 11 (STK11; also known as LKB1) [78]. On the other hand, AMPK may be the upstream of SIRT1 because AMPK activation increases NAD+/NADH ratio and SIRT1 is NAD+-dependent [79]. In AMPKα-null mice, resveratrol failed to increase the NAD+/NADH ratio or to reduce PGC1α acetylation in skeletal muscle [78].
Insulin resistance has been reported to be associated with mitochondrial dysfunction. Decrease in the expression of PGC-1α, which is post-translationally modulated by AMPK (phosphorylation) [80] and by SIRT1 (deacetylation) [62], and in mitochondrial DNA content was observed in type 2 diabetic patients [64] and individuals with an increased risk of developing diabetes [79, 81]. Resveratrol may attenuate the alterations of AMPK, SIRT1, PGC-1α, and mitochondrial dysfunction under insulin resistance [59].
In addition, estrogen receptor (ER) activation, which attenuates insulin resistance, impaired glucose tolerance and adipocyte hyperplasia in both male and female rodents, mediates resveratrol-stimulated glucose uptake in skeletal muscle [82–86]. The increased glucose uptake/metabolism normalizes serum glucose and the secondary hypertension or other histopathological changes in diabetes.
Effects on endothelial protection
Estrogenic activities
In addition to the effects on glucose metabolism and insulin sensitivity through ER, resveratrol also inhibits lipopolysaccharide-stimulated induction of iNOS in macrophages and improves endothelial function, which is similar to estradiol [34, 87]. Moreover, resveratrol enhances the interaction between ER, caveolin-1 (Cav-1), and c-Src (a tyrosine kinase), increases the phosphorylation of Cav-1, c-Src, extracellular signal–regulated kinase 1/2, and eNOS through non-genomic effect of ER, and stimulates NO production [88]. These effects are important in vasodilatation and endothelial protection.
Effects on endothelial progenitor cells
The integrity of the endothelium is very important to keep the normal function of the blood vessel and to avoid platelet aggregation. Circulating or resident endothelial progenitor cells (EPCs), which can differentiate into endothelial cells, exert an important impact as endogenously reparative mechanism to maintain the integrity of the blood vessel. Increasing evidence suggests that postnatal neovascularization relies not exclusively on resident cells but also involves the contribution of circulating EPCs [89, 90].
Bone marrow-derived mononuclear cells (BMMCs) are one of the sources of circulating stem cells. The consumption of resveratrol is associated with a significant increase in the number of bone marrow EPCs, which enter into circulating blood after hindlimb ischemia in hypercholesterolemic Apo E knockout mice [91]. In addition, resveratrol is able to enhance the EPCs migration and capillary-like tube formation through the angiogenic Akt (a serine/threonine protein kinase)/eNOS/NO/vascular endothelial growth factor (VEGF) pathway in endothelial cells exposed to oxidized LDL [90]. Similarly, the neovascularization capacity of BMMCs from diabetic mice is improved with resveratrol, resulting in a reduction in oxidative stress and an induction of angiogenic factors [91]. Resveratrol consistently augments the activation of telomerase through the phosphatidylinositol-3 kinase/Akt signaling pathway and attenuates EPCs reduction or senescence [92]. In general, resveratrol is important to the functions of EPCs, including proliferation, adhesion, migration, and tube formation [90, 92–95].
Effects on atherosusceptible sites of the endothelium
Atherosclerosis primarily occurs at a specific site where endothelial shear stress created by high-speed rotating blood flow contributes to the focal geometric progression of atherogenesis [96]. Resveratrol activates Kruppel-like factor (KLF) 2, KLF4, and nuclear factor erythroid 2-related factor 2 (Nrf2), which protect endothelial cells from oxidant injury and shear stress. Furthermore, Kruppel-like factors have been reported to be critical regulators of endothelial homeostasis [53–55, 97, 98]. Therefore, the targeting to KLFs or Nrf2 by resveratrol may be a promising therapeutic for an impaired endothelium at atherosusceptible sites.
Influence on preconditioning
Preconditioning has previously been shown to attenuate I/R-induced injuries due to the reduction in oxidative stress. Resveratrol-stimulated NO is able to protect against oxidative damage produced by oxoferryl-myoglobin, which is a potent oxidant found in I/R injury [35]. Although eNOS plays a role in the effects of resveratrol, iNOS, which is usually related to tissue injury, also mediates preconditioning because resveratrol fails to precondition hearts in iNOS knockout mice [35, 99, 100]. Controversial research showed that resveratrol was efficient and promising to prevent cardiac dysfunction after I/R injury, but the beneficial effect of resveratrol was not mediated by NO [101]. Besides the different experiment conditions, SIRT1 and PPARs may be involved in the preconditioning, since SIRT1 and PPARα have been shown to participate in brain protection achieved by resveratrol administration [102, 103].
In addition, autophage is a process that cells “eat/digest” autologous macromolecules or organelles, which are damaged under starvation, I/R injury, and other kinds of stress. Autophagy may be a homeostatic mechanism or a catabolic energy source by which apoptosis is inhibited and the deleterious effects of ischemia are limited. Resveratrol treatment induces autophage both in vivo and in vitro via mammalian target of rapamycin (mTOR) pathway [104, 105]. This helps cardiomyocytes to live and reduces the secondary impairment of cardiac function after I/R.
Resveratrol in cardiovascular remodeling and heart failure
To cardiac hypertrophy
Resveratrol did not lower high blood pressure directly in the spontaneously hypertensive rat, but it attenuated compliance and remodeling of small artery [106, 107]. Regression of pressure overload-induced cardiac hypertrophy and dysfunction has been reported in resveratrol-treated abdominal aortic-banded rats [108]. One of the determined anti-hypertrophic mechanisms of resveratrol is the upregulation of eNOS/NO [108].
Another anti-hypertrophic mechanism of resveratrol is via LKB1, which is an upstream signal of AMPK. Resveratrol prevents the inhibitory effect of oxidative stress on LKB1 and favors the activation of AMPK. Activation of AMPK and subsequent inactivation of mTOR/70-kDa ribosomal protein S6 kinase signaling pathway inhibit unnecessary protein synthesis and prevent remodeling in the heart [25, 109–111].
To heart failure
In failing heart, decompensation and cardiomyocyte deficiency turned to be the predominant problem. Under such a condition, an active DNA repair process instead of an inhibited protein synthesis process was potentiated by resveratrol, which preserved the genomic stability of cardiomyocytes [108]. Activation of SIRT1 limits premature cellular senescence, prolongs the life of myocytes from failing hearts, and decreases cardiac fibrosis [27, 112, 113].
Because catecholamines re-uptake is reduced, noradrenaline concentration increases in left ventricle of objects with chronic heart failure, resulting in a reduced sensitivity of myocardium to catecholamine [114]. Resveratrol administration normalizes β-adrenoceptors density, restores the sensitivity of myocardium to catecholamine, reduces infarct size, and improves heart function after myocardial infarction [115]. Moreover, resveratrol can improve sympathetic neural remodeling, thus causing less ventricular arrhythmias [116]. Significant reduction in norepinephrine is detected in resveratrol-loaded rats with myocardial infarction. In the hearts of these rats, a significant reduction in nerve sprouting and sympathetic hyperinnervation are observed [116], thus contributing to less secondary effects of renin-angiotensin system, better cardiac compliance and cardiac output. After receiving resveratrol treatment, the level of atrial natriuretic factor (ANF) is also attenuated in rats with myocardial infarction [115].
Cardiac sarcoplasmic reticulum Ca2+-ATPase 2 (SERCA2) is a direct regulator for cardiac systolic and diastolic functions through controlling sarcoplasmic reticulum Ca2+ uptake [117]. Sarcoplasmic reticulum Ca2+-ATPase 2 levels are decreased in mice with diabetic cardiomyopathy and rats with myocardial infarction, but they turned to be similar to normal control after resveratrol treatment, respectively [116, 118]. Intriguingly, overexpression of SIRT1 is sufficient to activate the SERCA2 promoter in cardiomyocytes [118], which means resveratrol may regulate SERCA2 level and subsequently improve heart function via activating SIRT1. All of these studies suggest that resveratrol not only delays the process of cardiac remodeling, but also directly improves cardiac function of failing heart.
To cardiomyocytes regeneration
Transplantation of stem cells into injured or failing heart is a hopeful procedure for cardiomyocytes regeneration. Endogenous ROS functions as signaling molecules for myogenic differentiation [119–121]. A recent report showed that resveratrol maintained a reduced cardiac environment by overexpressing Nrf2 and redox effector factor-1 in rats, resulting in an enhancement of the regeneration of the adult cardiac stem cells and leading to the improvement in cardiac function [122]. Cardiac stem cells pretreated with resveratrol were also showed better survival, proliferation, and differentiation toward cardiomyocyte in vivo. Transplantation of these resveratrol-modified cardiac stem cells improved cardiac function even at the end of 4 months [123].
To vascular remodeling
Resveratrol treatment can improve the morphology of the aortic elastic lamina via SIRT1 and AMPK in mice fed with a high-calorie diet [59]. Resveratrol also effectively inhibited proliferation and migration of aortic vascular SMCs by increasing ER-dependent NO production or decreasing the cross-talk between interleukin-18 and an inducer of MMPs [124, 125]. Vascular SMCs proliferation and migration, as well as excess deposition of extracellular matrix, are major factors contributing to vascular remodeling and luminal narrowing. The anti-proliferative properties of resveratrol have also been attributed to its inhibition of DNA synthesis and induction of p53 in SMCs [126, 127]. Moreover, resveratrol inhibits proliferation of cultured pulmonary arterial SMCs, which arrested the cell cycle in the S phase and prevented pulmonary hypertension [33].
Resveratrol behaving as a hormetin
Resveratrol, termed as hormetin, can bring about biologically/physiologically beneficial effects by activating pathways of stress response. Resveratrol interrupts different stress-induced hormesis, leading to a significant improvement of the living system [128]. Resveratrol possesses both angiogenic and anti-angiogenic properties. Resveratrol can both prevent cardiac remodeling and enhance cardiac regeneration. However, it is still elusive why resveratrol could be a bidirectional modulator. Numerous researches provided another possible interpretation that resveratrol enhanced proliferation and survival of cardiomyocytes at lower dose and depressesed cardiac function as well as inhibited proliferation at higher dose [25, 90, 92, 104, 111, 127, 129], like a J-curve. Higher dose of resveratrol not only inhibits endothelial cell growth and VEGF, but also hinders the synthesis of RNA, DNA, and protein, thus preventing tumor growth [63, 129–131].
Clinical implications
Resveratrol is a food supplement for eliminating free radical and keeping healthy, but it is not into a clinical stage yet currently. Resveratrol has a short initial half-life (~8–14 min for the primary molecule) and is extensively metabolized in the body. Blocking the metabolism of resveratrol, developing analogs with improved bioavailability, or searching for new and more potent compounds that mimic its effects will become increasingly important [20]. Fortunately, research on the structure of resveratrol is ongoing [52]. Screening the effective elements of resveratrol in vivo and determining the differences between in vivo and in vitro results are challenge for researchers [20]. Moreover, the effective dose in animals cannot be extrapolated to a human equivalent dose by a simple conversion based on body weight [132].
To investigate the dose, effect, and safety of resveratrol, numerous clinical trials are on going (see ClinicalTrails.gov), and some results are being published. In one phase I study, resveratrol intake for 4 weeks by healthy volunteers caused a small but significant decrease in circulating IGF-1 and IGF-binding protein 3 compared with pre-dosing values [133]. Reduction in ROS and tumor necrosis factor-α as well as inflammation markers interleukin-6 and C-reactive protein is achieved in participants who received resveratrol [134]. Serious side-effects are not observed [133–136] in the known human experiments. In phase I dose escalation pharmacokinetic study in healthy volunteers, the increase in blood bilirubin and alanine aminotransferase is observed, which are potentially related to resveratrol administration [135]. In a rising multiple-dose study in healthy volunteers, adverse events including frontal headache, myalgia, epididymitis, dizziness, and occipital headache are found. However, these symptoms are mild in severity and similar between groups of different does. Repeated administration is also well tolerated [137].
Through correction of oxidative stress, inflammation, hypercoagulative state, dyslipidemia, obesity, hyperglycemia, hyperinsulinemia, I/R injury, sympathetic tone, unnecessary cell loss/proliferation, and endothelial/cardiac dysfunction, resveratrol shows a surprising potential to treat atherosclerosis and heart failure (Fig. 3). We hope that we will be able to use resveratrol to prevent and treat cardiovascular disease in the future.
Multiple targets of resveratrol in cardiovascular disease. Resveratrol plays an import role in maintaining stationarity of the cardiovascular system. The risk factors colored gray make coronary atherosclerosis, myocardial ischemia, and end-stage heart failure happen. Resveratrol, by interrupting these factors and events, may be possible to prevent or slow the development of cardiovascular disease. In addition, resveratrol at lower dose prolongs cardiomyocytes survival in failing hearts, enhances angiogenesis under post-ischemic conditions, and improves cardiac stem cells homing, proliferation, and differentiation [122, 123], thus may be possible to reverse heart dysfunction
References
Griendling KK, FitzGerald GA (2003) Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS. Circulation 108:1912–1916
Griendling KK, FitzGerald GA (2003) Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation 108:2034–2040
Kawai J, Ando K, Tojo A, Shimosawa T, Takahashi K, Onozato ML, Yamasaki M, Ogita T, Nakaoka T, Fujita T (2004) Endogenous adrenomedullin protects against vascular response to injury in mice. Circulation 109:1147–1153
Matsui H, Shimosawa T, Itakura K, Xing G, Ando K, Fujita T (2004) Adrenomedullin can protect against pulmonary vascular remodeling induced by hypoxia. Circulation 109:2246–2251
Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY (2006) Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res 1090:182–189
Wang H, Shimosawa T, Matsui H, Kaneko T, Ogura S, Uetake Y, Takenaka K, Yatomi Y, Fujita T (2008) Paradoxical mineralocorticoid receptor activation and left ventricular diastolic dysfunction under high oxidative stress conditions. J Hypertens 26:1453–1462
Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, Nagase M, Fujita T (2008) Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension 52:287–294
Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T (2009) Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119:978–986
Houston MC (2010) The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidants and minerals in the prevention and treatment of hypertension and cardiovascular disease. Ther Adv Cardiovasc Dis 4:165–183
Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P (2000) Vitamin E supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 342:154–160
Marchioli R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L, Tognoni G, Valagussa F, GISSI-Prevenzione Investigators (2006) Vitamin E increases the risk of developing heart failure after myocardial infarction: results from the GISSI-prevenzione trial. J Cardiovasc Med (Hagerstown) 7:347–350
Miller ER III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142:37–46
Bertrand ME (2004) Provision of cardiovascular protection by ACE inhibitors: a review of recent trials. Curr Med Res Opin 20:1559–1569
Ono H, Minatoguchi S, Watanabe K, Yamada Y, Mizukusa T, Kawasaki H, Takahashi H, Uno T, Tsukamoto T, Hiei K, Fujiwara H (2008) Candesartan decreases carotid intima-media thickness by enhancing nitric oxide and decreasing oxidative stress in patients with hypertension. Hypertens Res 31:271–279
Ishimitsu T, Numabe A, Masuda T, Akabane T, Okamura A, Minami J, Matsuoka H (2009) Angiotensin-II receptor antagonist combined with calcium channel blocker or diuretic for essential hypertension. Hypertens Res 32:962–968
Sasaki H, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohhira M, Oyama T, Miyashita Y, Shirai K (2009) Protective effects of efonidipine, a T- and L-type calcium channel blocker, on renal function and arterial stiffness in type 2 diabetic patients with hypertension and nephropathy. J Atheroscler Thromb 16:568–575
Li J, Sun YM, Wang LF, Li ZQ, Pan W, Cao HY (2010) Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clin Cardiol 33:222–227
Papanas N, Maltezos E (2009) Oral antidiabetic agents: anti-atherosclerotic properties beyond glucose lowering? Curr Pharm Des 15:3179–3192
Hopkins AL (2008) Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4:682–690
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506
Opie LH, Lecour S (2007) The red wine hypothesis: from concepts to protective signalling molecules. Eur Heart J 28:1683–1693
Karlsson J, Emgard M, Brundin P, Burkitt MJ (2000) Trans-resveratrol protects embryonic mesencephalic cells from tert-Butyl hydroperoxide: electron paramagnetic resonance spin trapping evidence for a radical scavenging mechanism. J Neurochem 75:141–150
Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H (2009) Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol 60(Suppl 4):111–116
Yildiz F, Terzi A, Coban S, Celik H, Aksoy N, Bitiren M, Cakir H, Ozdogan MK (2009) Protective effects of resveratrol on small intestines against intestinal ischemia-reperfusion injury in rats. J Gastroenterol Hepatol 24:1781–1785
Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR (2009) Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation 119:1643–1652
Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N (2007) Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin and heme oxygenase. Free Radic Biol Med 43:720–729
Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y (2010) Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem 285:8375–8382
Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A (2007) Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292:H2417–H2424
Ferretti G, Bacchetti T, Menanno F, Curatola G (2004) Effect of genistein against copper-induced lipid peroxidation of human high density lipoproteins (HDL). Atherosclerosis 172:55–61
Belguendouz L, Fremont L, Linard A (1997) Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem Pharmacol 53:1347–1355
Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Förstermann U (2002) Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106:1652–1658
Zou JG, Wang ZR, Huang YZ, Cao KJ, Wu JM (2003) Effect of red wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int J Mol Med 11:317–320
Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z (2009) Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54:668–675
Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I (2010) SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem Biophys Res Commun 393:66–72
Hattori R, Otani H, Maulik N, Das DK (2002) Pharmacological preconditioning with resveratrol: role of nitric oxide. Am J Physiol Heart Circ Physiol 282:H1988–H1995
Lin KY, Lin SC (2004) A tale of two molecules: nitric oxide and asymmetric dimethylarginine. Acta Cardiol Sin 20:201–211
DeMarco VG, Johnson MS, Whaley-Connell AT, Sowers JR (2010) Cytokine abnormalities in the etiology of the cardiometabolic syndrome. Curr Hypertens Rep 12:93–98
Rizvi AA (2010) Hypertension, obesity, and inflammation: the complex designs of a deadly trio. Metab Syndr Relat Disord 8:287–294
Frémont L (2000) Biological effects of resveratrol. Life Sci 66:663–673
Sharma S, Chopra K, Kulkarni SK, Agrewala JN (2007) Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin Exp Immunol 147:155–163
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Szewczuk LM, Penning TM (2004) Mechanism-based inactivation of COX-1 by red wine m-hydroquinones: a structure-activity relationship study. J Nat Prod 67:1777–1782
Martinez J, Moreno JJ (2000) Effect of resveratrol, a natural polyphenoliccompound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol 59:865–870
Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ (1998) Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem 273:21875–21882
Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, Abramson SB (2008) The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum 58:2786–2797
Kang SS, Cuendet M, Endringer DC, Croy VL, Pezzuto JM, Lipton MA (2009) Synthesis and biological evaluation of a library of resveratrol analogues as inhibitors of COX-1, COX-2 and NF-kB. Bioorg Med Chem 17:1044–1054
Leiro J, Arranz JA, Fraiz N, Sanmartin ML, Quezada E, Orallo F (2005) Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int Immunopharmacol 5:393–406
Karlsen A, Paur I, Bøhn SK, Sakhi AK, Borge GI, Serafini M, Erlund I, Laake P, Tonstad S, Blomhoff R (2010) Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr 49:345–355
Shen MY, Hsiao G, Liu CL, Fong TH, Lin KH, Chou DS, Sheu JR (2007) Inhibitory mechanisms of resveratrol in platelet activation: pivotal roles of p38 MAPK and NO/cyclic GMP. Br J Haematol 139(3):475–485
Yang YM, Chen JZ, Wang XX, Wang SJ, Hu H, Wang HQ (2008) Resveratrol attenuates thromboxane A2 receptor agonist-induced platelet activation by reducing phospholipase C activity. Eur J Pharmacol 583:148–155
Lin KH, Hsiao G, Shih CM, Chou DS, Sheu JR (2009) Mechanisms of resveratrol-induced platelet apoptosis. Cardiovasc Res 83:575–585
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122
Sancho J, Villarreal G Jr, Zhang Y, García-Cardeña G (2010) Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res 85:514–519
Villarreal G Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, García-Cardeña G (2010) Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun 391:984–989
Ungvari ZI, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson KJ, de Cabo R, Csiszar A (2010) Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299:H18–H24
Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW (2010) Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation 122(11 Suppl):S142–S149
Le Brocq M, Leslie SJ, Milliken P, Megson IL (2008) Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal 10:1631–1674
Das DK, Mukherjee S, Ray D (2010) Resveratrol and red wine, healthy heart and longevity. Heart Fail Rev 15:467–477
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342
Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107:149–159
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434:113–118
Subramaniam D, Ramalingam S, Houchen CW, Anant S (2010) Cancer stem cells: a novel paradigm for cancer prevention and treatment. Mini Rev Med Chem 10:359–371
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D (2003) Groop LC (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273
Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429:771–776
Bai L, Pang WJ, Yang YJ, Yang GS (2008) Modulation of Sirt1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. Mol Cell Biochem 307:129–140
Mader I, Wabitsch M, Debatin KM, Fischer-Posovszky P, Fulda S (2010) Identification of a novel proapoptotic function of resveratrol in fat cells: SIRT1-independent sensitization to TRAIL-induced apoptosis. FASEB J 24:1997–2009
Miura D, Miura Y (2003) Yagasaki K (2003) Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatomabearing rats. Life Sci 73:1393–1400
Fremont L, Belguendouz L, Delpal S (1999) Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci 64:2511–2521
Araim O, Ballantyne J, Waterhouse AL, Sumpio BE (2002) Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J Vasc Surg 35:1226–1232
Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY (2008) Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun 367:190–194
Weber O, Bischoff H, Schmeck C, Böttcher MF (2010) Cholesteryl ester transfer protein and its inhibition. Cell Mol Life Sci 67:3139–3149
Berrougui H, Grenier G, Loued S, Drouin G, Khalil A (2009) A new insight into resveratrol as an atheroprotective compound: inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis 207:420–427
Do GM, Kwon EY, Kim HJ, Jeon SM, Ha TY, Park T, Choi MS (2008) Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun 374:55–59
Iannelli P, Zarrilli V, Varricchio E, Tramontano D, Mancini FP (2007) The dietary antioxidant resveratrol affects redox changes of PPARα activity. Nutr Metab Cardiovasc Dis 17:247–256
Zhang Y, Luo Z, Ma L, Xu Q, Yang Q, Si L (2010) Resveratrol prevents the impairment of advanced glycosylation end products (AGE) on macrophage lipid homeostasis by suppressing the receptor for AGE via peroxisome proliferator-activated receptor gamma activation. Int J Mol Med 25:729–734
Schimmack G, Defronzo RA, Musi N (2006) AMP-activated protein kinase: role in metabolism and therapeutic implications. Diabetes Obes Metab 8:591–602
Fullerton MD, Steinberg GR (2010) SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes 59:551–553
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T (2010) Adiponectin and AdipoR1 regulate PGC-1a and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464:1313–1319
Ja¨ger S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1a. Proc Natl Acad Sci USA 104:12017–12022
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471
Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM (2008) Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 57:1814–1823
Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL (2010) Impaired oxidative metabolism and inflammation are associated with insulin resistance in ER{alpha} deficient mice. Am J Physiol Endocrinol Metab 298:E304–E319
Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA (2009) Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab 297:E124–E133
Lundholm L, Bryzgalova G, Gao H, Portwood N, Fält S, Berndt KD, Dicker A, Galuska D, Zierath JR, Gustafsson JA, Efendic S, Dahlman-Wright K, Khan A (2008) The estrogen receptor {alpha}-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol 199:275–286
Barros RP, Machado UF, Gustafsson JA (2006) Estrogen receptors: new players in diabetes mellitus. Trends Mol Med 12:425–431
Cho DI, Koo NY, Chung WJ, Kim TS, Ryu SY, Im SY, Kim KM (2002) Effects of resveratrol-related hydroxystilbenes on the nitric oxide production in macrophage cells: structural requirements and mechanism of action. Life Sci 71:2071–2082
Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM (2008) Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22:2185–2197
Urbich C, Dimmeler S (2005) Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int 67:1672–1676
Lefèvre J, Michaud SE, Haddad P, Dussault S, Ménard C, Groleau J, Turgeon J, Rivard A (2007) Moderate consumption of red wine (cabernet sauvignon) improves ischemia-induced neovascularization in ApoE-deficient mice: effect on endothelial progenitor cells and nitric oxide. FASEB J 21:3845–3852
Gan L, Matsuura H, Ichiki T, Yin X, Miyazaki R, Hashimoto T, Cui J, Takeda K, Sunagawa K (2009) Improvement of neovascularization capacity of bone marrow mononuclear cells from diabetic mice by ex vivo pretreatment with resveratrol. Hypertens Res 32:542–547
Xia L, Wang XX, Hu XS, Guo XG, Shang YP, Chen HJ, Zeng CL, Zhang FR, Chen JZ (2008) Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br J Pharmacol 155:387–394
Huang PH, Chen YH, Tsai HY, Chen JS, Wu TC, Lin FY, Sata M, Chen JW, Lin SJ (2010) Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol 30:869–877
Balestrieri ML, Schiano C, Felice F, Casamassimi A, Balestrieri A, Milone L, Servillo L, Napoli C (2008) Effect of low doses of red wine and pure resveratrol on circulating endothelial progenitor cells. J Biochem 143:179–186
Wang XB, Huang J, Zou JG, Su EB, Shan QJ, Yang ZJ, Cao KJ (2007) Effects of resveratrol on number and activity of endothelial progenitor cells from human peripheral blood. Clin Exp Pharmacol Physiol 34:1109–1115
Dhawan SS, Avati Nanjundappa RP, Branch JR, Taylor WR, Quyyumi AA, Jo H, McDaniel MC, Suo J, Giddens D, Samady H (2010) Shear stress and plaque development. Expert Rev Cardiovasc Ther 8:545–556
Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA Jr (2007) Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res 101:723–733
Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M (2005) Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem 280:27244–27250
Imamura G, Bertelli AA, Bertelli A, Otani H, Maulik N, Das DK (2002) Pharmacological preconditioning with resveratrol: an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol 282:H1996–H2003
Penumathsa SV, Maulik N (2009) Resveratrol: a promising agent in promoting cardioprotection against coronary heart disease. Can J Physiol Pharmacol 87:275–286
Mokni M, Limam F, Elkahoui S, Amri M, Aouani E (2007) Strong cardioprotective effect of resveratrol, a red wine polyphenol, on isolated rat hearts after ischemia/reperfusion injury. Arch Biochem Biophys 457:1–6
Raval AP, Lin HW, Dave KR, Defazio RA, Della Morte D, Kim EJ, Perez-Pinzon MA (2008) Resveratrol and ischemic preconditioning in the brain. Curr Med Chem 15:1545–1551
Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S (2003) Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett 352:203–206
Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM, Das DK (2010) Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res 86:103–112
Gurusamy N, Das DK (2009) Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal 11:1975–1988
Behbahani J, Thandapilly SJ, Louis XL, Huang Y, Shao Z, Kopilas MA, Wojciechowski P, Netticadan T, Anderson HD (2010) Resveratrol and small artery compliance and remodeling in the spontaneously hypertensive rat. Am J Hypertens 23:1273–1278
Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD, Netticadan T (2010) Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens 23:192–196
Juric D, Wojciechowski P, Das DK, Netticadan T (2007) Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. Am J Physiol Heart Circ Physiol 292:H2138–H2143
Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR (2004) Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem 279:32771–32779
Noga AA, Soltys CL, Barr AJ, Kovacic S, Lopaschuk GD, Dyck JR (2007) Expression of an active LKB1 complex in cardiac myocytes results in decreased protein synthesis associated with phenylephrine-induced hypertrophy. Am J Physiol Heart Circ Physiol 292:H1460–H1469
Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR (2008) Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem 283:24194–24201
Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T (2002) Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 21:2383–2396
Pillai JB, Isbatan A, Imai S, Gupta MP (2005) Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 280:43121–43130
Gaemperli O, Liga R, Spyrou N, Rosen SD, Foale R, Kooner JS, Rimoldi OE, Camici PG (2010) Myocardial beta-adrenoceptor down-regulation early after infarction is associated with long-term incidence of congestive heart failure. Eur Heart J 31:1722–1729
Burstein B, Maguy A, Clement R, Gosselin H, Poulin F, Ethier N, Tardif JC, Hebert TE, Calderone A, Nattel S (2007) Effects of resveratrol (trans-3,5,4′-Trihydroxystilbene) treatment on cardiac remodeling following myocardial infarction. J Pharmacol Exp Ther 323:916–923
Xin P, Pan Y, Zhu W, Huang S, Wei M, Chen C (2010) Favorable effects of resveratrol on sympathetic neural remodeling in rats following myocardial infarction. Eur J Pharmacol 649:293–300
Schmidt AG, Zhai J, Carr AN, Gerst MJ, Lorenz JN, Pollesello P, Annila A, Hoit BD, Kranias EG (2002) Structural and functional implications of the phospholamban hinge domain: impaired SR Ca2+ uptake as a primary cause of heart failure. Cardiovasc Res 56:248–259
Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M (2010) Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 298:H833–H843
Ding Y, Choi KJ, Kim JH, Han X, Piao Y, Jeong JH, Choe W, Kang I, Ha J, Forman HJ, Lee J, Yoon KS, Kim SS (2008) Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation. Am J Pathol 172:1529–1541
Yang YJ, Qian HY, Geng YJ, Gao RL, Dou KF, Yang GS, Li JJ, Shen R, He ZX, Lu MJ, Zhao SH (2008) Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur Heart J 29:1578–1590
Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Yang GS, Willerson JT, Geng YJ (2009) Simvastatin improves microenvironment and facilitates survival and activities of the bone marrow mesenchymal stem cells implanted in post-infarct swine hearts. Arterioscler Thromb Vasc Biol 29:2076–2082
Gurusamy N, Ray D, Lekli I, Das DK (2010) Red wine antioxidant resveratrol-modified cardiac stem cells regenerate infarcted myocardium. J Cell Mol Med 14:2235–2239
Gorbunov N, Petrovski G, Gurusamy N, Ray D, Kim DH, Das DK Regeneration of infarcted myocardium with resveratrol-modified cardiac stem cells. J Cell Mol Med. doi:10.1111/j.1582-4934.2011.01281.x
Ekshyyan VP, Hebert VY, Khandelwal A, Dugas TR (2007) Resveratrol inhibits rat aortic vascular smooth muscle cell proliferation via estrogen receptor dependent nitric oxide production. J Cardiovasc Pharmacol 50:83–93
Venkatesan B, Valente AJ, Reddy VS, Siwik DA, Chandrasekar B (2009) Resveratrol blocks interleukin-18-EMMPRIN cross-regulation and smooth muscle cell migration. Am J Physiol Heart Circ Physiol 297:H874–H886
Wang Z, Chen Y, Labinskyy N, Hsieh TC, Ungvari Z, Wu JM (2006) Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem Biophys Res Commun 346:367–376
Zou J, Huang Y, Cao K, Yang G, Yin H, Len J, Hsieh TC, Wu JM (2000) Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci 68:153–163
Rattan SI, Fernandes RA, Demirovic D, Dymek B, Lima CF (2009) Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose Response 7:90–103
Juhasz B, Mukherjee S, Das DK (2010) Hormetic response of resveratrol against cardioprotection. Exp Clin Cardiol 15:e134–e138
Cecchinato V, Chiaramonte R, Nizzardo M, Cristofaro B, Basile A, Sherbet GV, Comi P (2007) Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol 74:1568–1574
Majumdar AP, Banerjee S, Nautiyal J, Patel BB, Patel V, Du J, Yu Y, Elliott AA, Levi E, Sarkar FH (2009) Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr Cancer 61:544–553
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K (2011) Clinical trials of resveratrol. Ann N Y Acad Sci 1215:161–169
Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P (2010) An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum Cuspidatum containing resveratrol. J Clin Endocrinol Metab 95:E1–E8
Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE (2007) Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 16:1246–1252
Camins A, Sureda FX, Junyent F, Verdaguer E, Folch J, Beas-Zarate C, Pallas M (2010) An overview of investigational antiapoptotic drugs with potential application for the treatment of neurodegenerative disorders. Expert Opin Investig Drugs 19:587–604
Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P (2009) Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res 53(Suppl 1):S7–S15
Acknowledgments
Yue-Jin Yang was supported by grants from National Natural Science Foundation of China [81070169], China Health & Medical Development Foundation [2008-zhfj2], and Research Fund of Capital Medical Development [2007–2018]. Hai-Yan Qian was supported by grants from National Natural Science Foundation of China [81000091] and Beijing Novel Program [2008B78]. Hong Wang was supported by grants from China Postdoctoral Science Foundation [20100470010].
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, H., Yang, YJ., Qian, HY. et al. Resveratrol in cardiovascular disease: what is known from current research?. Heart Fail Rev 17, 437–448 (2012). https://doi.org/10.1007/s10741-011-9260-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-011-9260-4