Abstract
The adequate reconstitution of human soft tissue wounds requires the coordinated interaction of endothelial cells and fibroblasts during the proliferation phase of healing. Endothelial cells assure neoangiogenesis, fibroblasts fill the defect and provide extracellular matrix proteins, and myofibroblasts are believed to support the reconstitution of microvessels. In the present study, we combined in vitro-wound size measurement and multicolour immunocytochemical staining of co-cultured human dermal microvascular endothelial cells and normal human dermal fibroblasts, recently introduced as co-culture scratch-wound migration assay. Applying antibodies for α-smooth-muscle actin, von Willebrand factor, extra domain A fibronectin and endothelin-1, we were able to monitor proliferation, migration and the differentiation process from fibroblasts to myofibroblasts as a response to hypoxia. Furthermore, we verified, whether transforming growth factor β1 (TGFβ1) and endothelin-1 are able to mediate this response. We show, that proliferation and migration of endothelial cells and fibroblasts decreased under hypoxia. The additional administration of TGFβ1 did not significantly attenuate this decrease. Solely the myofibroblast population in co-culture adapted well to hypoxia, when cultures were supplemented with TGFβ1. Considerating the data concerning TGFβ1 and endothelin-1, we propose a model explaining the cellular interaction during early and late proliferation phase of human wound healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human wound healing, which can be subdivided into four phases, affects a variety of different cell types. During the first phase of haemostasis a provisional matrix is formed by platelet aggregates and fibrin clots in order to stop bleeding. The inflammation phase, starting in parallel, is coordinated by immunoactive cells such as granulocytes, monocytes and macrophages, and assures wound debridement. During this phase, the enzymatic digestion of debris and bacteria leads to acidosis and tissue hypoxia.
Hypoxia is one of the most critical parameters in wound healing regulation (Trabold et al. 2003) and is also a pivotal physiological factor triggering angiogenesis in the damaged area (Chin et al. 2005). Nevertheless it is also crucial to minimize hypoxia by neoangiogensis during the third phase of wound healing, termed as proliferation phase. During this phase, the vessel network has to be rebuilt by endothelial cells, and the damaged or lost tissue has to be replaced, mainly by fibroblasts.
Fibroblasts present the largest cell mass in soft tissue healing and provide different extracellular matrix (ECM) proteins. Especially one differentiated type of fibroblasts, the myofibroblast (MF) deposits large amounts of ECM (Gabbiani 2003). Collagens and fibronectin are important in order to fill the tissue defect, to increase tissue tension and to prepare the framework required for new vessel formation by endothelial cells. The adequate regulation of the cellular interaction of microvascular endothelial cells and fibroblasts is obligatory during the proliferation phase in order to prepare the basement for the final re-epithelialization and remodelling phase, which finally ensures soft tissue healing.
During the proliferation phase the microvasculature has to be reconstructed in order to assure the nutrient supply to the regenerating tissue (Diegelmann and Evans 2004). An intact microvasculature furthermore prevents permanent tissue hypoxia, which is one of the most common reasons for the delayed or the complete absence of wound healing.
In vivo, hypoxia was shown to deteriorate proliferation of both of the relevant cell types, microvascular endothelial cells and fibroblasts, when they were cultured in mono-culture (Oberringer et al. 2005). In the present study, we examined, whether the co-culture of these cell types, is affected by hypoxia in the same way, or whether their interaction leads to the attenuation of hypoxia induced proliferation decrease.
In order to improve the potentials of current wound healing models, which are suitable to investigate proliferation, migration and the micromorphological cell interaction (reviewed by Amadeu et al. 2003), we recently introduced a new in vitro model, which is composed of a scratch-wound healing assay and the usage of co-cultured human dermal microvascular endothelial cells (HDMEC) and normal human dermal fibroblasts (NHDF), termed co-culture scratch-wound migration assay (CCSWMA) (Oberringer et al. 2007). This model serves to characterize the responses of HDMEC and NHDF to hypoxia in co-culture and to compare them to those in mono-culture, identifying the cellular interplay during the proliferation phase of wound healing.
Because a variety of different cytokines play a key role in the regulation of the hypoxia response, and because they influence all relevant cytophysiologic parameters such as proliferation, migration and differentiation, we also examined the role of one of the most important cytokine during wound healing, namely transforming growth factor β1 (TGFβ1).
Among other cytokines such as basic fibroblast growth factor and insulin like growth factor 2, TGFβ1 is released by platelets and activated macrophages during the phase of inflammation, and by fibroblasts and MF during the proliferation phase (Gharaee-Kermani and Phan 2001). TGFβ1 has been shown to be a prerequisite for regular wound healing (Brunner and Blakytny 2004). Its broad spectrum of activity also includes the improvement of the survival of endothelial cells during hypoxia (Zhao and Eghbali-Webb 2001). Although TGFβ1 triggers angiogenesis in vivo (Iruela-Arispe and Sage 1993), endothelial cells respond to TGFβ1 by a decreased migration (Petzelbauer et al. 1996) and proliferation (Merwin et al. 1991; Sankar et al. 1996) in most of the in vitro studies.
The reports concerning the effects of TGFß1 on fibroblast migration in vivo and in vitro are also quite differing. In vivo, TGFβ1 is crucial for the migration of fibroblasts into the damaged tissue area, but in vitro TGFβ1 provokes both a migration increase (Cordeiro et al. 2000; Mogford et al. 2002) as well as a migration decrease depending on the origin of the fibroblast (Ellis et al. 1997). In vitro-supplementation of dermal fibroblasts with TGFβ1 reduces proliferation activity in association with enhanced differentiation to the MF type (Ronnov-Jessen and Petersen 1993), which is characterized by the expression of extra domain A fibronectin (ED-A-fn) (Serini et al. 1998) and α-smooth-muscle actin (α-SMA) (Desmouliere et al. 1993).
The functions of the MF in vitro, namely enhanced ECM production and the generation of tissue forces with subsequent wound contraction are important during early stages of wound healing (Desmouliere et al. 1995). Moreover, MF are believed to take part in the reconstitution of microvessels during the proliferation phase (Powell et al. 1999). The occurrence of MF is triggered by the synergistic action of hypoxia and TGFβ1 (Chen et al. 2005). In the present study we therefore also focused on the differentiation of MF from fibroblasts and their interaction with HDMEC, also taking in to account a possible influence of HDMEC released endothelin-1 (ET-1) on the enhancement of MF differentiation (Sun et al. 1997; Kernochan et al. 2002; Shi-Wen et al. 2004).
Material and methods
Cell culture
Human dermal microvascular endothelial cells (HDMEC, Promocell, Heidelberg, Germany) were cultured in endothelial cell growth medium MV (Promocell) under standard incubator conditions (humid atmosphere, T = 37°C, 5% CO2). Identical cryo-cultures were stored in a “master cell bank” in passage 6. Normal human dermal fibroblasts (NHDF, Promocell) were cultured in Quantum 333 medium (PAA, Pasching, Austria) and stored in passage 5. Then, identical co-cultures (n = 10) were produced by pooling both cell types in a defined cell number ratio (HDMEC:NHDF = 4:1) and culturing in a medium mix (endothelial cell growth medium MV:Quantum 333 medium = 4:1). Additional mono-cultures, where HDMEC and NHDF/MF were cultured seperately (HDMEC: n = 3; NHDF: n = 3) in the medium mix, served to measure the cellular responses and to compare them to those in co-culture.
Co-culture scratch-wound migration assay (CCSWMA)
The CCSWMA is described in brief, for more details see (Oberringer et al. 2007). About 24 h before the in vitro-wounds were induced, each of the 16 cultures was seeded on 7 sterile glass slides in quadriperm dishes (Greiner Bio-One, Frickenhausen, Germany) and cultured under standard conditions. This setup resulted in 112 specimens. About 24 h after seeding we used a pipette tip to induce two in vitro-wounds by scratching the cell layers. In vitro-wounds were approx. 900 μm wide with respect to the mono-cultures and approx. 1150 μm wide in the co-cultures. The difference in the initial wound size might be due to different tensions of the cell layers in mono and co-culture. Slides were scratched in duplicate in order to obtain two areas for subsequent immunocytochemical staining.
For each of the 16 cultures 7 slides were required: One slide per experiment was fixed at the starting time (0 h) immediately after wounding in order to determine the initial values for wound size and cell numbers per mm2 of each cell type (HDMEC, NHDF, MF).
Two slides were cultured for 24 h under standard normoxic conditions (medium O2-saturation: >130 mmHg), one of them received additional medium-supplementation with TGFβ1 (c = 1 ng/ml, Promokine, Heidelberg, Germany). Another two slides, one of them also supplemented with TGFβ1, were cultured under hypoxic atmosphere (medium O2-saturation: <5 mmHg) for 24 h in a chamber with a permanent gas flow (N2: 95%, CO2: 5%) (Oberringer et al. 2005).
Two slides, one of them supplemented with TGFβ1, were cultured under normoxic atmosphere and were fixed after 48 h. After fixation, all slides were stored in buffered glycerine at −20°C until immunocytochemical staining was performed.
The following terms for the slides are used in this paper: 0 h; 24 h normoxia; 24 h normoxia/TGFβ 1 ; 24 h hypoxia; 24 h hypoxia/TGFβ 1 ; 48 h normoxia; 48 h normoxia/TGFβ 1 .
Immunocytochemistry
In order to determine the cell numbers per mm2 of the different cell populations, we applied antibodies against von Willebrand factor (vWF; rabbit polyclonal anti-von Willebrand factor Ab-1, 1:500, no. 12881, Dianova, Hamburg, Germany) to identify HDMEC and against α-SMA (mouse monoclonal anti-α-SMA, 1:800, no. A2547, Sigma, St. Louis, USA) in the first staining area, to identify MF (Skalli et al. 1986). The vWF antibody served to recognize HDMEC in the co-cultures (n = 70) and in the HDMEC mono-cultures (n = 21). The α-SMA antibody served to identify MF in both the co- (n = 70) and the NHDF mono-cultures (n = 21). With respect to the fact, that all HDMEC were positive for vWF and all MF were positive for α-SMA, non-stained cells were defined to be NHDF.
A second staining area was used to visualize ED-A-fibronectin (ED-A-fn; mouse monoclonal anti-ED-A-fibronectin, 1:400, no. ab6328, Acris Antibodies, Hiddenhausen, Germany) in combination with vWF. ED-A-fn is a fibronectin splice variant and its expression among fibroblasts was shown to be a prerequisite of MF formation (Serini et al. 1998).
The standard staining procedure was performed as follows: Specimens were washed 3 × 5 min in PBS/0.5% Tween 20. Both staining areas were incubated with the first antibody (vWF) at room temperature for 30 min. After three washing steps specimens were incubated for another 30 min with a Cy3-labelled antibody (CyTM3-conjugated goat anti-rabbit, 1:200, no. 111-166-045, Dianova) in order to visualize the first antibody. After washing, the first staining area was incubated with the α-SMA antibody. The second staining area was incubated with the ED-A-fn antibody simultaneously. After washing, a FITC-labelled antibody (FITC-conjugated goat anti-mouse, 1:100, no. 115-095-003, Dianova) was applied in order to detect α-SMA and ED-A-fn antibodies. After final washing steps slides were fixed in 4% paraformaldehyde. Dehydrated slides were covered with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI, Vectashield, Vector Laboratories, Burlingame, USA) for nuclear counterstaining.
Some additionally prepared mono- and co-cultures were used in order to determine the localization pattern of ET-1. These specimens were stained with a combination of the antibodies against vWF and ET-1 (monoclonal mouse, clone TR.ET. 48.5, Acris Antibodies). vWF was visualized by Cy3, ET-1 was visualized by the FITC-conjugated antibody.
Microscopic analysis and data acquisition
Cell numbers of the HDMEC-, NHDF- and MF-population were determined by counting a minimum of 1000 cells per glass slide using a Zeiss Axioskop 2 with suitable fluorescence filter sets. By counting the cell number in 20 viewing fields, we were able to calculate the total cell density of each population (HDMEC, NHDF, MF) expressed in cells/mm2.
Cell migration into the in vitro-wound was determined as follows: 10 photomicrographs were taken on 10 previously defined slide positions along each wound in the first staining area of each of the 112 slides. These photomicrographs served to measure the wound size, which was defined to be the widest distance between those two cells, which were lying on the horizontal line of the defined slide position. The values of those 10 distances were then averaged to obtain a specimen’s wound size (for details see Oberringer et al. 2007).
Endothelin-1 enzyme linked immunosorbent assay (ELISA)
A commercially available ELISA (ET-1 detection kit, titer zyme® EIA, Assay designs, Ann Arbor, USA) was used to determine ET-1 concentrations in the medium supernatant of all 7 slides of 6 co-cultures (n = 42) were. Therefore all culture media were collected before fixation of the cells and were frozen at −80°C.
We previously determined the ET-1 release of mono-cultured HDMEC of all 7 slides of 2 mono-cultures (n = 14) under the different experimental conditions. These ET-1 values were expressed in fg secreted per cell. Because the time dependant development of the HDMEC cell density was comparable in mono- and co-culture, the ET-1 values determined in the HDMEC mono-culture were transferred to the co-culture taking into account the actual HDMEC numbers in the co-culture. The resulting value of totally secreted ET-1 (ET-1 HDMEC release ) was used in the following term, where the calculated resorption of ET-1 by NHDF is given as:
In consideration of the actual NHDF numbers in the co-cultures, the calculated resorption was also expressed in fg/cell.
Statistical analysis
Cell densities of each cell type (HDMEC, NHDF, MF) were expressed as mean values with standard deviation in each of the 7 experimental groups (HDMEC: n = 10, NHDF: n = 3, MF = 3). Experimental groups were: 0 h; 24 h normoxia; 24 h normoxia/TGFβ 1 ; 24 h hypoxia; 24 h hypoxia/TGFβ 1 ; 48 h normoxia; 48 h normoxia/TGFβ 1 .
Wound sizes were also expressed as mean values with standard deviation in each of the 7 experimental groups (HDMEC: n = 10, NHDF: n = 3, MF: n = 3).
Differences in cell density and wound size between the groups were assessed by applying analysis of variance (ANOVA) with post-hoc analysis including Bonferroni α-correction. P-values of less than 0.05 were regarded as significant.
The same test procedure was applied to assess the difference in ET-1 resorption by NHDF in the different groups of the co-cultures (n = 6).
Results
Cell population development
24 h after seeding, defined as starting time 0 h, the mean densities of HDMEC (73 cells/mm2) and NHDF (72 cells/mm2) were approximately equal in co-culture (Fig. 1A–C). Among the NHDF population there were 20 MF/mm2, which were predominantly located in the vicinity of HDMEC (Fig. 2). In co-culture the HDMEC population was stable after 24 h (72 cells/mm2) and slightly increasing after 48 h (81 cells/mm2).
Cell density (mean cell number per mm2 with sd) of the different cell cultures dependent on culture time, hypoxia and TGFβ1-supplementation (*: P < 0.05). (A–C) The different populations in co-culture (n = 10); (D) HDMEC in mono-culture (n = 3); (E) NHDF in mono-culture (n = 3); (F) MF in mono-culture (n = 3). Time = 24 h and 48 h; normoxia = N; hypoxia = H; TGF = TGFβ1-supplementation [1 ng/ml]
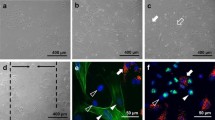
Immunocytochemical staining of cells in co-culture. (A–D) Clusters of HDMEC showing vWF expression (red), surrounded by NHDF and MF, the latter characterized by α-SMA expression (green). MF are preferentially located near HDMEC clusters. Cell nuclei appear in blue due to DAPI counterstaining. (A) Cell cluster appearing within in the cell layer at 24 h. (B) cell cluster located at the edge of an in vitro wound at 24 h. (C/D) HDMEC clusters at higher magnification showing the interaction of HDMEC and MF at the cluster margins. (D) A mitotic MF near clustered HDMEC
HDMEC: In co-culture, we detected a significant reduction of HDMEC density (36 cells/mm2) under the influence of hypoxia (24 h), compared to normoxic cultures. This represented a decrease of approx. 50%. The reduction was also significant among those cultures supplemented with TGFβ1 (hypoxia: 38 cells/mm2; normoxia: 62 cells/mm2), resulting in a decrease of approx. 39%. TGFß1-supplementation did not significantly influence HDMEC proliferation in the normoxic cultures after 24 and 48 h.
In mono-culture, HDMEC proliferation was enhanced compared to that in co-culture (Fig. 1D). Starting with 184 cells/mm2 at 0 h, HDMEC densities increased up to 257 cells/mm2 after 24 h and to 286 cells/mm2 after 48 h. The calculated generation time in mono-culture was 72.5 h and 3.7-fold lower than the generation time of HDMEC in co-culture (282.5 h). In response to hypoxia, HDMEC densities in mono-culture were also significantly reduced to 144 cells/mm2 (normoxia: 257 cells/mm2). A significant reduction was detected for the TGFß1-supplemented mono-cultures, too (hypoxia: 149 cells/mm2; normoxia: 253 cells/mm2). This displayed a decrease in cell density of 44% in the case of the non-supplemented and of 41% in the case of the TGFβ1-supplemented cultures. The comparison of cell densities in non-supplemented and TGFβ1-supplemented mono-cultures did reveal the same effects as previously described for HDMEC in co-culture: TGFβ1-supplementation did not significantly influence HDMEC proliferation in the normoxic cultures after 24 and 48 h.
NHDF: In co-culture the fibroblast density increased significantly to 667 cells/mm2 during 48 h (Fig. 1B). In co-culture hypoxia led to a decrease among NHDF of 32% (hypoxia: 132 cells/mm2; normoxia: 193 cells/mm2). The density of TGFβ1-supplemented cultures decreased to 34% under hypoxia (hypoxia: 127 cells/mm2; normoxia: 192 cells/mm2). TGFβ1-supplementation did not significantly influence NHDF proliferation in both the normoxic (24 and 48 h) and the hypoxic co-cultures (24 h).
In mono-culture the NHDF generation time of 13.4 h was approximately the same as in co-culture (15.0 h) (Fig. 1E). Hypoxia led to a density decrease of 46% (hypoxia: 332 cells/mm2; normoxia: 614 cells/mm2). The density of the TGFβ1-supplemented cultures decreased to 55% after hypoxia (hypoxia: 309 cells/mm2; normoxia: 689 cells/mm2). TGFß1-supplementation did also not significantly influence NHDF density in mono-culture.
MF: In normoxic co-culture, the MF density remained quite stable during 48 h (0 h: 20 cells/mm2, 24 h: 26 cells/mm2, 48 h: 19 cells/mm2) (Fig. 1C). Hypoxia induced a very weak proliferation decrease of 27% (hypoxia: 19 cells/mm2; normoxia: 26 cells/mm2) for the non-supplemented cultures. TGFβ1-supplementation had no significant effect on MF densities in the co-culture. Nevertheless, we realized, that MF were the only cells in this setup, which were not reduced in number during hypoxia (hypoxia: 22 cells/mm2; normoxia: 22 cells/mm2). In fact, MF were able to slightly increase during hypoxia (2%), when supplemented with TGFβ1.
In mono-culture the MF population was also quite stable (0 h: 18 cells/mm2, 24 h: 30 cells/mm2, 48 h: 26 cells/mm2) (Fig. 1F). In contrast to the co-cultures, hypoxia decreased the MF number among the non-supplemented mono-cultures by 35% (hypoxia: 20 cells/mm2; normoxia: 30 cells/mm2) and among the TGFβ1-supplemented mono-cultures by 52% (hypoxia: 19 cells/mm2; normoxia: 39 cells/mm2). TGFβ1 had no detectable effect on MF proliferation in mono-culture.
Cell migration
An overview of the process of cell migration into the in vitro-wound is shown in Fig. 3.
Immunocytochemical staining of co-cultured cells migrating into the in vitro wound. (A–C) HDMEC are characterized by vWF expression (red), NHDF and MF by the expression of ED-A-fn (green). Cell nuclei appear in blue due to DAPI counterstaining. The wound is located at the right side of the microphotographs. (A) Cells and wound edge immediately after wounding (0 h). (B) Cell migration into the wound at 24 h. Please note, that both cell types, NHDF and HDMEC, repopulate the wound space. (C) Localization of the cells at 48 h: The cell density in the wound is lower (right) than in the monolayer (left)
HDMEC: The mean wound size was 933 μm at 0 h in the HDMEC-culture (Fig. 4A). The remaining wound size after 24 h normoxia was 631 μm and 414 μm after 48 h. A significant decrease of migration was detected for the non-supplemented and TGFβ1-supplemented hypoxic HDMEC-cultures. TGFβ1 slightly stimulated migration after 24 h of both hypoxic and normoxic cultures and slightly decelerated migration after 48 h. Nevertheless, these effects were not significant.
Migration activity of (A) HDMEC in mono-culture (n = 3), (B) NHDF in mono-culture (n = 3) and (C) Cells in co-culture (n = 10) in dependence on time, hypoxia and TGFβ1-supplementation (*: P < 0.05, mean wound sizes). For better comparability and comprehension, wound closure is expressed in % of the initial wound diameter at 0 h (means with sd). Time = 24 and 48 h; normoxia = N; hypoxia = H; TGF = TGFβ1-supplementation [1 ng/ml]
NHDF: The initial wound size in NHDF-mono-culture was 894 μm after 24 h. The wound was closed after 48 h normoxic cultivation (Fig. 4B). There was also a significant deterioration of migration after 24 h hypoxia, which was also true for the TGFβ1-supplemented cultures. Again, TGFβ1 had no significant effect on the migration capacity. The complete wound closure after 48 h reflects the overall higher migration potential of NHDF compared to HDMEC.
HDMEC-NHDF-co-culture: The co-culture showed an initial wound size of 1147 μm (Fig. 4C). Wound closure after 24 h (757 μm) and after 48 h (182 μm) normoxic cultivation were faster compared to the HDMEC-mono-culture and slower compared to the NHDF-mono-culture. TGFβ1 had no significant influence on cell migration in co-culture, but slightly stimulated migration after 24 h of both hypoxic and normoxic cultivated cells and decelerated migration after 48 h.
Hypoxia only decelerated cell migration significantly, when co-cultures were supplemented with TGFβ1.
Cell culture morphology
During cell culturing we detected several special morphologic cell patterns. These cell patterns were not altered by the influence of hypoxia or TGFβ1.
HDMEC in mono-culture initially showed a clustered formation 24 h after seeding (0 h), when cell density was low. Afterwards (24 h, 48 h), when cell densities became higher, the clustered distribution disappeared so that HDMEC were uniformly distributed. NHDF were evenly distributed at each point of time and showed no tendency to cluster in the mono-culture.
In co-culture, HDMEC were also initially clustered (0 h), but this morphology was persistent throughout the experiments up to 48 h. Initial clusters usually were composed of a few (3–5) HDMEC, large clusters at 48 h showed up to 30 HDMEC (Fig. 2; Fig. 5).
ET-1 expression pattern of cells in co-culture revealed by immunostaining. (A/B) Clustered HDMEC with vWF expression (red) and ET-1 expression (green), the latter more prominent in cells located at the margin of the cluster. Cell nuclei are DAPI counterstained. (A) NHDF are characterized by absent vWF expression and do not express ET-1 (arrowheads)
Large clusters were seldom located within the cell layer (Fig. 2A), but developed preferentially at the edges of the in vitro wounds (Fig. 2B). In those clusters located at the wound edge, MF were often detectable above HDMEC, indicating an expansion of the clusters into the third dimension (Fig. 2B).
In co-culture, fibroblasts located around the HDMEC cluster margin were characterized by a high tendency to differentiate into MF (Fig. 2A–D). Fibroblasts located distant from the HDMEC clusters only showed little tendency to differentiate into MF (Fig. 2A/B).
ET-1 localization, release and resorption
The characteristic ET-1 expression pattern detected by immunocytochemical staining—the ubiquitous, but slightly enhanced expression of ET-1 in those cells located at the HDMEC cluster margin—was not altered by TGFβ1 nor by hypoxic treatment. Because the ET-1 expression pattern by HDMEC were identical in mono- and co-cultures and because NHDF were negative for ET-1 expression, as visualized by immunocytochemistry (Fig. 5) and measured by ELISA (data not shown), we assumed the amount of ET-1 released per HDMEC in the mono-cultures to be transferable to the co-cultures.
HDMEC in mono-culture secreted 4.00 fg ET-1 per cell at 0 h (Fig. 6). ET-1 values at 24 h and at 48 h were 4.15 fg/cell and 5.27 fg/cell, respectively. Although the differences of the groups were not significant, hypoxia decreased the amount of ET-1 after 24 h (3.73 fg/cell), which was also obvious for the TGFβ1-supplemented cultures (3.97 fg/cell). TGFß1 did not only slightly trigger the ET-1 release by HDMEC after hypoxia compared to the non-supplemented cultures. The release during normoxic culture at 24 h (4.59 fg/cell) and at 48 h (5.70 fg/cell) was also slightly enhanced by TGFβ1.
The subsequently calculated resorption of ET-1 by NHDF was 2.49 fg/cell at 0 h, decelerating at 24 h (1.12 fg/cell) and at 48 h (0.63 fg/cell) (Fig. 6). Hypoxia led to a—non-significant—decreased resorption compared to the normoxic culture (0.63 fg/cell vs. 1.12 fg/cell). TGFβ1 had a slightly positive effect on ET-1 resorption exclusively after hypoxia (0.68 fg/cell vs. 0.63 fg/cell), but had a slightly negative effect on ET-1 resorption at 24 h (0.92 fg/cell vs. 1.10 fg/cell) and at 48 h (0.54 fg/cell vs. 0.63 fg/cell).
Discussion
Proliferation
The influence of hypoxia on the proliferation of adherent cells in vitro is quite heterogeneous. We have shown before, that both HDMEC and NHDF mono-cultures decrease proliferation in response to hypoxia (Oberringer et al. 2005). These results were reproduced in part in the present study on the basis of the CCSWMA. Whereas mono-cultured NHDF were not significantly reduced under hypoxia, the significant reduction of mono-cultured HDMEC might imply the increased occurrence of cell death (Hogg et al. 1999). When HDMEC and NHDF were cultured together in co-culture, the hypoxia driven decrease of the cell density was more moderate compared to that in mono-culture in the case of NHDF (decrease co-culture 32%; decrease mono-culture: 46%). In the case of HDMEC, the hypoxia driven decrease was more profound (decrease co-culture 50%; decrease mono-culture: 44%).
Proliferation of NHDF and HDMEC was not significantly influenced by TGFβ1 under normoxia in our setup. TGFβ1-supplementation did neither reduce NHDF proliferation (Ronnov-Jessen and Petersen 1993), nor HDMEC proliferation (Merwin et al. 1991; Sankar et al. 1996) as described previously. The non-effectiveness of TGFβ1 on proliferation in our setup might be caused by the relatively high initial cell density, which is necessary to produce adequate in vitro wounds. The co-culture of HDMEC and NHDF did also not reveal any significant effect of TGFβ1 under normoxic conditions.
Although TGFß1 was described to be oxygen-responsive apart from its functions as a cytokine (Sakuda et al. 1992), there was also no detectable significant hypoxia induced effect of TGFß1 on mono- and co-cultured cells in our setup.
Migration
Migration of mono-cultured HDMEC was significantly decreased by hypoxia and was nearly absent without TGFβ1-supplementation. TGFβ1-supplemented HDMEC cultures showed at least a weak migration under hypoxia.
The migration decrease of mono-cultured NHDF was not significantly attenuated by TGFβ1 in hypoxia. These findings differ from other studies (Mogford et al. 2002). Also hypoxia alone did not trigger NHDF migration, but significantly inhibit, a result, which also disagrees to the current literature (Li et al. 2007). One reason for this different behaviour might be the fact, that TGFβ1 rather affects cells in poor condition, which was warranted in other studies by conducting the experiments with starved fibroblasts as control groups (Li et al. 2007), whereas we used complete medium for cell culture, ensuring optimal growth conditions.
In co-culture cell migration was generally faster compared to mono-cultured HDMEC, but slower compared to mono-cultured NHDF. Hypoxia significantly reduced migration only with TGFβ1-supplementation in co-culture. The weak and non-significant migration decrease without TGFβ1-supplementation indicates that TGFβ1 triggers the hypoxia induced migration decrease in co-culture. The slightly faster migration without TGFß1 might be explained by the fact, that the formation of rather immobile MF is suppressed under TGFß1 deprivation, which is also supporting enhanced NHDF migration (Moulin et al. 1998).
Furthermore, the MF population in co-culture—as the sole population—was able to absolutely increase during hypoxia compared to the normoxic culture, when supplemented with TGFβ1. Under TGFβ1 deprivation, MF slightly decreased during hypoxia. This underscores again, that TGFβ1 deprivation supports NHDF proliferation and migration, whereas TGFß1-supplementation supports the development of MF under hypoxia. The presence of HDMEC in the co-culture also seems to be essential for MF differentiation, because MF in the TGFß1-supplemented mono-culture behave different manifesting in a population decrease during hypoxia.
This in vitro situation described on the basis of the results of the CCSWMA is comparable to the in vivo situation during the early proliferation phase of wound healing, where the newly formed tissue has to be conditioned by fibroblasts, enabling endothelial cell migration and subsequent neoangiogenesis supported by MF.
MF differentiation
The well known effect of TGFβ1 to trigger MF differentiation in vitro (Desmouliere et al. 1993, Ronnov-Jessen and Petersen 1993) was detectable in normoxic mono-culture, but reversed in the co-culture. These effects were not significant, which might be due to the quite high cell density, which was necessary in our setup in order obtain suitable in vitro wounds, but which is counterproductive to the MF differentiation process in general (Masur et al. 1996). The combination of hypoxia, TGFβ1 and the presence of HDMEC seem to have slightly triggered MF differentiation among fibroblasts in our setup, although hypoxia alone was shown before not to be effective in triggering MF differentiation (Eul et al. 2006).
Role of ET-1
It is very likely, that the influence of HDMEC on MF differentiation is mediated by ET-1 (Sun et al. 1997; Kernochan et al. 2002; Shi-Wen et al. 2004), which is acting synergistic with TGFß1 (Shephard et al. 2004). ET-1 expression is regulated by a hypoxia inducible factor 1 binding hypoxia responsive element in the ET-1 gene (Minchenko and Caro 2000). Although most endothelial cells respond to in vitro hypoxia with an increase in secreted ET-1 (Kourembanas et al. 1991; Basilico et al. 2002), there exists evidence of decreasing ET-1 release in response to hypoxia, too (Saito et al. 1998). We also measured an ET-1 decrease after hypoxia in the HDMEC medium supernatant. Therefore, based on our setup, we have to assume, that rather low ET-1 concentrations in combination with TGFβ1 and hypoxia lead to enhanced differentiation to MF.
Relevance for in vivo wound healing
The summarized conclusions of the study result in a model proposed in Fig. 7, which might give some new insights into the interaction of endothelial cells, fibroblasts and MF and into the role of HDMEC released ET-1 in enhancing the MF differentiation process during wound healing in vivo (Thiemermann and Corder 1992). The milieu present in the early proliferation phase of in vivo wound healing—a combination of low ET-1 and high TGFβ1 concentration with additional hypoxia—favours the establishment of MF arising from ED-A-fn expressing proto-myofibroblasts and stellate cells (Serini and Gabbiani 1999; Tomasek et al. 2002) rather than fibroblast proliferation (Dube et al. 2000; Gallelli et al. 2005).
Cellular, molecular and physiologic interaction in the early and late proliferation phase of soft tissue healing. NHDF prefer differentiation to MF in the early stage due to the distinct milieu, which is charged with TGFβ1 mainly provided by platelets, but which lacks ET-1 and sufficient amounts of oxygen. MF accompany HDMEC in order to assure sufficient neoangiogenesis. During late proliferation phase, when TGFβ1 concentrations, now mainly provided by MF, are still high and ET-1 and oxygen concentrations have increased, NHDF proliferation and migration are prominent. This assures final wound healing by provision of sufficient cell mass and suitable ECM
The function of the MF during this early phase is the mechanical support of microvascular endothelial cells—a function, which is taken over by pericytes in the macrovasculature—in order to efficiently trigger neoangiogenesis (Powell et al. 1999). In our co-culture setup the interaction of MF and HDMEC manifested in a distinct micromorphological pattern, where MF were generally located in vicinity of HDMEC.
During the late proliferation phase of wound healing, when the milieu changes to a more normoxic state with higher amounts of ET-1 and constantly high amounts of TGFβ1, the NHDF population is able to reactivate migration and proliferation mechanisms in order to assure filling of the defect by sufficient cell mass and ECM proteins.
In conclusion, the results show that essential parts of the regulation of soft tissue wound healing in vivo are explainable on the basis of the CCSWMA. In the future, the assay will be a tool for the investigation of the interaction of endothelial cells and MF with respect to the role of a variety of other mediators, which are important for the understanding of neoangiogenesis during soft tissue healing.
Abbreviations
- α-SMA:
-
α-Smooth-muscle actin
- bFGF:
-
Basic fibroblast growth factor
- CCSWMA:
-
Co-culture scratch-wound migration assay
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- ECM:
-
Extracellular matrix
- ED-A-fn:
-
Extra domain A fibronectin
- ET-1:
-
Endothelin-1
- HDMEC:
-
Human dermal microvascular endothelial cell/s
- NHDF:
-
Normal human dermal fibroblast/s
- MF:
-
Myofibroblast/s
- sd:
-
Standard deviation
- TGFβ1 :
-
Transforming growth factor β1
- vWF:
-
von Willebrand factor
References
Amadeu TP, Coulomb B, Desmouliere A, Costa AMA (2003) Cutaneous wound healing: myofibroblastic differentiation and in vitro models. Int J Low Extrem Wounds 2(2):60–68
Basilico N, Speciale L, Parapini S, Ferrante P, Taramelli D (2002) Endothelin-1 production by a microvascular endothelial cell line treated with Plasmodium falciparum parasitized red blood cells. Clin Sci 103(48):464–466
Brunner G, Blakytny R (2004) Extracellular regulation of TGF-ß activity in wound repair: growth factor latency as a sensor mechanism for injury. Thromb Haemost 92:253–261
Chen CP, Yang YC, Su TH, Chen CY, Aplin JD (2005) Hypoxia and transforming growth factor-β1 act independently to increase extracellular matrix production by placental fibroblasts. J Clin Endocrinol Metab 90:1083–1090
Chin GA, Diegelmann RF, Schultz GS (2005) Cellular and molecular regulation of wound healing. Wound Healing 2005:17–39
Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT (2000) TGF-β1, -β2, and -β3 in vitro: biphasic effects on tenon’s fibroblast contraction, proliferation and migration. Invest Ophthalmol Vis Sci 41:756–763
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111
Desmouliere A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146(1):56–66
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
Dube J, Chakir J, Dube C, Grimard Y, Laviolette M, Boulet LP (2000) Synergistic action of endothelin (ET)-1 on the activation of bronchial fibroblast isolated from normal and asthmatic subjects. Int J Exp Path 81:429–437
Ellis I, Banyard J, Schor SL (1997) Differential response of fetal and adult fibroblasts to cytokines: cell migration and hyaluronan synthesis. Development 124:1593–1600
Eul B, Rose F, Krick S, Savai R, Goyal P, Klepetko W, Grimminger F, Weissmann N, Seeger W, Hänze J (2006) Impact of HIF-1α and HIF-2α on proliferation and migration of human pulmonary artery fibroblasts in hypoxia. FASEB J 20(1):163–165
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200(4):500–503
Gallelli L, Pelaia G, D’Agostino B, Cuda G, Vatrella A, Fratto D, Gioffre V, Galderisi U, De Nardo M, Mastruzzo C, Salinaro ET, Maniscalco M, Sofia M, Crimi N, Rossi F, Caputi M, Costanzo FS, Maselli R, Marsico SA, Cancheri C (2005) Endothelin-1 induces proliferation of human lung fibroblasts and IL-11 secretion through an ET(A) receptor-dependent activation of MAP kinases. J Cell Biochem 96(4):858–68
Gharaee-Kermani M, Phan SH (2001) Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des 7:1083–1103
Hogg N, Browning J, Howard T, Winterford C, Fitzpatrick D, Gobe G (1999) Apoptosis in vascular endothelial cells caused by serum deprivation, oxidative stress and transforming growth factor-beta. Endothelium 7(1):35–49
Iruela-Arispe ML, Sage EH (1993) Endothelial cells exhibiting angiogenesis in vitro proliferate in response to TGF-beta 1. J Cell Biochem 52(4):414–430
Kernochan LE, Tran BN, Tangkijvanich P, Melton AC, Tam SP, Yee HF (2002) Endothelin-1 stimulates human colonic myofibroblast contraction and migration. Gut 50:65–70
Kourembanas S, Marsden PA, McQuillan LP, Faller DV (1991) Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest 88:1054–1057
Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT (2007) Extracellular heat shock protein-90a: linking hypoxia to skin cell motility and wound healing. The EMBO Journal 2007:1–13
Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S (1996) Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA 93(9):4219–4223
Merwin JR, Newman W, Beall LD, Tucker A, Madri J (1991) Vascular cells respond differentially to transforming growth factors beta 1 and beta 2 in vitro. Am J Pathol 138(1):37–51
Minchenko A, Caro J (2000) Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: Role of hypoxia responsive element. Mol Cell Biochem 208:53–62
Mogford JE, Tawil N, Chen A, Gies D, Xia Y, Mustoe TA (2002) Effect of age and hypoxia on TGFß1 receptor expression and signal transduction in human dermal fibroblasts: impact on cell migration. J Cell Physiol 190:259–265
Moulin V, Castilloux G, Auger FA, Garrel D, O`Connor-McCourt MD, Germain L (1998) Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res 238:283–293
Oberringer M, Jennewein M, Motsch SE, Pohlemann T, Seekamp A (2005) Different cell cycle responses of wound healing protagonists to transient in vitro hypoxia. Histochem Cell Biol 123(6):595–603
Oberringer M, Meins C, Bubel M, Pohlemann T (2007) A new in vitro wound model based on the co-culture of human dermal microvascular endothelial cells and human dermal fibroblasts. Biol Cell 99(4):197–207
Petzelbauer E, Springhorn JP, Tucker AM, Madri JA (1996) Role of plasminogen activator inhibitor in the reciprocal regulation of bovine aortic endothelial and smooth muscle cell migration by TGF-beta 1. Am J Pathol 149(3):923–931
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB (1999) Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277:C1–C19
Ronnov-Jessen L, Petersen OW (1993) Induction of α-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasis. Lab Invest 68:696–707
Saito T, Itoh H, Chun T, Igaki T, Mori Y, Yamashita J, Doi K, Tanaka T, Inoue M, Masatsugu K, Fukunaga Y, Sawada N, Tojo K, Saito Y, Hosoya T, Nakao K (1998) Oxidative stress suppresses the endothelial secretion of endothelin. J Cardiovasc Pharmacol 31(Suppl 1):345–347
Sakuda H, Nakashima Y, Kuriyama S, Sueishi K (1992) Media conditioned by smooth muscle cells cultured in a variety of hypoxic environments stimulates in vitro angiogenesis. A relationship to transforming growth factor-beta 1. Am J Pathol 141:1507–1516
Sankar S, Mahooti-Brooks N, Bensen L, McCarthy TL, Centrella M, Madri JA (1996) Modulation of transforming growth factor beta receptor levels on microvascular endothelial cells during in vitro angiogenesis. J Clin Invest 97(6):1436–1446
Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G (1998) The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 142(3):873–881
Serini G, Gabbiani G (1999) Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 250:273–283
Shephard P, Hinz B, Smola-Hess S, Meister JJ, Krieg T, Smola H (2004) Dissecting the roles of endothelin, TGF-ß and GM-CSF on myofibroblast differentiation by keratinocytes. Thromb Haemost 92:262–274
Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ (2004) Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell 15:2707–2719
Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G (1986) A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103:2787–2796
Sun G, Stacey M, Bellini A, Marini M, Mattoli S (1997) Endothelin-1 induces bronchial myofibroblast differentiation. Peptides 18(9):1449–1451
Thiemermann C, Corder R (1992) Is endothelin-1 the regulator of myofibroblast contraction during wound healing?. Lab Invest 67(6):667–669
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechanoregulation of connective tissue remodelling. Mol Cell Biol 3:349–363
Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain Z, Rosen N, Seremetiev A, Becker HD, Hunt TK (2003) Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Rep Reg 11:504–509
Zhao L, Eghbali-Webb M (2001) Release of pro- and anti-angiogenic factors by human cardiac fibroblasts: effects on DNA synthesis and protection under hypoxia in human endothelial cells. Biochim Biophys Acta 1538(2–3):273–82
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oberringer, M., Meins, C., Bubel, M. et al. In vitro wounding: effects of hypoxia and transforming growth factor β1 on proliferation, migration and myofibroblastic differentiation in an endothelial cell-fibroblast co-culture model. J Mol Hist 39, 37–47 (2008). https://doi.org/10.1007/s10735-007-9124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-007-9124-3