Abstract
Hypertrophic scar (HS) is a cutaneous fibrotic disorder characterized by persistent inflammation, excessive proliferation of fibroblasts, and abundant accumulation of extracellular matrix (ECM) proteins. Pleiotrophin (PTN) is a highly conserved and secreted ECM-associated protein that belongs to a novel family of heparin-binding cytokines with multiple biological functions. The aim of this study was to detect and compare the expression and localization of PTN in HS tissues and normal skin tissues. Surgically removed HS tissue samples and site-matched normal skin specimens were obtained from 18 patients during the scar excision and reconstructive surgery. Semi-quantitative RT-PCR, Western blot analysis and immunohistochemistry were used to determine PTN gene expression and localization in skin tissues. Compared with the normal skin tissues, PTN was highly expressed at both mRNA and protein levels in HS tissues (P < 0.01). In immunohistochemical staining, PTN protein was localized in the cells of both epidermis and dermis in skin tissues, and there were increased staining intensity of PTN in HS tissues than in normal skin samples. In conclusion, elevated expression of PTN is likely to be involved in the pathogenesis of HS. Further studies are still required to elucidate the exact role of PTN in HS formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertrophic scar (HS) is a cutaneous fibrotic disorder that occurs usually within 8 weeks following wound closure with excess tension, wound infection, hypoxia, or other traumatic skin injury (Niessen et al. 1999). In addition to a cosmetic concern, HS is frequently associated with symptoms of pain, pruritus, contractures, and other functional problems (Bock et al. 2006). Despite considerable research efforts in recent years, the underlying pathophysiology of HS remains to be elucidated. It is, however, generally accepted that persistent inflammation, excessive proliferation of fibroblasts, and abundant accumulation of extracellular matrix (ECM) proteins are key processes in the formation of HS (Tredget et al. 1997; Gauglitz et al. 2011). Therefore, understanding the changes in ECM molecules and cytokines involved in these processes may provide valuable information about the pathophysiology of this disease.
Pleiotrophin (PTN) (also known as heparin-binding growth-associated molecule [HB-GAM] or osteoblast specific factor-1 [OSF-1]) is a highly conserved and secreted ECM-associated protein that belongs to a novel family of heparin-binding cytokines with similar biological activities (Deuel et al. 2002). PTN was initially described as a growth factor produced by the bovine uterus (Milner et al. 1989) and as a neurite outgrowth promoting factor present in the neonatal rat brain (Rauvala 1989). Subsequent studies showed that PTN is strongly expressed in multiple tissues during later stages of embryogenesis, suggesting a critical role of PTN in development (Vanderwinden et al. 1992). Recently, considerable accumulated evidence suggests that activation of PTN is involved in various physiopathological processes, such as angiogenesis (Perez-Pinera et al. 2008), odontogenesis (Erlandsen et al. 2012), atherosclerosis (Li et al. 2010), bone formation (Imai et al. 2009), peritoneal fibrosis (Yokoi et al. 2012), wound healing (Florin et al. 2005), and tumorigenesis (Papadimitriou et al. 2009). In particular, Florin et al. have reported that PTN exerts a mitogenic effect on primary human keratinocytes, and activation of PTN is observed during cutaneous wound healing in vivo (Florin et al. 2005). Furthermore, PTN has been shown to trigger inflammation and increased peritoneal permeability, thereby leading to peritoneal fibrosis (Yokoi et al. 2012). Given the role of PTN in growth- and inflammation-promoting activities, it is possible to hypothesize that the expression of PTN may be changed in HS tissues.
In the present study, we analyzed the expression of PTN in the specimens of HS and normal skin samples from 18 patients by using semi-quantitative RT-PCR, Western blot analysis, and immunohistochemistry. Our data showed increased expression of PTN in HS specimens compared to the normal skin tissues, suggesting that PTN is likely to be involved in the pathophysiology of HS.
Materials and methods
Patients and clinical specimens
Surgically excised HS tissue samples and site-matched normal skin specimens were obtained from 18 patients who received scar excision and reconstructive surgery at 6–12 months after thermal injury. These patients consisted of 7 male and 11 female, with a median age of 32 years (range, 12–79 years). None of these patients received preoperative glucocorticosteroids or radiotherapy. All the scars were in proliferative phase confirmed pathologically. All tissues were divided into two proportions, one immersed in 10 % formalin solution for histopathological examination and immunohistochemistry, and the remaining part in liquid nitrogen for semi-quantitative RT-PCR and Western blot analysis. Written informed consent was obtained from either patients or their guardians, and this study was approved by the Ethics Committee of The General Hospital of Shenyang Military Area Command.
Histological examination
Skin tissues were fixed in 10 % formalin solution overnight, embedded in paraffin, and sectioned with a thickness of 5 μm. The tissue sections were placed on slides and stained with routine haematoxylin and eosin (H&E) for histology and Mason’s Trichrome for distribution of collagen. The slides were then viewed under a light microscope (BA400 Binocular Microscope; Motic, Xiamen, China).
Immunohistochemistry
Paraffin-embedded tissue sections were pretreated at 60 °C for 2 h, deparaffinized in xylene, and hydrated gradually through a series of graded ethanol. Thereafter, antigen retrieval was carried out by microwaving the sections in 10 mmol/l citrate buffer (pH 6.0) for 10 min, and endogenous peroxidase activity was blocked by using 0.3 % hydrogen peroxide in methanol. The sections were treated with 10 % normal goat serum to reduce non-specific binding, and then incubated with a goat polyclonal antibody against human PTN (1:100 diluted; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in a humidified chamber at 4 °C overnight, followed by the corresponding streptavidin-biotinylated secondary antibody. Finally, immunolabeled sections were visualized by using 3, 3′-diaminobenzidine (DAB), then counterstained with hematoxylin, dehydrated, and mounted. Negative controls were achieved by an isotype matched IgG in each of the immunostaining.
Semi-quantitative RT-PCR
Total RNA was isolated from frozen tissue samples using the RNA simple total-RNA kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. One microgram of total RNA from each sample was reverse-transcribed to single-strand cDNA with an oligo dT primer. Semi-quantitative RT-PCR was performed in a final volume of 20 μl of containing 2 μl of 10 × Taq buffer, 0.5 μM of forward and reverse primers, 1.6 μl of dNTPs, 0.1 μl of Taq polymerase (Tiangen), and 1 μl of template cDNA. The forward and reverse primers were 5′-TCTCCATTTCCCTTCCGTTCC-3′ and 5′-AGGTTGCTACCGCTGAGTCC-3′ for the target gene PTN and were 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and 5′-CTGTCACCTTCACCGTTCCAGTTT-3′ for the reference gene β-actin. The PCR products were visualized by electrophoresis on a 1.5 % agarose gel stained with ethidium bromide.
Western blot analysis
Total proteins were isolated from frozen tissues using ice-cold radioimmunoprecipitation (RIPA) lysis buffer (Beyotime Institute of Biotechnology, Haimen, China), and concentrations of soluble proteins were quantified using the Bradford method. Equal amounts of protein were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA) using a semi-dry transfer apparatus. The membranes were blocked with 5 % skimmed milk at room temperature for 2 h and then immunoblotted overnight at 4 °C with anti-PTN polyclonal antibody (1:1,000 diluted; Santa Cruz Biotechnology) or anti-β-actin antibody (dilution 1: 5,000, Sigma, St. Louis, MO, USA), followed by incubation with their respective horseradish peroxidase-conjugated secondary antibodies. Antibody binding was probed using an ECL plus chemiluminescence kit (Millipore) and visualized by exposing membranes to X-ray films. The intensity of the bands was then quantified by densitometric analysis and normalized to the loading control β-actin.
Statistical analysis
All data are presented as mean ± standard deviation (SD), and raw data were analyzed by the unpaired Student t test using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The values of P less than 0.05 were considered statistically significant.
Results
Morphological characteristics of HS tissues
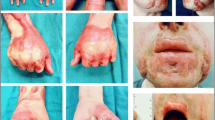
Specimens were obtained from patients who developed clinical signs of HT including thickened, hyperemic, raised, pruritic, and itchy scars confined to the site of injury. Site-matched normal skin samples from the same patients were served as controls. Histological observations were conducted in both HS and normal skin tissues, and histological characteristics of HT compared with normal skin are illustrated in Fig. 1. H&E staining showed a thick layer of keratinocytes in epidermis and an abundance of dermal cell density in HS specimens (Fig. 1a), whereas a thin epidermal layer and less dense of cells were found in normal skin samples (Fig. 1b). Masson’s staining also revealed that the typical feature of HS tissues was fibrosis characterized by excessive accumulation of ECM resulting from the excessive of hypercellularity proliferation in dermis (Fig. 1c). The collagen fibers appeared flexible and arranged in a network in normal skins (Fig. 1d), while the collagen fibers appeared swirl-shaped in thick collagen fibers instead of a network in HS tissues (Fig. 1c). Moreover, in HS tissues, loosely packed collagen fiber bundles showed to be thicker with a parallel noodle-shaped structure oriented in one direction (Fig. 1c). In contrast, in normal skin, thin collagen fibers were organized in a basket-weave orientation throughout the connective tissue (Fig. 1d).
High expression of PTN in HS tissues compared with matched normal tissues
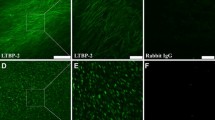
Immunohistochemical staining showed that PTN protein was localized in the cells of both epidermis and dermis in skin tissues. In comparison to normal skin tissues, which showed scarce PTN staining in the cells of both epidermis and dermis (Fig. 2b), HS tissues exhibited intensive PTN positive staining in epidermal and dermal layer as well as the extracellular matrix (Fig. 2a). To further clarify the above findings, mRNA and protein levels of PTN in all the tissues were analyzed by semi-quantitative RT-PCR and Western blot analysis, respectively. As shown in Fig. 3a and b, the relative expression of PTN mRNA in HS tissues was markedly higher than that in the normal skin samples (P < 0.01). Figure 3c shows representative blots of PTN protein expression by Western blot analysis. β-actin expression was used as the internal control for each sample. Relative band densities for PTN expression after normalized by β-actin expression is shown in Fig. 3d. The results of Western blot analysis revealed that the protein expression levels of PTN were significantly up-regulated in HS tissues compared with normal skins (P < 0.01). Taken together, our data suggest that elevated expression of PTN is likely to be involved in the pathogenesis of HS.
Semi-quantitative RT-PCR (a and b) and Western blot analysis (c and d) of PTN expression in 18 cases of HS tissues and site-matched normal skin samples. a Representative ethidium bromide-stained agarose gel is shown. b Band intensities were quantified and the intensities of the bands corresponding to PTN were compared to those corresponding to β-actin (n = 18). c Representative blots are shown, and the protein size is expressed in kDa. d Densitometric values were normalized by β-actin levels (n = 18). HS, hypertrophic scar tissue; N, normal skin tissues
Discussion
In this study, we investigated the expression of PTN mRNA and protein, and observed the differential expression of PTN between HS and normal skin tissues. From the results of immunohistochemical observations, we found that PTN protein was localized in the cells of both epidermis and dermis in skin tissues, and there were increased staining intensity of PTN in HS tissues than in normal skin samples. In addition, PTN was significantly up-regulated at both mRNA and protein levels in HS tissues as compared with the site-matched normal skin specimens.
As an important secreted and heparin-binding cytokine, PTN has been reported to exert various biological functions. Although the role of PTN in other systems has been extensively studied, to our knowledge this study represents, for the first time, evidence for increased expression of PTN in HS tissues. Florin et al. have demonstrated that PTN exerts a mitogenic effect on primary human keratinocytes, and activation of PTN is observed during cutaneous wound healing in vivo (Florin et al. 2005). In agreement with these observations, the present study also showed that PTN protein was localized in both epidermis and dermis in HS and normal skin tissues. These findings suggest a key physiological role of PTN in various tissue types.
Notably, we found increased PTN expression in HS tissues compared with normal skin samples. Increased PTN expression was demonstrated by both immunostaining of skin tissue sections and by total protein expression of snap frozen skin tissue homogenates. However, the mechanisms by which aberrant PTN expression contributes to the pathogenesis of HS remain unknown. Angiogenesis has long been known to play an essential role in the early stage of wound healing, and there are reports of microvascular abnormalities in pathological scars (Kischer 1992; Kurokawa et al. 2010). In addition, it has been suggested that anti-angiogenesis may inhibit the proliferation of fibroblasts and production of collagen, and might thus be used as an effective strategy for early intervention of HS formation (Song et al. 2009). Recently, mounting evidence has shown that PTN is a multifunctional angiogenic factor that stimulates both normal and pathological angiogenesis (Perez-Pinera et al. 2008). Thus, we speculated that stimulation of angiogenesis may represent as one of the mechanisms for the role of PTN in the pathogenesis of HS formation. Furthermore, recent evidence also suggests that several signaling pathways, such as the Wnt/β-catenin and Notch pathways, are also involved in the pathogenesis of HS formation (Sato 2006; Xia et al. 2009; Diao et al. 2009). Weng et al. have documented that PTN regulates lung epithelial cell proliferation and differentiation during fetal lung development via β-catenin and Notch pathways (Weng et al. 2009). Therefore, PTN is likely to contribute to the pathogenesis of HS formation via integration of multiple signaling pathways, including the β-catenin and Notch pathways. Nonetheless, it remains unknown whether the increased PTN is a cause or a consequence of HS formation. Further studies are also necessary to elucidate the functional roles of PTN in the pathophysiology of HS, and to explain whether PTN could be used to prevent the formation of HS.
In summary, the present study demonstrated that PTN is highly expressed at both mRNA and protein levels in HS tissues, and there is a significant increase in PTN expression HS tissues compared with that in normal skin tissues. Further studies are still required to elucidate the exact role of PTN in HS formation.
References
Bock O, Schmid-Ott G, Malewski P, Mrowietz U (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297(10):433–438. doi:10.1007/s00403-006-0651-7
Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY (2002) Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys 397(2):162–171. doi:10.1006/abbi.2001.2705
Diao JS, Zhang X, Ren J, Zeng HF, Liu B, Ma FC, Wang YM, Yang XT, Guo SZ, Xia W (2009) Blocking of Notch signaling decreased scar formation in rabbit ear hypertrophic scar model. Zhonghua Yi Xue Za Zhi 89(16):1088–1092
Erlandsen H, Ames JE, Tamkenath A, Mamaeva O, Stidham K, Wilson ME, Perez-Pinera P, Deuel TF, Macdougall M (2012) Pleiotrophin expression during odontogenesis. J Histochem Cytochem 60(5):366–375. doi:10.1369/0022155412439316
Florin L, Maas-Szabowski N, Werner S, Szabowski A, Angel P (2005) Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell Sci 118(Pt 9):1981–1989. doi:10.1242/jcs.02303
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG (2011) Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 17(1–2):113–125. doi:10.2119/molmed.2009.00153
Imai S, Heino TJ, Hienola A, Kurata K, Buki K, Matsusue Y, Vaananen HK, Rauvala H (2009) Osteocyte-derived HB-GAM (pleiotrophin) is associated with bone formation and mechanical loading. Bone 44(5):785–794. doi:10.1016/j.bone.2009.01.004
Kischer CW (1992) The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol 24(2):281–296
Kurokawa N, Ueda K, Tsuji M (2010) Study of microvascular structure in keloid and hypertrophic scars: density of microvessels and the efficacy of three-dimensional vascular imaging. J Plast Surg Hand Surg 44(6):272–277. doi:10.3109/2000656X.2010.532923
Li F, Tian F, Wang L, Williamson IK, Sharifi BG, Shah PK (2010) Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN-{gamma}/JAK/STAT1 signaling is critical for the expression of PTN in macrophages. FASEB J 24(3):810–822. doi:10.1096/fj.09-140780
Milner PG, Li YS, Hoffman RM, Kodner CM, Siegel NR, Deuel TF (1989) A novel 17 kD heparin-binding growth factor (HBGF-8) in bovine uterus: purification and N-terminal amino acid sequence. Biochem Biophys Res Commun 165(3):1096–1103
Niessen FB, Spauwen PH, Schalkwijk J, Kon M (1999) On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 104(5):1435–1458
Papadimitriou E, Mikelis C, Lampropoulou E, Koutsioumpa M, Theochari K, Tsirmoula S, Theodoropoulou C, Lamprou M, Sfaelou E, Vourtsis D, Boudouris P (2009) Roles of pleiotrophin in tumor growth and angiogenesis. Eur Cytokine Netw 20(4):180–190. doi:10.1684/ecn.2009.0172
Perez-Pinera P, Berenson JR, Deuel TF (2008) Pleiotrophin, a multifunctional angiogenic factor: mechanisms and pathways in normal and pathological angiogenesis. Curr Opin Hematol 15(3):210–214. doi:10.1097/MOH.0b013e3282fdc69e
Rauvala H (1989) An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J 8(10):2933–2941
Sato M (2006) Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol 86(4):300–307. doi:10.2340/00015555-0101
Song B, Zhang W, Guo S, Han Y, Zhang Y, Ma F, Zhang L, Lu K (2009) Adenovirus-mediated METH1 gene expression inhibits hypertrophic scarring in a rabbit ear model. Wound Repair Regen 17(4):559–568. doi:10.1111/j.1524-475X.2009.00514.x
Tredget EE, Nedelec B, Scott PG, Ghahary A (1997) Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg Clin North Am 77(3):701–730
Vanderwinden JM, Mailleux P, Schiffmann SN, Vanderhaeghen JJ (1992) Cellular distribution of the new growth factor pleiotrophin (HB-GAM) mRNA in developing and adult rat tissues. Anat Embryol (Berl) 186(4):387–406
Weng T, Gao L, Bhaskaran M, Guo Y, Gou D, Narayanaperumal J, Chintagari NR, Zhang K, Liu L (2009) Pleiotrophin regulates lung epithelial cell proliferation and differentiation during fetal lung development via beta-catenin and Dlk1. J Biol Chem 284(41):28021–28032. doi:10.1074/jbc.M109.052530
Xia W, Pan BH, Liu B, Zhang X, Ma FC, Wang YM, Yang XT, Liu D, Guo SZ (2009) Expression of Notch receptors, ligands and downstream target genes in epidermis of hypertrophic scar. Zhonghua Zheng Xing Wai Ke Za Zhi 25(1):41–45
Yokoi H, Kasahara M, Mori K, Ogawa Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Kato Y, Mori KP, Toda N, Ohno S, Muramatsu H, Muramatsu T, Sugawara A, Mukoyama M, Nakao K (2012) Pleiotrophin triggers inflammation and increased peritoneal permeability leading to peritoneal fibrosis. Kidney Int 81(2):160–169. doi:10.1038/ki.2011.305
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Tao, K., Huang, W. et al. Elevated expression of pleiotrophin in human hypertrophic scars. J Mol Hist 44, 91–96 (2013). https://doi.org/10.1007/s10735-012-9453-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-012-9453-8