Abstract
Glucose-regulated protein 75 (GRP75), a member of the heat-shock protein 70 family, is known to protect cells from stress-induced injury. However, information regarding its distribution and possible function in the retina is limited. In this study, we performed an optic nerve crush (ONC) model in adult rats and found that GRP75 was significantly upregulated in the retina after ONC. Double immunofluorescent staining revealed that GRP75 was localized in the retinal ganglion cells (RGCs). We also examined the expression profile of active caspase3, whose change was correlated with the expression profile of GRP75. In addition, we utilized co-staining of GRP75 and active caspase3 and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) to study their correlation in the retina following ONC. Furthermore, the expressions of Bax, cytochrome c (Cytc), p-extracellular-signal-regulated kinases (ERK)1/2, and p-AKT were enhanced in the retina after ONC, and they were parallel with the expression profile of GRP75. Based on our data, we speculated that GRP75 might play an important role in RGCs apoptosis following ONC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinal ganglion cells (RGCs) are sensitive to optic nerve injuries caused by various etiologies such as glaucoma (Joachim et al. 2014). It is well accepted that RGCs of glaucomatous eyes die due to apoptosis (Joachim et al. 2014). The mechanism of RGCs apoptosis in glaucoma is controversial and not fully understood; many signaling pathways are involved in this process (Chen et al. 2013; Wada et al. 2013).
The apoptotic mechanism of RGCs has been investigated in this regard, and much effort has been put to the effects of mitochondria on apoptosis (Zhang et al. 2012). The activation of mitochondrial pathway (Bcl-2 family) results in the release of mitochondrial cytochrome c (Cytc) into the cytoplasm. Cytc combines with the caspase9 precursor to form an apoptosis complex. This activation of caspase9 then drives caspase3 to induce RGCs apoptosis (Wang et al. 2014; Zhang et al. 2012).
A widely used animal model for investigating the mechanism of RGCs death is optic nerve crush (ONC) (Huang et al. 2014; Xu et al. 2014; Wu et al. 2014). It triggers RGC death by inducing apoptosis and is characterized by the presence of fragmented nuclei and apoptotic bodies, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, and the activation of caspase family and Bcl-2 family (Huang et al. 2014; Xu et al. 2014; Wu et al. 2014). This model has been used for elucidating the cellular and molecular events leading to RGC death and for testing neuroprotective strategies (Zhu et al. 2013).
Glucose-regulated protein 75 (GRP75), a member of the HSP70 family, is primarily of mitochondrial origin and is involved in multiple functions that are required to maintain cell metabolism, including the stress response and cell proliferation and differentiation (Kaul et al. 2007). Stress causes protein damage and may also trigger the cellular defense mechanisms, among which the GRP75 has being well described. Of note, GRP75 is triggered by glucose deprivation (GD), oxidative injury, ionizing radiation, calcium ionophores, and hyperthyroidism (Liu et al. 2005; Taurin et al. 2002; Yang et al. 2008). Over-expression of GRP75 in PC12 cells could prevent cells from apoptosis by reducing the expression of Bax and delaying the release of Cytc (Yang et al. 2008). GRP75 also could inhibit the Bax conformational change and subsequent cell apoptosis through activating AKT pathway and extracellular-signal-regulated kinases (ERK)1/2 pathway (Yang et al. 2011). Up to now, the biological function of GRP75 in the retina is still with little acquaintance. Because of the mitochondria-dependent anti-apoptotic effect of GRP75 in neurons and the activation of mitochondrial pathway in RGCs apoptosis, it is reasonable to hypothesize that GRP75 may be associated with RGC apoptosis after ONC.
In the present study, we reported the expression and distribution of GRP75 in rat retina after ONC for the first time. Our research is conducted to gain a better insight into the physiologic functions of GRP75 in the retina and its association with the mitochondrial pathway after ONC.
Materials and Methods
Animals
Male Sprague-Dawley rats (10 weeks; Laboratory of Nanjing Traditional Chinese Medicine University, Nanjing, China) with an average body weight of 250 g (220–275) were used in this study. All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996, USA) and were approved by the Ethics Committee of Nanjing Traditional Chinese Medicine University.
Optic Nerve Crush
All animals underwent ONC injury or sham operation in the left eye. ONC was performed as previously described with slight modification (Huang et al. 2014; Xu et al. 2014; Wu et al. 2014). For ONC, the rats were deeply anesthetized with chloral hydrate (10 % solution) and surgery was performed under aseptic conditions. A conjunctival incision was made over the dorsal aspect of one eye, which was then gently rotated downward in the orbit. The superior and external rectus muscles were removed to expose 3–4 mm of the optic nerve. The left optic nerve was exposed under a surgical microscope and injured by partially crushing for 8 s with a cross-action calibrated crush forceps placed 2 mm behind the bulbus according to a well-established method (Vigneswara et al. 2012). Before wound closure, the retinal perfusion was checked funduscopically. Animals with severe reduction of the perfusion were excluded. Sham operation was done with the same procedures but without crushing the optic nerve.
Experimental Design
One hundred SD rats were used in this study. In the ONC groups (normal, 1, 2, 3, and 5 days) and the SHAM groups (normal and 2 days), all animals were killed at different survival times after injury. In the ONC groups, no animals were lost before these determined time points. The animals were used for Western blot analysis (n = 10 at each group), TUNEL staining (n = 10 at each group), and immunofluorescent staining (n = 10 at each group).
Western Blot Analysis
For Western blot analysis, eyes were enucleated. Retina tissues were harvested and stored at −80 °C until use. Total protein was obtained by lysing in a buffer (containing 1 M Tris-HCl at pH 7.5, 1 % Triton X-100, 1 % Nonidet p-40, 10 % SDS, 0.5 % sodium deoxycholate, 0.5 M EDTA, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF) and then centrifuged at 10,000 × g for 30 min to collect the supernatant. Protein was separated with sodium dodecyl sulfate-PAGE and transferred to polyvinylidene difluoride filter membranes (Millipore, Bedford, MA). The membranes were incubated overnight with GRP75 (anti-rabbit, 1:500; Cell Signaling), active caspase3 (anti-mouse, 1:500; Cell Signaling), Bax (anti-rabbit, 1:500; Santa Cruz), Cytc (anti-rabbit, 1:500; Santa Cruz), p-ERK (anti-rabbit, 1:500; Cell Signaling), ERK (anti-rabbit, 1:500; Cell Signaling), p-AKT (anti-rabbit, 1:500; Cell Signaling), AKT (anti-rabbit, 1:500; Cell Signaling), or β-actin (anti-mouse, 1:1000; Sigma) at 4 °C. At last, the membrane was incubated with second antibody goat-anti-rabbit or goat-anti-mouse conjugated horseradish peroxidase (1:2000; Southern-Biotech) for 2 h and visualized using an enhanced chemiluminescence system (ECL; Pierce Company, USA).
Sections and Immunofluorescent Staining
After defined survival times, rats were terminally anesthetized and perfused through the ascending aorta with saline, followed by 4 % paraformaldehyde. After perfusion, the sham and injured retina were removed and post fixed in the same fixative for 6 h and then replaced with 20 % sucrose for 1 day, following 30 % sucrose for 2–3 days. The whole eyeball was embedded in OCT (Sakura Finetek, Inc., CA, USA) and fast frozen in liquid nitrogen, and 7-μm-thick sections of the tissues were cut. The cryosections were blocked with 10 % normal goat serum containing 3 % (w/v) bovine serum albumin, 0.1 % Triton X-100, and 0.05 % Tween 20 overnight at 4 °C in order to avoid unspecific staining. Sections were incubated with polyclonal antibodies specific for GRP75 (anti-rabbit, 1:100; Cell Signaling). The co-incubated antibodies were monoclonal antibody for active caspase3 (anti-mouse, 1:100; Cell Signaling), separately for 12–24 h at 4 °C. After washing in phosphate-buffered saline (PBS), a mixture of fluorescein isothiocyanate- and Cy3-conjugated secondary antibodies and Hoechst were added in a dark room and incubated for 2–3 h at 4 °C. Photomicrographs were obtained using a Leica TCS SP2 confocal spectral microscope.
TUNEL Staining and Quantitative Analysis
TUNEL staining was performed using the In Situ Cell Death Detection Kit, Fluorescence (Roche Applied Science, Mannheim, Germany). Frozen tissue sections were rinsed with PBS and treated with 1 % Triton-100 in PBS for 2 min on ice. Slides were rinsed in PBS and incubated for 60 min at 37 °C with 50 μl of TUNEL reaction mixture. After washing with PBS, the slides were analyzed by Leica TCS SP2 confocal spectral microscope.
Cell quantitation in ganglion cell layer (GCL) was performed in an unbiased manner as previously described (Huang et al. 2014). Two or three adjacent sections (50 μm apart) per animal sections for every animal were sampled. The number of TUNEL- and GRP75/TUNEL-positive cells in the GCL was counted at ×400 magnification. The cell counts in the three or four sections were used to determine the total number of TUNEL-positive cells or GRP75-TUNEL-positive cells per square millimeter. A minimum of 200 TUNEL-positive cells was counted in each section.
Statistical Analysis
All data were analyzed with Stata 7.0 statistical software. The OD of the immunoreactivity is represented as mean ± SEM; one-way ANOVA followed by the Tukey’s post-hoc multiple comparison tests was used for statistical analysis. The parametric Spearman’s correlation coefficient was applied to evaluate the strength of the relationship between TUNEL-positive RGCs and GPR75-positive RGCs. P values <0.05 were considered statistically significant. Each experiment consisted of at least three replicates per condition.
Results
The Expression Profile of GRP75 in the Retina After ONC
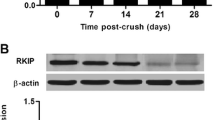
To examine the expression pattern of GRP75, Western blot analysis was performed on the retina. The GRP75 expression was low in normal retina, gradually increased on the 1 day after ONC, and had its peak after 2 days. Then, GRP75 expression tended to decline (Fig. 1a, b). In the sham group, GRP75 protein did not show significant differences between the normal and 2 days after sham operation (Fig. 1a).
Analysis of GRP75 expression following ONC by Western blot. ONC or sham operation was performed rats for indicated times. a Western blot analysis of GRP75 in the retina with or without ONC. β-actin protein level was used as the internal control. But there was no change in the sham group. b Quantification graphs for GRP75. Optical density from each band was normalized by the relative β-actin level. Data of relative GRP75 protein level to β-actin are presented as the mean ± SD of three independent experiments (*P < 0.05; **P < 0.01 significantly different from the normal group)
Phenotype of GRP75-Positive Cells in Retina After ONC
To determine the cellular localization GRP75 in the retina after ONC, we then performed immunofluorescent staining experiments on transverse cryosections of the retinal tissues. In normal retina, weakly positive signals of GRP75 protein were detected in the ganglion cell layer (GCL) and inner nuclear layer (INL) (Fig. 2a). At 2 days, a significant increase in GRP75 protein was detected in the GCL only (Fig. 2b). Furthermore, to investigate the cell types expressing GRP75 after ONC, we used double fluorescence staining confocal microscope technique with a RGC-specific marker, NeuN, to identify the cell type. We found that positive GRP75 was mostly in the cytoplasm of RGCs (Fig. 2c–f). These results indicated that the expression and distribution of GRP75 appeared to be associated with the biological function of RGCs after ONC.
Double immunofluorescence analyzes the localization of GRP75 in the retina after ONC. a The normal retina showed weak immunoreactivity for GRP75 antibody. b At 2 days after ONC, the number of GRP75-positive cells increased in the GCL. c–f At 2 days after ONC, increased GRP75 co-stained with RGCs in the GCL. GRP75 (green, c), NeuN (red, d), nuclear Hoechst staining (blue, e), and merged image (yellow, f). Scale bars 50 μm. ONL outer nuclear layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer
Association of GRP75 with RGCs Apoptosis After ONC
As mentioned above, GRP75 is involved in the regulation of neuronal apoptosis (Liu et al. 2005; Taurin et al. 2002; Yang et al. 2008). Therefore, we performed Western blot to examine the expression profile of active caspase3 to further detect the association between GRP75 and RGC apoptosis (Fig. 3a–b). The expression of active caspase3 was upregulated, which was parallel with that of GRP75 in the retina after ONC in a time-dependent manner. Furthermore, immunofluorescent staining showed that the co-localization of active caspase3 and GRP75 was detected in RGCs at 2 days after ONC (Fig. 3c–f). These results indicated that GRP75 might be involved in RGC apoptosis via a caspase-dependent way after ONC.
Association of GRP75 with active caspase3 in retina after ONC. a Western blot analysis of active caspase3 in retina after ONC. The expression of active caspase3 was increased after ONC and peaked at 2 days. There was no change in the sham group. b Ratio of active caspase3 to β-actin at each time point; these data were mean ± SEM. Illustrations were representative of three separate experiments. (**P < 0.01, significantly different from the normal group). c–f At 2 days after ONC, the increasing expression of GRP75 was co-localized with active caspase3. GRP75 (green, c), active caspase3 (red, d), nuclear Hoechst staining (blue, e), and the merged image (yellow, f). Scale bar 50 μm
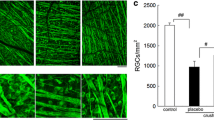
The TUNEL staining was widely used to identify RGCs apoptosis (Huang et al. 2014; Xu et al. 2014; Wu et al. 2014). Next, we performed TUNEL staining to examine the expression of RGC apoptosis after ONC. TUNEL-positive cells were weakly detected in normal RGCs (Fig. 4a), while a significantly increasing number of TUNEL-positive cells (Fig. 4b) were observed in RGCs at 2 days after ONC. Moreover, the co-localization of TUNEL-positive RGCs and GRP75 was detected at 2 days after ONC (Fig. 4b–e). In addition, semiquantitative analysis showed an increase of the density of TUNEL and GRP75/TUNEL-immunopositive RGCs after ONC (Fig. 4f–g). Finally, TUNEL-positive RGCs were positively correlated with GPR75-positive RGCs between these two periods (Fig. 4h; r = 0.8182, P = 0.0011). These results further indicated that GRP75 might be involved in RGC apoptosis after ONC.
Double staining with anti-GRP75 and TUNEL. a The immunoreactivity of TUNEL staining (green) in normal retina. b The immunoreactivity of TUNEL staining in the RGCs at 2 days after ONC. TUNEL (green, b), GRP75 (red, c), nuclear Hoechst staining (blue, d), and merged image (yellow, e). Scale bars 50 μm. A semiquantitative analysis of f TUNEL and g GRP75/TUNEL—immunoreactive RGCs. This figure showed an increase of the density of TUNEL and GRP75/TUNEL-immunopositive RGCs in the RGCs at 2 days after ONC (*P < 0.05, significantly different from the normal group). h The parametric Spearman’s correlation coefficient was applied to evaluate the strength of the relationship between number of TUNEL-positive RGCs/fields (200 μm) and GPR75-positive RGCs/fields (200 μm) between control group and ONC group. Between these two periods, TUNEL-positive RGCs were positively correlated with GPR75-positive RGCs (r = 0.8182, P = 0.0011)
Association of GRP75 with Mitochondrial Pathway After ONC
GRP75 has been shown to have anti-apoptotic effect via reducing the expression of Bax and delaying the release of Cytc in PC12 cells (Yang et al. 2011). Furthermore, we performed Western blot to examine the expressions of Bax and Cytc. After ONC, both the expressions of Bax and Cytc were upregulated, which kept increasing until 2 days then it decreased gradually (Fig. 5a–c). It was noteworthy that the increased expressions of Bax and Cytc were parallel with the expression of GRP75 and active caspase3 in the retina after ONC. These results were consistent with the previous studies, suggesting that mitochondrial pathway might participate in the RGC apoptosis (Wang et al. 2014; Zhang et al. 2012). These data indicated that GRP75 had a temporally change caused by ONC, and it might be relevant to mitochondrial pathway.
Association of GRP75 with Bax and Cytc in the retina after ONC. a The time courses of Bax and Cytc in retina after ONC. The expressions of Bax and Cytc were increased in the retina after ONC and peaked at 2 days. b, c Ratio of Bax and Cytc to β-actin at each time point; these data were mean ± SEM. Illustrations were representative of three separate experiments (*P < 0.05; **P < 0.01 significantly different from the normal group)
Association of GRP75 with AKT- and ERK-Dependent Mechanisms After ONC
GRP75 suppresses mitochondrial-dependent apoptosis via activating AKT- and ERK-dependent mechanisms (Yang et al. 2011). Thus, the association of GRP75 with AKT- and ERK-dependent pathway in the retina after ONC needs to be proved. Western blot analysis revealed that the protein levels of p-AKT and p-ERK1/2 were low in normal retina, increased the most at 2 days after ONC (Fig. 6a–c). But there was no change in the expressions of total AKT and ERK1/2 in retina after ONC. At the same time, the expressions of p-AKT and p-ERK1/2 were coincidence with the expression of GRP75, active caspase3, Bax, and Cytc in the retina after ONC. Based on these results, we further found that GRP75 might be related with RGC apoptosis after ONC, which process might be relevant to AKT- and ERK-dependent pathway.
Association of GRP75 with p-AKT and p-ERK1/2 in the retina after ONC. a The time courses of p-AKT and p-ERK1/2 in the retina after ONC. The expressions of p-AKT and p-ERK1/2 were increased in the retina after ONC and peaked at 2 days. But there was no change in the expression of total AKT and ERK1/2. b, c Ratio of p-AKT and p-ERK1/2 to β-actin at each time point; these data were mean ± SEM. Illustrations were representative of three separate experiments (*P < 0.05; **P < 0.01 significantly different from the normal group)
Discussion
The present study mimicked the acute RGC damage of glaucoma in rats and explored the cell mechanism after ONC. Here, we investigated the protein level of GRP75 in both normal and injured rat retina. Western blot and immunofluorescent staining analysis showed that the expression of GRP75 was increased in retina after ONC. The temporal change of GRP75 was significant in RGCs. We also found that the expression profile of active caspase3 was parallel with that of GRP75. Besides, the co-localization of GRP75/active caspase3 and GRP75/TUNEL was detected in RGCs after ONC. Additionally, expression patterns of Bax, Cytc, p-AKT, and p-ERK1/2 were in parallel with that of GRP75. Based on our data, we speculated that GRP75 might play an essential role in RGC apoptosis after ONC.
In glaucoma, RGCs execute a typical mitochondrial-dependent apoptotic program (Lin and Kuang 2014; Wang et al. 2014). Changes of several mitochondrial-dependent genes in transcriptional level in injured RGCs have been shown in experimental glaucoma and the optic nerve after being acutely injured to. Genes that increased expression in RGCs include several specifical pro-apoptotic or stress response genes, such as Bax (Harder et al. 2012; Das et al. 2006), Bid Cytc (Kim et al. 2013) and caspases (Huang et al. 2014; Wu et al. 2014; Xu et al. 2014). These changes in the pattern of gene expressions in RGCs occur before detectable cell loss presents and can also be induced by ONC (Huang et al. 2014; Kim et al. 2013; Wu et al. 2014; Xu et al. 2014), indicating that this event occurs at the early phase of the apoptotic pathway. Our present study also showed that the expression levels of active caspase3, Bax, and Cytc were significantly increased in the retina after ONC. Therefore, focusing on the molecules prior to mitochondrial pathway is an important element of developing strategies to intervene in RGC death.
GRP75 (mortalin/mtHsp70/PBP74/HSPA9B) is primarily of mitochondrial origin, though it is also found in endoplasmic reticulum, cytosol, and cytoplasmic vesicles (Bhattacharyya et al. 1995; Ran et al. 2000). The various names given to GRP75 reflect the multifunctional nature of the protein such as the regulation of the glucose response in rats (GRP75), the involvement in antigen processing in mice (PBP74), the mitochondrial protein transport pathway in humans (mtHSP70), and in-cell mortality in mice (mortalin) (Wadhwa et al. 2002). GRP75 expression also can be induced by cerebral ischemia (Massa et al. 1995), GD (Liu et al. 2005), and low doses of ionizing radiation (Sadekova et al. 1997). Previous studies demonstrated that over-expression of GRP75 could attenuate GD-induced apoptosis in PC12 cells, which is due to the inhibition of mitochondrial activity and biogenesis (Ornatsky et al. 1995; Yang et al. 2008). Notably, in our study, we found that the expression of GRP75 was enhanced obviously in the injured retina compared with the sham control. These changes were strikingly located in apoptotic RGCs and also be paralleled with increased expressions of active caspase3, Bax, and Cytc. Collectively, our data were consistent with the hypothesis that GRP75 was involved in the RGC apoptosis after ONC, and it might be relevant to mitochondrial pathway.
Accumulated evidence has demonstrated the role of GRP75 in regulating cellular stress responses, mitochondrial homeostasis, intracellular trafficking, antigen presenting, cell proliferation, differentiation, and tumorigenesis (Wadhwa et al. 2002). So GRP75 might play its vital role both in the cytoplasm (mitochondrion) and nuclear. In our present study, most of increased GRP75 was located in the cytoplasm (mitochondrion) in apoptotic RGCs after ONC. Moreover, both the expressions of Bax and Cytc were upregulated at 2 days after ONC. These results indicated that GRP75 might be involved in RGC apoptosis via a mitochondrial-dependent pathway after ONC. In addition, GRP75 also plays an important role in cell proliferation and differentiation. But in our present study, only a little GRP75 was located in the nucleus of RGCs, which might be involved in the RGC proliferation and differentiation. Previous studies have shown that optic nerve crush (ONC) can induce RGC death, and ONC has been used as an animal model to investigate axonal degeneration of the CNS (Huang et al. 2014). The animal model of ONC was an ideal model to explore the mechanism of RGCs apoptosis, so we did not pay attention to the little location of GRP75 in nuclear.
Members of the Bcl-2 family have been shown to be targets of the kinases that actively respond to stress. GRP75 may affect the Bcl-2 family and caspase family through its interaction with the members of some signal pathway (Lin and Kuang 2014). The AKT pathway is an important signal transduction pathway that regulates cell survival of neurons and of other cell types (Huang et al. 2008; Tsai et al. 2010). Various cellular stresses can activate AKT through phosphorylation. Once activated, AKT phosphorylates the downstream targets in various subcellular locations. A wide variety of putative downstream effectors have been identified that could contribute to the anti-apoptotic effects of AKT, which is involved in Bcl-2 family and caspase family. The interaction of AKT pathways with ERK pathway has shown crosstalk on multiple levels. The AKT pathway (Chen et al. 2008; Dong and Larner 2000; Naito et al. 2009; Schneider et al. 2005) and ERK pathway (Huang et al. 2007; Schneider et al. 2005) have all been reported for its anti-apoptotic effects in the CNS injury models. Phosphorylation events occurring in these pathways have rescue effects on RGCs after an ON injury (Huang et al. 2008; Kermer et al. 2000; Luo et al. 2007; Nakazawa et al. 2003; Tsai et al. 2010). Previous study showed that the expressions of p-AKT and p-ERK1/2 were universally upregulated in the RGCs after ONC (Tsai et al. 2010; Zhang et al. 2012). In this study, we observed the similar results. The AKT could also prevent apoptosis by inhibiting the Bax conformations (Yamaguchi and Wang 2001). Our present study also showed that increased expressions of p-AKT and p-ERK1/2 were consistent with the result of Bax in the retina after ONC. Recent report showed that the activated AKT and ERK1/2 by GRP75 inhibited the Bax conformational change and subsequent apoptosis (Yang et al. 2011). Therefore, it is reasonable to believe that GRP75 was implicated in the RGC apoptosis after ONC through modulating the AKT- and ERK-dependent pathway.
In conclusion, this study provides novel evidence that GRP75 was implicated in the Bcl-2- and caspase-dependent apoptosis of RGCs after ONC through modulating the AKT- and ERK-dependent pathway (Fig. 7). However, the detailed mechanism through which GRP75 participates in RGC apoptosis requires further studies. A better understanding of its contribution in future investigations may extend our knowledge in glaucoma.
References
Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI (1995) Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem 270:1705–1710
Chen WF, Jean YH, Sung CS, Wu GJ, Huang SY, Ho JT, Su TM, Wen ZH (2008) Intrathecally injected granulocyte colony-stimulating factor produced neuroprotective effects in spinal cord ischemia via the mitogen-activated protein kinase and Akt pathways. Neuroscience 153:31–43
Chen C, Xu Y, Zhang J, Zhu J, Zhang J, Hu N, Guan H (2013) Altered expression of nNOS/NIDD in the retina of a glaucoma model of DBA/2J mice and the intervention by nNOS inhibition. J Mol Neurosci: MN 51:47–56
Das A, Garner DP, Del Re AM, Woodward JJ, Kumar DM, Agarwal N, Banik NL, Ray SK (2006) Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res 1084:146–157
Dong F, Larner AC (2000) Activation of Akt kinase by granulocyte colony-stimulating factor (G-CSF): evidence for the role of a tyrosine kinase activity distinct from the Janus kinases. Blood 95:1656–1662
Harder JM, Fernandes KA, Libby RT (2012) The Bcl-2 family member BIM has multiple glaucoma-relevant functions in DBA/2J mice. Sci Rep 2:530
Huang HY, Lin SZ, Kuo JS, Chen WF, Wang MJ (2007) G-CSF protects dopaminergic neurons from 6-OHDA-induced toxicity via the ERK pathway. Neurobiol Aging 28:1258–1269
Huang Y, Li Z, Wang N, van Rooijen N, Cui Q (2008) Roles of PI3K and JAK pathways in viability of retinal ganglion cells after acute elevation of intraocular pressure in rats with different autoimmune backgrounds. BMC Neurosci 9:78
Huang Y, Xu Y, Cheng Q, Yu S, Gao Y, Shu Q, Yang C, Sun Y, Wang J, Xu F, Liang X (2014) The expression changes of myelin and lymphocyte protein (MAL) following optic nerve crush in adult rats retinal ganglion cells. J Mol Neurosci: MN
Joachim SC, Mondon C, Gramlich OW, Grus FH, Dick HB (2014) Apoptotic retinal ganglion cell death in an autoimmune glaucoma model is accompanied by antibody depositions. J Mol Neurosci: MN 52:216–224
Kaul SC, Deocaris CC, Wadhwa R (2007) Three faces of mortalin: a housekeeper, guardian and killer. Exp Gerontol 42:263–274
Kermer P, Klocker N, Labes M, Bahr M (2000) Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci: Off J Soc Neurosci 20:2–8
Kim KA, Shim SH, Ahn HR, Jung SH (2013) Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress-induced retinal damage. Toxicol Appl Pharmacol 269:109–120
Lin WJ, Kuang HY (2014) Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy 10
Liu Y, Liu W, Song XD, Zuo J (2005) Effect of GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol Cell Biochem 268:45–51
Luo JM, Cen LP, Zhang XM, Chiang SW, Huang Y, Lin D, Fan YM, van Rooijen N, Lam DS, Pang CP, Cui Q (2007) PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci 26:828–842
Massa SM, Longo FM, Zuo J, Wang S, Chen J, Sharp FR (1995) Cloning of rat grp75, an hsp70-family member, and its expression in normal and ischemic brain. J Neurosci Res 40:807–819
Naito T, Goto K, Morioka S, Matsuba Y, Akema T, Sugiura T, Ohira Y, Beppu M, Yoshioka T (2009) Administration of granulocyte colony-stimulating factor facilitates the regenerative process of injured mice skeletal muscle via the activation of Akt/GSK3alphabeta signals. Eur J Appl Physiol 105:643–651
Nakazawa T, Shimura M, Tomita H, Akiyama H, Yoshioka Y, Kudou H, Tamai M (2003) Intrinsic activation of PI3K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr Eye Res 26:55–63
Ornatsky OI, Connor MK, Hood DA (1995) Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle. Biochem J 311(Pt 1):119–123
Ran Q, Wadhwa R, Kawai R, Kaul SC, Sifers RN, Bick RJ, Smith JR, Pereira-Smith OM (2000) Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res Commun 275:174–179
Sadekova S, Lehnert S, Chow TY (1997) Induction of PBP74/mortalin/Grp75, a member of the hsp70 family, by low doses of ionizing radiation: a possible role in induced radioresistance. Int J Radiat Biol 72:653–660
Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR (2005) The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 115:2083–2098
Taurin S, Seyrantepe V, Orlov SN, Tremblay TL, Thibault P, Bennett MR, Hamet P, Pshezhetsky AV (2002) Proteome analysis and functional expression identify mortalin as an antiapoptotic gene induced by elevation of [Na+]i/[K+]i ratio in cultured vascular smooth muscle cells. Circ Res 91:915–922
Tsai RK, Chang CH, Sheu MM, Huang ZL (2010) Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Exp Eye Res 90:537–545
Vigneswara V, Berry M, Logan A, Ahmed Z (2012) Pharmacological inhibition of caspase-2 protects axotomised retinal ganglion cells from apoptosis in adult rats. PLoS One 7:e53473
Wada Y, Nakamachi T, Endo K, Seki T, Ohtaki H, Tsuchikawa D, Hori M, Tsuchida M, Yoshikawa A, Matkovits A, Kagami N, Imai N, Fujisaka S, Usui I, Tobe K, Koide R, Takahashi H, Shioda S (2013) PACAP attenuates NMDA-induced retinal damage in association with modulation of the microglia/macrophage status into an acquired deactivation subtype. J Mol Neur: MN 51:493–502
Wadhwa R, Taira K, Kaul SC (2002) An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones 7:309–316
Wang Y, Xu K, Zhang H, Zhao J, Zhu X, Wang Y, Wu R (2014) Retinal ganglion cell death is triggered by paraptosis via reactive oxygen species production: a brief literature review presenting a novel hypothesis in glaucoma pathology. Mol Med Rep 10:1179–1183
Wu Y, Xu F, Huang H, Chen L, Wen M, Jiang L, Lu L, Li L, Song D, Zeng S, Li L, Li M (2014) Up-regulation of SKIP relates to retinal ganglion cells apoptosis after optic nerve crush in vivo. J Mol Histol
Xu F, Huang H, Wu Y, Lu L, Jiang L, Chen L, Zeng S, Li L, Li M (2014) Upregulation of Gem relates to retinal ganglion cells apoptosis after optic nerve crush in adult rats. J Mol Histol 45:565–571
Yamaguchi H, Wang HG (2001) The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20:7779–7786
Yang L, Liu X, Hao J, Yang Y, Zhao M, Zuo J, Liu W (2008) Glucose-regulated protein 75 suppresses apoptosis induced by glucose deprivation in PC12 cells through inhibition of Bax conformational change. Acta Biochim Biophys Sin 40:339–348
Yang L, Guo W, Zhang Q, Li H, Liu X, Yang Y, Zuo J, Liu W (2011) Crosstalk between Raf/MEK/ERK and PI3K/AKT in suppression of Bax conformational change by Grp75 under glucose deprivation conditions. J Mol Biol 414:654–666
Zhang ZZ, Gong YY, Shi YH, Zhang W, Qin XH, Wu XW (2012) Valproate promotes survival of retinal ganglion cells in a rat model of optic nerve crush. Neuroscience 224:282–293
Zhu J, Zhang J, Ji M, Gu H, Xu Y, Chen C, Hu N (2013) The role of peroxisome proliferator-activated receptor and effects of its agonist, pioglitazone, on a rat model of optic nerve crush: PPARgamma in retinal neuroprotection. PLoS One 8:e68935
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, G., Han, M., Wang, X. et al. GRP75 Involves in Retinal Ganglion Cell Apoptosis After Rat Optic Nerve Crush. J Mol Neurosci 56, 422–430 (2015). https://doi.org/10.1007/s12031-015-0493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0493-x