Abstract
Age-related jawbone loss directly impact the function of oral cavity resulted from tooth loss, implant failure, and jaw fracture. Numerous evidences show that age-related senescence of bone marrow stromal cells (BMSCs) play a critical role in bone loss, but little attention has been paid to jawbone. Here, we delineated the critical role of sirtuin family protein 6 (SIRT6) in senescence, autophagy, and osteogenesis of BMSCs from jawbones. Radiography analysis showed less jawbone quality in elderly than young people. We also showed that SIRT6 expression decreased in bone tissue and BMSCs from the elderly by immunochemical staining. BMSCs from the elderly exhibited decreased osteogenic differentiation and inclined senescence which these phenotypes could be simulated by SIRT6 knockdown. Furthermore, accompanied with the inhibition of SIRT6, the autophagy level and ostogenesis of BMSCs was also decreased. However, using rapamycin, an autophagy activator, could rescue these adverse effects of BMSCs caused by SIRT6 inhibition. Mechanistically, SIRT6 regulated the autophagy and osteogenesis of BMSCs by activating AKT-mTOR pathway, at least in part. Finally, a decreased jawbone quality was shown in SIRT6 haploinsufficiency mice by Wnt1 specific tissue knockdown (Wnt1-Cre;SIRT6fl/+) model. Taken together, our data revealed that SIRT6 adjusted senescence and osteogenesis of BMSCs via altering autophagy level, and associated with age-related bone loss. SIRT6 could be as a promising therapeutic target for age-related osteoporosis of jawbone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jawbone quantity loss includes periodontitis induced alveolar bone absorption, residual alveolar bone absorption and maxillofacial osteoporosis, which are related to systemic osteoporosis and occur in the process of chronic bone loss or bone absorption in the elderly. The loss of jawbones can cause alveolar bone absorption and jaw atrophy, which makes it difficult for clinical prosthodontics. Therefore, it is meaningful to determine the cause of jawbone loss and guide the treatment (Bodic et al. 2005). Bone remodeling is maintained by the coordinated activity of bone cells including bone marrow stromal cells (BMSCs), which are multipotent stromal cells as defined by their self-renewal ability and the capability of multilineage mesenchymal differentiation (osteoblasts, chondrocytes and adipocytes) (Bianco et al. 2008). Decreased bone formation caused by retarded osteogenic differentiation of BMSCs could be one of the reasons for osteoporosis (McClung et al. 2017). Maxillofacial bones derive from migrating cranial neural crest cells (NCCs), which is distinct from the appendicular bones deriving from mesoderm (Chai et al. 2000). These differences imply that site-specific BMSCs from different origins may present phenotypic and functional differences.
The sirtuin family proteins (SIRT1-7) are nicotinamide adenine dinucleotide dependent deacetylase enzymes, which are classified into the Class III HDACs family (Gertler et al. 2013). Among these seven mammalian sirtuins, of interest, SIRT6 has been found to involve in various biological processes such as metabolism, inflammation and aging by targeting specific sites on histone H3 lysine deacetylase (Mostoslavsky et al. 2006; Mu et al. 2018). SIRT6 deficiency leads to the degenerative aging-like phenotype in mice and cynomolgus monkeys (Mostoslavsky et al. 2006; Zhang et al. 2018). Some studies have identified the relationship between the SIRT6 gene and bone remodeling, and found that SIRT6 is involved in the BMSCs differentiation by modulating nuclear factor-kB (NF-kB) transcriptional activity and bone morphogenetic protein (BMP) signaling (Sun et al. 2014; Zhang et al. 2017). Mechanistically, SIRT6 interacts with Runx2 and osterix and deacetylates histone H3 at Lysine 9 (H3K9) at their promoters (Sugatani et al. 2015). These findings suggest a crucial role of SIRT6 in the osteogenesis of BMSCs with aging. However, the underlying mechanisms behind jawbone aging remain largely undefined.
Autophagy is a self-degrading process in which autophagosomes separate organelles or parts of the cytoplasm and fuse with lysosomes or vacuoles for decomposition by resident hydrolases (García-Prat et al. 2016; He et al. 2009). Defective autophagy in aged cells leads to diminished autophagosome formation and insufficient clearance of autophagosomes to some extent (Cuervo et al. 2005). Regulation of autophagy has an impact on the functions of BMSCs, which includes differentiation and immunoregulatory capacities (Song et al. 2014). Our previous results revealed that simvastatin can increase osteoblastic differentiation, which is via enhanced autophagy (Xu et al. 2018). Autophagy plays a vital role in the aging of BMSCs and autophagy activation could partially reserve this aging (Ma et al. 2018). However, the relationship between aging, osteogenesis and autophagy is still unclear.
In this study, we aimed to explore the age-dependent role of SIRT6 in jawbone. Our findings showed that the deficiency of SIRT6 in BMSCs with the jawbone aging inhibited their osteogenesis and autophagy, which AKT-mTOR pathway participates in the process. Wnt1-Cre;SIRT6fl/+ mice model, craniomaxillofacial bone-specific SIRT6 haploinsufficiency also showed a key of SIRT6 in jawbone aging. SIRT6 could be as a promising therapeutic target for age-related jawbone loss.
Materials and methods
Patients and CBCT measurements
Twenty young (20- to 30-year-old) and twenty old (60- to 70-year-old) men were enrolled as the subjects in this study. The exclusion criteria are pathological damages to the mandible, such as tumor, cyst, inflammation, etc. or periodontitis, periapical disease in the lower anterior teeth area and systemic and metabolic diseases. Informed consent was obtained before. CBCT images were obtained using the CBCT device (Planmeca Pro Max 3D, Planmeca OY Company, Finland) at 90 kV, 14 mA, 13.779 s, and the pixel size was 0.2 mm. Two experienced maxillofacial radiologist performed the measurements using the software Mimics Research 19.0. The terms “CTI(S)”, “CTI(I)”, “CTMI” were used in our present study (Koh et al. 2011). CTI(S): computed tomography mandibular index (superior). CTI(I): computed tomography mandibular index (inferior). CTMI: computed tomography mental index. As is shown in Fig. 1a, on the coronal image of the mental foramen, the lowest point D of the lower edge of the mandible directly below the mental foramen was tangent a, while the perpendicular B of the tangent line was tangent D. The parallel lines of tangent a and perpendicular b intersect at C, B and A along the lowest point of the upper edge of the cortical bone of the lower margin of the mandible and the upper and lower margin of mental foramen respectively. CTI(S) = CD/AD, CTI(I) = CD/BD, CTMI = CD.
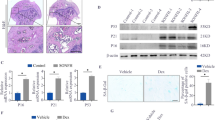
Loss of mineralization and less SIRT6 expression in the jaw bones of the elderly. a Representive coronal view images of the young and old groups’ mandibles by CBCT. b Quantitative analysis of CTI(S), CTI(I), CTMI, n = 20. c, d SIRT6-expressing cells determined by anti-SIRT6 immunohistochemistry of the mandibular bones, n = 3. Scale bar 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001. All data are presented as mean ± SD
Isolation and culture of BMSCs
Under a protocol approved by the Ethics and Research Committee of Nanjing Medical University, jaw bones was obtained from two groups (20- to 30-year-old and 60-to 70-year-old) of donors when they underwent impacted tooth extraction or dental implantation at our hospital. Informed consent was obtained before volunteers were enrolled in his study. BMSCs were harvested as we previously described in an earlier study (Zhou et al. 2016). Primary BMSCs were cultured in 25 cm2 plastic flasks with medium consisting of DMEM (Gibco, Grand Island, NY, USA), 100 μg/ml streptomycin, 100 U/ml penicillin, and 10% fetal bovine serum (ScienCell, Carlsbad, CA, USA) at 37 °C maintained in 5% CO2. Once BMSCs reached 85–95% confluence, they were harvested and passaged routinely. Cells that had undergone 3–6 population doublings were used in subsequent experiments.
RNA interference
Interference small interfering RNA (siRNA) targeting SIRT6 and scrambled siRNA were purchased from GenePharma Co.Ltd (Shanghai, China). Two sequences of siRNAs targeting human SIRT6 were listed as follows: siRNA1, 5′-UCAUGACCCGGCUCAUGAAdTdT-3′, 3′-dTdTAGUACUGGGCCGAGUACUU-5′; siRNA2, 5′-GGAAGAAUGUGCCAAGUGUdTdT-3′, 3′-dTdT CCUUCUUACACGGUUCACA-5′. The final concentration of all these siRNA duplexes was 50 nM, which were transfected into BMSCs using Lipofectamine2000 (Invitrogen, San Diego, CA, USA) according to the manufacturer’s instructions. Cells were harvested 48 h later, and the knockdown efficiency was determined via real-time quantitative-PCR analysis and Western blot analysis.
Protein extraction and immunoblotting
As we described before (Fu et al. 2014), total protein lysates were obtained from harvested cells in protein RIPA lysis buffer (Beyotime, Shanghai, China) containing 10 mM phenylmethylsulphonyl fluoride as a protease inhibitor (PMSF; Beyotime) on ice for 30 min. Coomassie blue staining was used to measure the concentrations of total proteins. The total proteins were separated on 10% SDS-PAGE gels and transferred to 0.45 μm Immobilon-P Transfer Membranes (Millipore Corpora-tion, Billerica, MA, USA). The membranes were blocked in 5% skim milk dissolved in Tris-buffered saline containing Tween and then immunoblotted with primary antibodies.
Real-time quantitative-PCR analysis
RNA was extracted from BMSCs using a RNA isolation kit (Takara, Dalian, China) according to the manufacturer’s instructions. A quantitative RT-PCR analysis was performed using the SYBR Green PCR Master Mix (Takara Bio). The primer sequences included: GAPDH 5′-GGAGATTACTGCCCTGGCTCCTA-3′ (forward) and 5′-GACTCATCGTACTCCTGCTTGCTG-3′ (reverse); SIRT6 5′-CCATCCTAGACTGGGAGGACT-3′ (forward) and 5′-GGATCTGCAGCGATGTACCC -3′ (reverse).
Alizarin Red staining and cell senescence-associated β-galactosidase staining
As we described previously (Xu et al. 2016), after osteogenic induction for 14 days in vitro, cells were stained using alizarin red at room temperature for 10 min then rinsed with PBS. The deposition of calcium was identified under light microscope. Calcified nodules were eluted with 10% cetylpyridinium chloride (CPC) and the absorbance at 562 nm was compared to calcium standards. Senescent cells were identified according to their cellular senescence-associated β-galactosidase activity (SA-β-gal) by the β-Gal Staining Kit (GenMed Scientifics Inc., Shanghai, China), which was described before (Zhou et al. 2016). The ImageJ software was used to quantify the senescent cells.
Immunofluorescence
Immunocytochemical analysis of SIRT6 and LC3B were performed using a standard protocol as we described previously (Fu et al. 2014). Briefly, BMSCs were fixed with 4% paraformalde-hyde and permeabilized with 0.2% Triton X-100 (Sigma) for 15 min, followed by incubation with primary antibodies against SIRT6 (Ab191385; Abcam, Cambridge, MA, USA) (1:200) and LC3B (Ab51520; Abcam, Cambridge, MA, USA) (1:200) at 4 °C overnight. Cells were then incubated with anti-mouse CY3 tagged secondary antibody (Bioworld; Louis Park, MN, USA) (1:50) for 60 min at 37 °C. Nuclei were stained with DAPI (Beyotime). Immunofluorescent images were captured using a fluorescencemicroscope (Leica Microsystems, Mannheim, Germany).
Immunohistochemistry
The paraffin-embedded sections were deparaffinized, and sodium citrate buffer solution was used for tissue antigen retrieval. Endogenous peroxidase activity of each sections were blocked using 3% H2O2 for 20 min and incubation of sections were conducted in serum block for 60 min (10% normal goat serum, Solarbio), followed by incubating with SIRT6 primary antibody overnight at 4 °C. Next, the sections were incubated with appropriate secondary antibody and diaminobenzidine (DAB) was performed according to the manufacturer’s instructions to visualize the SIRT6-expressing cells. The percentage of positive cells compared to the total BMSCs or osteoblasts at the bone boundary in each sample were counted with Image-Pro Plus 5.0 software (Media Cybernetics, MD, USA) (Yu et al. 2012).
Experimental animals
Wnt1-cre and SIRT6fl/fl mice have been described somewhere else (Xiao et al. 2012). To specifically knockdown SIRT6 in NCCs-derived BMSCs, Wnt1-Cre males were crossed with SIRT6fl/fl females (C57BL/6). We used SIRT6fl/+ mice and Wnt1-Cre mice as controls and Wnt1-Cre;SIRT6fl/+ as mutants. All experiments were performed with the approval of the Ethics Committee of the School of Stomatology of Nanjing Medical University. All procedures were carried out in compliance with the guidelines of the Animal Care Committee of Nanjing Medical University.
Micro computed tomography (micro-CT) analysis
The micro-CT analysis was performed as we described previously (Zhu et al. 2019). Briefly, the bones were scanned at high resolution (18 μm) and with an energy of 50 kV and 456 μA. We used NRecon v1.6 and CTAn v1.13.8.1 software for constructing and analyzing the 3D images of the bones. The region of interest was defined to focus on the mandibular bones and the femora of the mice. The following four parameters were calculated to analyze the bone structures: bone volume ratio (BV/TV), trabecular thickness (Tb.Th.), trabecular number (Tb.N.) and trabecular separation (Tb.Sp.).
Statistical analysis
All experiments were repeated independently at least three times. Data in our study were expressed as the means ± SD. Student’s t-test or ANOVA analysis was used to assess the statistical significance as indicated. P-value < 0.05 was considered statistically significant.
Results
Jawbone loss and decreased SIRT6 expression with aging
As is known to all, the loss of bone mass is related with age-related changes (Shih et al. 1993). Twenty young and twenty old male patients were enrolled in our study as we described before. Representative coronal image of mandibles was shown (Fig. 1a). As is shown in Fig. 1b, there were significant differences between the young and old groups in the CTI(S), CTI(I) and CTMI (P < 0.05), which means reduced bone mass in the old group. The loss of SIRT6 has been reported to be related to some aging-associated degenerative processes (Mostoslavsky et al. 2006). Jaw bones obtained from 2 groups of donators (young and old) were prepared into tissue specimens. We examined the expression patterns of SIRT6 in the jaw bones from the two different groups using immunohistochemistry, and found that SIRT6 positive staining was showed in BMSCs and osteoblast in jaw bones from young group. As anticipated, SIRT6 expression in jaw bones from the old group presented a drastic decrease (Fig. 1c, d).
Age-related properties of BMSCs from jawbones of the old group
To investigate the properties of BMSCs from the two groups (young and old) mentioned above, we successfully isolated and cultured human BMSCs from jaw bones of the two groups in vitro. Our data revealed that more SA-β-gal positive cells were gained in the old group (Fig. 2a, b). In addition, Western blot manifested reduced SIRT6 protein expression in the old group, which was also confirmed by our immunofluorescence results (Fig. 2c–e). Together, these results demonstrated that as individuals get older, BMSCs from jaw bones get more senescent and the expression of SIRT6 in the BMSCs may reduce, implying the critical role of SIRT6 in the regulation of BMSCs differention.
Age-related properties of BMSCs from jaw bones of the old group. a, b SA-β-gal staining showed more senescent cells in the Old group. Scale bar 100 μm. c, d Western blot results revealed declined SIRT6 expression in BMSCs from the old group. e Immunofluorescence of BMSCs exhibited similar results. Scale bar 100 μm. **P < 0.01. All data are presented as mean ± SD, n = 3
SIRT6 deficiency affects osteogenic differentiation of BMSCs and promotes senescence
To figure out whether SIRT6 is involved in the osteogenic differentiation of BMSCs, the osteogenic differentiation of BMSCs following SIRT6 knockdown using small interfering RNA was determined (siSIRT6-1 and siSIRT6-2). The knockdown efficiency was confirmed by qRT-PCR and Western blot. The efficiency of siSIRT6-2 was approximately 80 percent reduction of SIRT6 mRNA (Fig. 3a), and significant reduction of SIRT6 protein was also showed in siSIRT6-2 group (Fig. 3b). Our data revealed that SIRT6 knockdown reduces the expression of some osteogenesis-related protein such as RUNX2 and OPN (Fig. 3c). In addition, the BMSCs transfected with si-SIRT6 exhibited markedly decreased density of calcium nodes compared to the non-transfected BMSCs after 14 days of osteogenic induction (Fig. 3d, e). Furthermore, more SA-β-gal positive cells were obtained after transfecting with si-SIRT6 in BMSCs (Fig. 3f, g). Taken together, these results indicated that the ability of BMSCs osteogenic differentiation can be damaged after inhibiting the expression of SIRT6, which may also promote the senescence of BMSCs.
Effects of SIRT6 inhibition on the osteogenic differentiation and senescence of BMSCs. a The mRNA expression levels of SIRT6 were analyzed by qRT-PCR. b The protein expression levels of SIRT6 were analyzed by Western blot. c The patterns of RUNX2, OPN expression levels following the osteogenic culture of BMSCs treated with si-SIRT6 for 7 days were analyzed by Western blot. d, e Alizarin red staining revealed a reduced amount of calcium nodules in BMSCs treated with si-SIRT6. Scale bar 100 μm. f, g SA-β-gal staining showed an increased senescence trend after knockdown of SIRT6. Scale bar 100 μm. *P < 0.05, **P < 0.01, ****P < 0.0001. All data are presented as mean ± SD, n = 3
SITR6 inhibition impairs autophagy through the AKT-mTOR signaling pathway in BMSCs
The involvement of SIRT6 in the autophagy has been demonstrated in recent works (Huang et al. 2017; Lu et al. 2016; He et al. 2017; Wang et al. 2018). Here we investigated the effect of SIRT6 inhibition on the autophagy of BMSCs. As was shown in Fig. 4a, b, the protein level of two well-known effectors of autophagosome formation, LC3BII and Beclin1, were downregulated in the presence of small interfering RNA of SIRT6. Also the protein level of P62, was increased after the knockdown of SIRT6, which is a selective receptor of autophagy substrates. This trend was also demonstrated in our immunofluorescence staining data (Fig. 4c). The formation of LC3B punctate structure was inhibited after we silenced the expression of SIRT6, indicative of loss of autophagosome accumulation. Autophagy is regulated by some different signaling pathways including PI3K-AKT-mTOR. To investigate whether SIRT6 is involved in the AKT-mTOR signaling, we detected relative protein level in the BMSCs after inhibiting the expression of SIRT6. The expression of phosphorylated AKT and phosphorylated mTOR were markedly increased in the si SIRT6 group (Fig. 4d, e). To further explore the relationship between SIRT6 and autophagy, we used rapamycin (RA), an autophagy inducer which is a lipophilic macrolide antibiotic after the inhibition of SIRT6 in BMSCs. The levels of osteogenic proteins and autophagy-related proteins in the SI + RA group were obviously enhanced compared to the SI group (Fig. 4f, g). In summary, autophagy of BMSCs was damaged after the inhibition of SIRT6 via, at least in part, the AKT-mTOR signaling pathway.
The inhibition of SIRT6 impairs autophagy in BMSCs. a, b The protein expression levels of SIRT6, Beclin1, P62 and LC3B were detected by Western blot. c Representative Immunofluorescence microscopic images of LC3B positive puncta transfected with si-SIRT6. Scale bar: 10 μm. d, e The protein expression levels of AKT, P-AKT, mTOR and P-mTOR in SIRT6-knockdown BMSCs were analyzed by Western blot. f, g The patterns of some osteogenesis and autophagy markers were analyzed by Western blot in BMSCs treated with si-SIRT6 (SI) and rapamycin (RA) individually. *P < 0.05, **P < 0.01. All data are presented as mean ± SD, n = 3
Wnt1-Cre;SIRT6fl/+ mice exhibit maxillofacial rather than femoral bone defects
To explore whether the role of SIRT6 in the development of bones is of importance in vivo, we knocked down SIRT6 specially in NCCs and NCCs-derived cells and tissues such as BMSCs and most craniomaxillofacial bones by crossing Wnt1-Cre males with SIRT6fl/fl females (C57BL/6). As is shown in Fig. 5a, b, micro-CT scanning showed that the 3-week-old Wnt1-Cre;SIRT6fl/+ (Mutant) mice present a conspicuous decrease in mineralization of the mandibular and alveolar bones relative to their SIRT6fl/+ (Ctrl) littermates. However, there were no radiographic differences in femoral bone mass in Wnt1-Cre;SIRT6fl/+ mice compared to their SIRT6fl/+ littermates (Fig. 5c, d). The Masson trichrome staining demonstrated similar results (Fig. 5e, f). As a result, we have reasons to believe that the absence of SIRT6 in BMSCs influence the development and mineralization of bones.
Wnt1-Cre;SIRT6fl/+ mice exhibit mandibular and alveolar rather than femoral bone defects. a Representative micro-CT sagittal view images of mandibular and alveolar bones from SIRT6fl/+ (Ctrl) and Wnt1-Cre;SIRT6fl/+ (Mutant) mice. b Quantitative analysis of mandibles by micro-CT. c Representative micro-CT images of femora from the two groups of mice. d Quantitative analysis of the femora by micro-CT. e Representative Masson’s trichrome staining photographs of mandibles described above. Scale bar 500 μm. f Representative Masson’s trichrome staining photographs of the femora described above. Scale bar 500 μm. *P < 0.05, **P < 0.01. All data are presented as mean ± SD, n = 3
Discussion
In this study, we analyzed CBCT data of the elderly and found age-related jawbone loss. Then we characterized age-related properties of jawbone tissues and BMSCs from human jaws, diminished SIRT6 expression with aging was identified to be crucially involved in fates determination of jawbone-derived BMSCs. Furthermore, our data indicated that SIRT6 regulated autophagy and osteogenesis of BMSCs by activating AKT-mTOR pathway. Wnt1-Cre;SIRT6fl/+ mice model was established and we found impaired bone quality in their jawbones.
The lineage differention and fate decision of BMSCs is essential for bone formation, which is an orchestrated biological process with many protein molecules involved in it (Ono et al. 2017; Schlundt et al. 2018). Many osteogenic genes have been explored in vitro and in vivo. However, we are still far from a thorough understanding of osteogenesis process. The silencing information regulator 2 (Sir2) family was discovered to regulate aging genomic stability in budding yeast as a chromatin silencer (Gertler et al. 2013). Sirtuins are members of it in mammals which includes SIRT1-7. Among them SIRT6 is located in the nucleus and is best characterized as a NAD+-dependent deacetylase of H3K9 and H3K56 (Michishita et al. 2008; Yang et al. 2009). SIRT6−/− mice can present skeletal defects (Zhang et al. 2018; Fan et al. 2019). These results indicate SIRT6 may play a critical role in the prevention of age-related jawbone loss. Distinct from peripheral bones, jawbones derive from migrating cranial neural crest cells (Chai et al. 2000). In our study, because of the high fatality rate in the NCC-specific knockout mice Wnt1-Cre;SIRT6fl/fl, SIRT6 haploinsufficiency mice Wnt1-Cre;SIRT6fl/+ were used as an animal model to explore the role of SIRT6 in the osteogenesis of jawbones. We found that the mutant mice exhibit defects in the minerilization of mandibular bones. However, there was no significant difference in the bone quality of their femora compared with that of the control group. As is known to all, peripheral bones including the femur develop from mesoderm and are not derived from neural crest cells, which is consistent with our results. Our immunohistological analysis data indicated the expression of SIRT6 diminish in the elderly. Subsequently, similar results were found in BMSCs from jaw bones. After the inhibition of SIRT6 in BMSCs with related siRNA, we observed that the osteogenic differentiation of BMSCs was inhibited, which was consistent with some previous studies (Zhang et al. 2017; Sun et al. 2014).
Then we further explored the mechanism behind the phenomenon. Autophagy was reported to play a paramount role in cell homeostasis (Smith et al. 2017; Revuelta et al. 2017). In general pathological conditions, the disturbance of balance between bone resorption and bone formation is the root of osteoporosis, while autophagy is associated with bone metabolism-related degeneration process of cells from the beginning to the end (Hocking et al. 2012). In recent years, more and more evidences showed that autophagy plays an important role in maintaining the balance of bone metabolism and the change of autophagy level is an important cause of osteoporosis (Hocking et al. 2012; Qi et al. 2017). We found that autophagy-related protein expression levels decreased when we silenced the expression of SIRT6 with siRNA. The accumulation of autophagosome was reduced and the AKT-mTOR signaling pathway was activated, which plays an important role in the regulation of autophagy (Heras-Sandoval et al. 2014; Xue et al. 2017). Our study also revealed that the autophagy inducer rapamycin can rescue the decreased expression of osteogenesis-related genes caused by SIRT6 deficiency. However, how SIRT6 regulates autophagy to play the role of osteogenesis has not been known. Further work is needed to explore the specific mechanism between SIRT6 and autophagy.
In summary, we have reasons to conclude that diminished SIRT6 expression of BMSCs is a promoter of jawbone loss in the elderly, which may be affected by impaired autophagy. Our work presented a new perspective for understanding the causes of age-related jawbone loss. SIRT6-targeting agents such as pharmacological activation of SIRT6 may be an effective therapeutic strategy for preventing jawbone loss. Furthermore, detailed mechanisms underlying SIRT6 gene regulation should be elaborated to develop possible clinical treatments for osteoporosis or other bone-related diseases such as hyperostosis, osteoarthritis and osteonecrosis.
References
Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2:313–319. https://doi.org/10.1016/j.stem.2008.03.002
Bodic F, Hamel L, Lerouxel E, Baslé MF, Chappard D (2005) Bone loss and teeth. Joint Bone Spine 72:215–221. https://doi.org/10.1016/j.jbspin.2004.03.007
Chai Y et al (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127:1671–1679
Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A (2005) Autophagy and aging: the importance of maintaining "clean" cells. Autophagy 1:131–140
Fan Y et al (2019) Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through AMPK activation. Int J Biol Sci 15:701–713. https://doi.org/10.7150/ijbs.29323
Fu Y et al (2014) Histone deacetylase 8 suppresses osteogenic differentiation of bone marrow stromal cells by inhibiting histone H3K9 acetylation and RUNX2 activity. Int J Biochem Cell Biol 54:68–77. https://doi.org/10.1016/j.biocel.2014.07.003
García-Prat L et al (2016) Autophagy maintains stemness by preventing senescence. Nature 529:37–42. https://doi.org/10.1038/nature16187
Gertler AA, Cohen HY (2013) SIRT6, a protein with many faces. Biogerontology 14:629–639. https://doi.org/10.1007/s10522-013-9478-8
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. https://doi.org/10.1146/annurev-genet-102808-114910
He J et al (2017) SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. Febs J 284:1324–1337. https://doi.org/10.1111/febs.14055
Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Hocking LJ, Whitehouse C, Helfrich MH (2012) Autophagy: a new player in skeletal maintenance? J Bone Miner Res 27:1439–1447. https://doi.org/10.1002/jbmr.1668
Huang N et al (2017) Sirtuin 6 plays an oncogenic role and induces cell autophagy in esophageal cancer cells. Tumour Biol 39:1010428317708532. https://doi.org/10.1177/1010428317708532
Koh K, Kim K (2011) Utility of the computed tomography indices on cone beam computed tomography images in the diagnosis of osteoporosis in women. Imaging Sci Dent 41:101. https://doi.org/10.5624/isd.2011.41.3.101
Lu J et al (2016) SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl Res 172:96–112.e6. https://doi.org/10.1016/j.trsl.2016.03.002
Ma Y et al (2018) Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. https://doi.org/10.1111/acel.12709
McClung M, Baron R, Bouxsein M (2017) An update on osteoporosis pathogenesis, diagnosis, and treatment. Bone 98:37. https://doi.org/10.1016/j.bone.2017.02.013
Michishita E et al (2008) SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452:492–496. https://doi.org/10.1038/nature06736
Mostoslavsky R et al (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124:315–329. https://doi.org/10.1016/j.cell.2005.11.044
Mu W et al (2018) Metformin promotes the proliferation and differentiation of murine preosteoblast by regulating the expression of sirt6 and oct4. Pharmacol Res 129:462–474. https://doi.org/10.1016/j.phrs.2017.11.020
Ono T, Takayanagi H (2017) Osteoimmunology in bone fracture healing. Curr Osteoporos Rep 15:367–375. https://doi.org/10.1007/s11914-017-0381-0
Qi M et al (2017) Autophagy maintains the function of bone marrow mesenchymal stem cells to prevent estrogen deficiency-induced osteoporosis. Theranostics 7:4498–4516. https://doi.org/10.7150/thno.17949
Revuelta M, Matheu A (2017) Autophagy in stem cell aging. Aging Cell 16:912–915. https://doi.org/10.1111/acel.12655
Schlundt C et al (2018) Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone 106:78–89. https://doi.org/10.1016/j.bone.2015.10.019
Shih MS, Cook MA, Spence CA, Palnitkar S, McElroy H, Parfitt AM (1993) Relationship between bone formation rate and osteoblast surface on different subdivisions of the endosteal envelope in aging & osteoporosis. Bone 14:519–521
Smith M, Wilkinson S (2017) ER homeostasis and autophagy. Essays Biochem 61:625–635. https://doi.org/10.1042/EBC20170092
Song C, Song C, Tong F (2014) Autophagy induction is a survival response against oxidative stress in bone marrow-derived mesenchymal stromal cells. Cytotherapy 16:1361–1370. https://doi.org/10.1016/j.jcyt.2014.04.006
Sugatani T, Agapova O, Malluche HH, Hruska KA (2015) SIRT6 deficiency culminates in low-turnover osteopenia. Bone 81:168–177. https://doi.org/10.1016/j.bone.2015.07.018
Sun H, Wu Y, Fu D, Liu Y, Huang C (2014) SIRT6 regulates osteogenic differentiation of rat bone marrow mesenchymal stem cells partially via suppressing the nuclear factor-kappaB signaling pathway. Stem Cells 32:1943–1955. https://doi.org/10.1002/stem.1671
Wang L et al (2018) Aberrant SIRT6 expression contributes to melanoma growth: role of the autophagy paradox and IGF-AKT signaling. Autophagy 14:518–533. https://doi.org/10.1080/15548627.2017.1384886
Xiao C et al (2012) Progression of chronic liver inflammation and fibrosis driven by activation of c-JUN signaling in Sirt6 mutant mice. J Biol Chem 287:41903–41913. https://doi.org/10.1074/jbc.M112.415182
Xu R et al (2016) Transplantation of osteoporotic bone marrow stromal cells rejuvenated by the overexpression of SATB2 prevents alveolar bone loss in ovariectomized rats. Exp Gerontol 84:71–79. https://doi.org/10.1016/j.exger.2016.09.001
Xu R et al (2018) Simvastatin improves oral implant osseointegration via enhanced autophagy and osteogenesis of BMSCs and inhibited osteoclast activity. J Tissue Eng Regen Med 12:1209–1219. https://doi.org/10.1002/term.2652
Xue JF, Shi ZM, Zou J, Li XL (2017) Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother 89:1252–1261. https://doi.org/10.1016/j.biopha.2017.01.130
Yang B, Zwaans BM, Eckersdorff M, Lombard DB (2009) The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle 8:2662–2663. https://doi.org/10.4161/cc.8.16.9329
Yu Y et al (2012) Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol 137:513–525. https://doi.org/10.1007/s00418-011-0908-x
Zhang P et al (2017) SIRT6 promotes osteogenic differentiation of mesenchymal stem cells through BMP signaling. Sci Rep. https://doi.org/10.1038/s41598-017-10323-z
Zhang W et al (2018) SIRT6 deficiency results in developmental retardation in cynomolgus monkeys. Nature 560:661–665. https://doi.org/10.1038/s41586-018-0437-z
Zhou, P. et al. (2016) SATB2-Nanog axis links age-related intrinsic changes of mesenchymal stem cells from craniofacial bone. Aging 8:2006–2011. https://doi.org/10.18632/aging.101041
Zhu W et al (2019) Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw. Faseb J. https://doi.org/10.1096/fj.201801791RR
Acknowledgements
This work was supported by the National Natural Science Fundation of China (81970910) and a project funded by the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (2018-87).
Author information
Authors and Affiliations
Contributions
XS performed experiments, analyzed data, and wrote the manuscript. XC, JDH prepared the figures and analyzed data. RYX and JC contributed to the reagents and materials. HBJ designed the experimental study and analyzed the data. All authors participated in discussing and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, X., Chen, X., Huang, J. et al. Age-dependent role of SIRT6 in jawbone via regulating senescence and autophagy of bone marrow stromal cells. J Mol Hist 51, 67–76 (2020). https://doi.org/10.1007/s10735-020-09857-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-020-09857-w