Abstract
Polyploidization is a process present in cells of many different human tissues. Since it is also prominent in human wound healing in vivo and in vitro, we focused on the influence of hypoxia on the cells’ proliferation and polyploidization response. The proliferation response of two major cell types, involved in human wound healing, human dermal microvascular endothelial cells (HDMEC) and normal human dermal fibroblasts (NHDF) was quite similar in the in vitro setup: proliferation significantly decreased under the influence of 18 h of hypoxia and was reinitiated after 72 h of reoxygenation. The cells’ response concerning their tendency towards the development of polyploidy was different: NHDF did not generate any polyploid cells, which stands in contrast to former in vitro studies with human wound-derived fibroblasts, but HDMEC were characterized by the presence of both mononuclear and binuclear tetraploid cells. The number of tetraploids was downregulated during hypoxia and increased during reoxygenation, accompanied by proliferation onset. The immunomicroscopic survey of HDMEC opened up a cell cycle model, which might be useful in the future to evaluate cell cycle modulations leading to polyploidy without the need to apply any additional cell cycle inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyploidy and at its lowest level tetraploidy, which means an accurate duplication of the whole 2n chromosome-set, occurs in several cell types of the human body. At present, it is rather controversially discussed, if tetra- and polyploidization are beneficial for a stressed tissue or not and if they are causal for malignant development due to subsequent aneuploidization (Storchova and Pellman 2004). In recent studies in the yeast system, it has become clear that the state of ploidy indeed affects gene-expression (Galitski et al. 1999).
Besides several other tissues, the human wound granulation tissue also was identified to contain elevated numbers of tetraploid cells in vivo and in vitro. An increased occurrence of tetraploidization among wound fibroblasts was linked with a better healing prognosis (Ermis et al. 1998; Oberringer et al. 1999). At the cell cycle level, tetraploidization in the wound is supposed to be generated by mitotic slippage and it might be accompanied by a cell cycle stop in G0 of the following cell cycle under control of the tetraploidy checkpoint (Hanselmann and Oberringer 2001; Margolis et al. 2003).
Studies concerning teraploidization in the human wound still remain descriptive and a mechanism which forms the basis of tetraploidization at the level of cell cycle is not available so far. Furthermore, it is not evident which physiological factor out of the huge spectrum occurring during wound healing and tissue regeneration in other organs might be able to trigger this process. Besides the acute inflammation leading to an enhanced secretion of mediators and cytokines especially tissue, hypoxia is a characteristic condition during wound healing. Hypoxia is immediately established after a traumatic incident, due to blood vessel damage or collapse. Subsequently, tissue oxygen concentration reaches a minimum. As a consequence necrosis and apoptosis occur, but other cells succeed in surviving and getting established in this environment. The O2 gradient, which is formed between the edge and the centre of an open wound, additionally serves as a key stimulus for the direction of cell migration (Davis et al. 1988). Not only endothelial cells, but also subcutaneous fibroblasts, which are the most important cell types involved in wound healing, are influenced by hypoxia (Dawes et al. 1994).
Here, we present an in vitro approach, which serves to verify the influence of hypoxia and subsequent reoxygenation on proliferation, cell cycle regulation and polyploidization of the wound healing protagonists, human dermal microvascular endothelial cells (HDMEC) and normal human dermal fibroblasts (NHDF). The study also investigates whether hypoxia alone or in combination with reoxygenation triggers tetraploidization in these cell types; until now, this has been demonstrated solely for tumor cell lines (Loffler 1987; Rofstad et al. 1996).
Materials and methods
Cells and cell culture
Normal human dermal fibroblasts (PromoCell, Heidelberg, Germany) were grown in Quantum333 medium (PAA-laboratories, Pasching, Austria). Media were changed every 2–3 days and cells were passaged after trypsination (0.05% trypsin-EDTA; PAA-laboratories). Cells from one batch were used in different passages (P) for the experiments (single cultures in P5, P6, P7, P7, P8, P9, P10, P11, P13, P15 and P17). No morphological heterogeneity was detectable in different passages. HDMEC (PromoCell) were grown in complete endothelial cell growth medium (PromoCell). Cells were passaged after trypsination and used for the experiments in passages P5, P5, P6, P7, P8, P8, P9, P10, P11, P12, P13. In total, three different batches of HDMEC were used. In late passages factor VIII related antigen-expression was still detectable by immunocytochemistry in the majority of the cells. Cells were fixed at relevant times by washing twice with phosphate buffered saline (PBS), applying a 5-min incubation step in 0.05 M KCl at 37°C and dehydrogenation in methanolabs. for 10 min at −20°C.
Hypoxia
Cells from two 75-ml culture flasks were trypsinated and split into seven similar fractions. Cells were subsequently cultured on glass slides in quadriperm dishes (Greiner, Frickenhausen, Germany) and used for the hypoxia experiments if confluence was about 50%. One of the seven slides was fixed before the experiment to determine initial polyploidization and proliferation values. The medium of the remaining six slides was changed before the experiment: three of the remaining six slides were cultured in normal medium under standard incubator conditions, serving as controls. The other three slides were used for hypoxia treatment. Therefore, culture medium was made hypoxic (<5 mmHg) before medium change. During hypoxia, cells were aerated with a gas mixture containing 95% N2 and 5% CO2 (Messer, Griesheim, Germany), in a humidified incubator. A constant gas flow of 1 l/min assured a constant O2 saturation of <5 mmHg over a period of 18 h, which was monitored by a Lycox probe (Lycox, Kiel-Mielkendorf, Germany). Immediately after 18 h of hypoxia (H), one slide with hypoxic cells and one standard incubator control slide were fixed at the same time. Hypoxic cells on the remaining two slides were reoxygenated by transferring to a standard incubator, which generates an O2 saturation of 130 mmHg, and were cultured for an additional 24 h (H/R1) and 72 h (H/R2) respectively. After 24 h and 72 h, reoxygenated cells and cells from the standard incubator control slide were fixed at the same time.
Immunocytochemistry
Immunocytochemical staining was done prior to chromosome staining by fluorescence in-situ hybridization (FISH) according to the following standard procedures. After storage, the specimen were washed 3×5 min in PBS/Tween. The slides were incubated with 75 μl of the first antibody (monoclonal mouse anti-human Ki67, clone MIB-1, ready-to-use, DakoCytomation, Carpinteria, USA) in a humidified chamber at room temperature for 30 min. After three washing steps of 5 min each in PBS/Tween, specimens were incubated for another 30 min with a Cy3-labeled antibody (goat anti mouse Cy3, 1:400, Dianova, Hamburg, Germany). After that, the slides were washed 3×5 min in PBS/Tween, then fixed in 4% paraformaldehyde in PBS. After drying the slides in alcohol (70–80–90%), they were stored for at least 2 days at −20°C before they underwent FISH.
The staining procedure for centrosomal γ-tubulin (monoclonal anti-γ-tubulin Cy3 conjugate, 1:200; Sigma, St Louis, USA) was the same as that described for MIB-1, only omitting the additional detection step.
Fluorescence in-situ hybridization
The method applied to determine the degree of ploidy by using two centromere-specific probes simultaneously was described earlier (Ermis et al. 1998). To identify chromosome 2 and chromosome 8 copies in interphase nuclei, a biotin labeled chromosome 2 α-satellite probe (D2Z, Qbiogene, Heidelberg, Germany) and a digoxigenin-labeled chromosome 8 α-satellite probe (D8Z2, Qbiogene) were used simultaneously. After hybridization overnight, stringency washes were performed and the detection of the probes by using streptavidin-FITC (Fluorescein-streptavidin, 1:250, VectorLaboratories, Burlingame, USA) and an anti-dig-Cy3 antibody (Cy3 conjugated IgG fraction monoclonal mouse anti-digoxin, 1:100, Dianova) was done. Then, nuclear DNA was counterstained with DAPI (Vectashield, Vector Laboratories).
Data acquisition
A minimum of 300 cells, which were distributed over ten individual areas, were evaluated per microscopic slide. MIB-positive and -negative cells were counted and expressed in percent of all cells counted. In addition, MIB-stained cells were evaluated concerning their degree of polyploidy. Therefore cells were classified in diploid, tetraploid and higher polyploid populations, which were also expressed in percent of all cells counted.
In addition the number of centrosomes, identified by positive γ-tubulin-staining, was counted in at least 200 cells, according to the classification proposed by Dictenberg et al. (1998).
Statistics
We applied paired samples Student’s t-test to verify the significance of the effects of hypoxia and hypoxia/reoxygenation on cell proliferation and polyploidization compared to standard incubator controls.
Results
Proliferation
We compared the total number of resting cells, which was characterized by a missing expression of the Ki-67 antigen (Fig. 1), after hypoxia (H), after a 24-h (H/R1) and after a 72-h reoxygenation period (H/R2) to the reference cultures, which were cultured under standard conditions (Gerdes et al. 1984).
HDMEC increased the number of cells in cell cycle phase G0/early G1 during hypoxia (P=0.02; Fig. 2a, blue bars). A turning point at passage 11 indicates that HDMEC behave different in late passages: from passage 11 on, the number of cells in cell cycle phase G0/early G1 decreased. Absolute proliferation values did not differ between early and late passages. The behaviour of NHDF after hypoxia was similar to that of HDMEC (Fig. 2a, yellow bars): all cultures but one responded with an increase of resting cells (P=0.02). Only one culture, again the latest passage (P17), responded by a decrease of resting cells.
Development of the number of the cells being in G0/early G1 after: a hypoxia (H), b hypoxia and 24-h reoxygenation period (H/R1) and c hypoxia and 72-h reoxygenation period (H/R2) compared to control (as the difference of percentages). NHDF independent cultures of one batch in ascending passage order (P); HDMEC independent cultures of three different batches in ascending passage order (P)
The increase of cells in G0/early G1 was stable beyond the 1-day reoxygenation period (H/R1) among both different cell types (Fig. 2b). All cultures were characterized by an increase of cells in G0/early G1 with the only exception of one culture in the groups of HDMEC and NHDF. Statistics revealed P-values of P=0.005 for HDMEC, P=0.05 for NHDF.
The cellular response after hypoxia and a 72-h reoxygenation period (H/R2) was different between both cell types (Fig. 2c) and a statistically relevant tendency in any group could not be observed. Seven out of eleven NHDF cultures and six out of 11 HDMEC cultures reduced the amount of cells in G0/early G1, when compared to the cells under standard conditions, representing the re-establishment of enhanced proliferation activity in these cultures.
Additionally, we examined all culture slides microscopically in reference to signs of apoptosis like cellular or nuclear shrinking, chromatin condensation and the presence of apoptotic bodies. Apoptosis occurred only sporadically among both HDMEC and NHDF and seemed not to be induced by either hypoxia or reoxygenation.
Tetraploidization and centrosome data
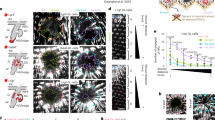
The acquired tetraploidization data were heterogeneous among NHDF and HDMEC. Whereas NHDF did not tend to develop binuclear cells, we could confirm that this is a common feature in HDMEC cultures. Binuclear cells had two diploid nuclei, resulting in a tetraploid cell (Fig. 3b). In addition to binuclear cells, we detected cells with one tetraploid nucleus.
a A tetraploid HDMEC nucleus showing four copies of chromosome 2 (green) and chromosome 8 (red), counterstained with DAPI. Amplified centrosomes, stained by an anti-γ-tubulin-Cy3 antibody, are divided in a single one (white arrowhead) and a bundle of three (green arrowhead). b Tetraploid HDMEC with two diploid nuclei. Amplified centrosomes are grouped as a bundle of four (arrowhead). c Octoploid HDMEC with two tetraploid nuclei, each showing four copies of chromosome 2 (green) and chromosome 8 (red). Scale bars 20 μm
Independent of hypoxia or reoxygenation, the overall fraction of binuclear cells among all tetraploids was stable and about 50%. We also detected octoploid cells, with the majority having two tetraploid nuclei (Fig. 3c). All different polyploid cells (binuclear, real tetraploids and octoploids) showed MIB-expression in up to 65% of all cells. This was again independent of hypoxia or reoxygenation.
An enhanced development of tetraploid cells in NHDF could not be induced by hypoxia or reoxygenation. The data taken immediately after hypoxia (H) and after the 24-h reoxygenation period (H/R1) and the 72-h reoxygenation period (H/R2) respectively, were heterogeneous. The absolute tetraploidization values in NHDF were low in general, ranging between 0% and maximum 4.5%. Statistical testing did not show any relevant effects of hypoxia and reoxygenation in the treated cultures, when compared to the cultures under standard atmosphere. Tetraploids were consistently mononuclear. Binuclear tetraploids were never detected among NHDF.
In contrast, HDMEC cultures showed a significant response to hypoxia (H): the amount of tetraploid cells was reduced (P=0.02). Figure 4 furthermore illustrates the HDMEC response after the 24-h reoxygenation period (H/R1) and the 72-h reoxygenation period (H/R2). After H/R1 the heterogeneous response can be seen when some cultures still remain in the status of reduced tetraploidization at this time, while other cultures have already re-established tetraploidization. Due to this heterogeneous response, statistics did not reveal any significance using acceptable P-values at H/R1. At H/R2, all HDMEC cultures but one were characterized by an increased number of tetraploid cells when compared to control cultures (P=0.01), showing the collective re-establishment of enhanced tetraploidization after 72 h of reoxygenation. The only culture, not responding with enhanced tetraploidization at H/R2 (asterisk) was characterized by the strongest absolute decrease of tetraploid cells at H/R1. The fact that this culture did not respond with an enhanced tetraploidization at H/R2 just might be explained by a delayed response, which came too late to be detected within the chosen time frame.
Considering both the proliferation and the tetraploidization HDMEC data together led to the results that (1) the inhibition of proliferation after hypoxia is accompanied by a decrease of tetraploid cells and that (2) proliferation onset during H/R2 is accompanied by the re-establishment of tetraploidization. Since proliferation onset is not given in all HDMEC cultures after H/R2 (Fig. 2c), but the re-establishment of tetraploidization is good (Fig. 4) at this point in time, tetraploidization seems to be a very early response of HDMEC to reoxygenation.
In order to investigate the cell cycle phase of binuclear and mononuclear tetraploid HDMEC, we determined the number of centrosomes per cell. As clarified in Table 1, the centrosome was amplified in 75–92% of all tetraploid cells, which were cultured in complete medium under a standard incubator atmosphere. Mostly, the centrosomes were duplicated to a number of exactly four. Amplified centrosomes were either completely (Fig. 3b) or incompletely clustered (Fig. 3a).
Discussion
Polyploidy is found in numerous mammalian cells. The existence of polyploid cells in the liver had been documented well and in recent years more and more tissues were found to contain polyploid cells. They were found in ventricular hypertrophy and especially smooth muscle cells that often respond to stress with polyploidization, i.e. in the uterus and the aorta (Owens and Schwarz 1982). In the placenta, in testis and in different glands, there is also a high incidence of polyploidy cells (reviewed in Hanselmann and Oberringer 2001).
It seems evident that cells develop polyploidy especially in times of stress. It is likely that polyploid cells then shift their efforts to an enhanced metabolic activity instead of wasting energy for cell division (Gahan 1977; Ravid et al. 2002). The absence of cell division is one of the characteristic events during the development of polyploidy. Any kind of endocycle, which is known to occur during the sequence of polyploidization, is based upon the absence of cell division. Besides endomitosis, endoreduplication, amitosis and C-mitois acytokinetic mitosis is especially relevant. Acytokinetic mitosis generates, in contrast to any of the aforementioned mechanisms, a binuclear cell (Oksala and Therman 1974; Therman and Susman 1993; Ravid et al. 2002). Binuclear cells were frequently detected among HDMEC in this study.
Since our group was able to show that the development of polyploidy and especially tetraploidy is a frequent event during the process of human wound healing (Ermis et al. 1998; Oberringer et al. 1999), we focused on the following questions:
-
1.
Do different cell types in the wound respond differently to hypoxia and reoxygenation considering their overall proliferation capability and polyploidization activity?
-
2.
Is the development of polyploid cells correlated to enhanced proliferation (Only regularly healing wounds were shown to contain a high number of tetraploid cells)?
-
3.
Is it possible to identify cell type specific cell cycle events leading to the development of polyploidy?
In the following part of the discussion we try to give some answers to the questions above under consideration of the experimental results.
Cells involved in tissue damage and wound healing are exposed to a variety of extraordinary circumstances like a massive change in tissue pH and the modulation of the cytokine composition. Wound cells are also affected by reduced oxygen saturation (hypoxia). Hypoxia and lactate concentration are discussed to trigger the healing process by enhanced proliferation of the involved cells (Hunt 1988; Trabold et al. 2003; Wagner et al. 2004). The experimental induction of tetraploidization was succesfully achieved by hypoxia and reoxygenation in different tumor cell lines (Loffler 1987; Rofstad et al. 1996). It was thus reasonable to evaluate the potency of hypoxia and reoxygenation to provoke polyploidization among primary cultures of the wound healing protagonists HDMEC and NHDF.
Normal human dermal fibroblasts accumulated cells in G0/early G1 after exposure to experimental hypoxia. This indicates that the G1-checkpoint, which is able to prevent the cells from entering the subsequent cell cycle phase, is activated by hypoxia (Graeber et al. 1994; Achison and Hupp 2003). If we consider the G1-checkpoint to be controlled by hypoxia, the re-entry of cells from G1 into the following cell cycle phases should be detectable after reoxygenation. A reoxygenation period of 24 h was not enough for the resting cells to re-enter the cell cycle, but 72-h reoxygenation indeed stimulated seven out of 11 cultures to get out of the G1 block, to re-enter the cell cycle and restart proliferation activity. The remaining cultures might need longer reoxygenation times, due to a possible hypoxia-induced damage and a delayed response.
Evaluation of tetraploidization among NHDF did not show any significant response to either hypoxia alone or hypoxia and reoxygenation. It was difficult to evaluate, because the basal tetraploidization rates were low in NHDF. The possibility that tetraploid NHDF, which potentially develop as a consequence of the reinitiated proliferation during reoxygenation, are sorted out by apoptosis, cannot be ruled out completely in our setup. However, we did not notice increased apoptosis among NHDF during reoxygenation.
The low tetraploidization rates were in strong contrast to the high rates of tetraploidization among in vitro cultures of wound-derived fibroblasts (Ermis et al. 1998; Oberringer et al. 1999) and were not expected. The different polyploidization tendency of wound-derived fibroblasts and NHDF in vitro might be explained by their different p53 status. P53 was shown to be suppressed during wound healing in vivo (Antoniades et al. 1994) and p53 downregulation enables both enhanced proliferation and polyploidization (Meek 2000). In contrast to wound-derived fibroblasts, NHDF likely have a normal p53 status. Another explanation for the different behaviour of these cell types concerning tetraploidization in vitro might be, that the wound-derived fibroblasts, which are characterized as contractile myofibroblasts in recent studies (Gabbiani 2003), have lost normal fibroblast cell cycle control elements during their differentiation, which is influenced by wound-specific stimuli.
Endothelial cells are known to be quite resistant towards a 1-day hypoxia period (Stempien-Otero et al. 1999). They obviously belong to those groups of cells, like cardiac myocytes also, which are not very sensitive to transient hypoxia alone, but are very sensitive to a combination of hypoxia and reoxygenation (Webster et al. 1999). However in our in vitro setup, HDMEC already showed a significant response to hypoxia without reoxygenation: the accumulation of cells in G0/early G1. The behaviour of HDMEC was comparable to the one of NHDF: HDMEC also were not able to re-enter into the cell cycle during 24-h reoxygenation. However, after 72-h reoxygenation, comparable to NHDF, six from the 11 HDMEC-cultures were able to re-establish proliferation. The remaining five cultures might need longer reoxygenation times to re-establish proliferation due to hypoxia-induced damage.
In contrast to NHDF, HDMEC showed relatively high amounts of mononuclear and binuclear tetraploid cells under standard incubation. This might be explained by considering the assumption that optimized HDMEC culture medium already leads to overstimulation. This situation is then comparable to the in vivo situation in the wound, which is characterized by enhanced cytokine release. These conditions might be responsible for an uncoupling of cell cycle and DNA cycle, finally leading to endoreduplication and tetraploidization. Indeed, we identified tetraploid binuclear cells, which is a hint that tetraploidization is generated via karyokinesis and subsequent acytokinesis. The number of tetraploid HDMEC significantly decreased during hypoxia, which was accompanied by a proliferation decrease. Hypoxia was proven to separate out tetraploid HDMEC, which seems to be achieved by preventing acytokinesis. Apoptosis must be considered as a possible mechanism to reduce the number of tetraploid cells, too. Apoptosis is likely to occur in the case of tetraploidy checkpoint activation in G0/G1 as response to hypoxia. G0/G1-checkpoint activation prevents tetraploid cells to enter subsequent cell cycle phases (Borel et al. 2002). However, we did not observe enhanced apoptosis in our setup. Once having reached a low level of tetraploidy after hypoxia, the occurrence of tetraploids restarted after 24-h reoxygenation and reached significant values after 72-h reoxygenation. This increase was accompanied by the reestablishment of proliferation activity.
Based on these results and under consideration of the recent fundamental knowledge about cell cycle checkpoints, we propose a model of HDMEC specific cell cycle events (Fig. 5), which is similar to results recently published for liver cells (Guidotti et al. 2003). As mentioned above, binuclear HDMEC develop via karyokinesis with subsequent acytokinesis under normal culture conditions. The mitotic spindle checkpoint, preventing cells from completing mitosis when activated, does not seem to be activated under these conditions: a great part of the binuclear cells is MIB-positive, indicating that these cells still proliferate and enter G1 of the subsequent cell cycle. Only a minority of the binuclear cells (8–24%) have the regular, not amplified number of centrosomes (Table 1). This indicates that these cells are resting in late mitosis or in G0/G1 of the subsequent cell cycle, since centrosome duplication occurs in S-phase. A rest in late mitosis is due to activation of the spindle checkpoint, a rest in G0/G1 is due to activation of the G1-tetraploidy checkpoint. If mitotic spindle checkpoint inactivity, which is also called mitotic spindle checkpoint adaptation, is achieved very quickly, the subsequent G1-tetraploidy checkpoint will not function properly either (Andreassen et al. 2003; Margolis et al. 2003). The majority of binuclear HDMEC does not rest at the G1 tetraploidy checkpoint. This is supported by the fact that these cells were MIB positive. Furthermore, their G2-phase entry is shown by centrosome amplification, which happened during S-phase and occurred in up to 92% of binuclear HDMEC (Fig. 3b). Cells in G2 have duplicated the chromosomes in both the nuclei leading to a binuclear octoploid cell (Fig. 3c). Another checkpoint, which was recently shown to exist at G2 for polyploid cells (Vogel et al. 2004), also seems not to be active, since octoploid HDMEC are able to enter mitosis. Consequently, the chromosomes of both nuclei are aligned all together in one metaphase plate resulting in two tetraploid mononuclear cells (Fig. 3a). These tetraploid cells contain a regular centrosome, because amplified centrosomes were clustered during mitosis at the poles of the cell and one cluster is present in each of the daughter cells subsequent to cytokinesis. This special kind of 8n chromosome segregation is described exactly the same way for liver cells (Guidotti et al. 2003). That it also holds true in the HDMEC system is supported by the fact that nearly all mononuclear tetraploid HDMEC were detected in clusters and seldom as single cells.
The above proposed HDMEC model is a very useful system to investigate cell cycle relevant modulations leading to polyploidy without the need to apply additional chemical spindle inhibitors. Polyploidization was shown to occur under standard culture conditions and could be limited by hypoxia, resulting in a strong decrease of the binuclear cell fraction. In addition binuclear HDMEC seem to be able to align an 8n chromosome set in a single metaphase plate. Figure 6 outlines a possible scenario of a tetrapolar 8n chromosome segregation leading to four normal diploid daughter cells. Please note that this sequence is composed of different cell types, which were targets of our long lasting microscopic survey and is not restricted to the proposed HDMEC model.
Summarizing the results in the context of human wound healing, we finally answer the questions that had been posed in the very beginning of the Discussion section:
-
1.
Do different cell types in the wound respond differentially to hypoxia and reoxygenation considering their overall proliferation capability and polyploidization activity? Proliferation characteristics of both cell types as a consequence of hypoxia alone and hypoxia/reoxygenation were similar: proliferation drastically decreases under hypoxic conditions (Niinikoski 1969) and is reestablished under standard oxygenation conditions. In the context of wound healing, this means that acute and transient hypoxic conditions can be handled very well by both the cell types. Saddiqui et al. (1996) showed this before on cultured wound fibroblasts. Chronic hypoxia might lead to a more serious cellular damage, but chronic hypoxia has not been investigated in this setup yet. HDMEC and NHDF showed a different polyploidization tendency. NHDF were characterized by low polyploidization rates under normal conditions, hypoxia and hypoxia/reoxygenation, which stands in strong contrast to the high polyploidization rates of wound-derived fibroblasts in vitro (Ermis et al. 1998; Oberringer et al. 1999). HDMEC were characterized by extensive binuclear polyploidy. This might be caused by cytokine overstimulation in the medium used in this setup. Polyploidy was clearly shown to be reduced by hypoxia and re-established during reoxygenation. This indicates that a combination of cytokine overstimulation and reoxygenation in vivo in the wound, which generally occur a few days post-trauma, might lead to endothelial polyploidization accompanying proliferation.
-
2.
Is the development of polyploid cells correlated to enhanced proliferation? Initiation of NHDF proliferation during reoxygenation did not cause polyploidy, but HDMEC responded in such a way that proliferation inhibition during hypoxia was accompanied by a decrease of polyploid cells and that reoxygenation caused both proliferation and polyploidization enhancement. The strong correlation of polyploidization and proliferation is known from a variety of different human tissues like the liver or the wound and was confirmed for the HDMEC cell type in this in vitro setup.
-
3.
Is it possible to identify cell type specific cell cycle events leading to the development of polyploidy? Cultured HDMEC seem to have a very special cell cycle, which is outlined in Fig. 5 and which certainly will serve in the future as a useful system to investigate cell cycle relevant modulations leading to polyploidy without the need to apply any additional cell cycle inhibitors.
References
Achison M, Hupp TR (2003) Hypoxia attenuates the p53 response to cellular damage. Oncogene 22(22):3431–3440
Andreassen PR, Lohez OD, Margolis RL (2003) G2 and spindle assembly checkpoint adaptation, and tetraploidy arrest: implications for intrinsic and chemically induced genomic instability. Mutat Res 27(532):245–253
Antoniades HN, Galanopoulus T, Neville-Golden J, Kiritsi CP, Lynch SE (1994) P53 expression in normal tissue regeneration in exposure to acute cutaneous injury in swine. J Clin Invest 93:2206–2214
Borel F, Lohez OD, Lacroix FB, Margolis RL (2002) Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein compromised cells. Proc Natl Acad Sci USA 99(15):9819–9824
Davis JC, Buckley CJ, Barr PO (1988) Compromised soft tissue wounds: correction of wound hypoxia. In: Davis JC, Hunt TK (eds) Problem wounds. The role of oxygen. Elsevier, New York, pp 143–152
Dawes KE, Peacock AJ, Gray AJ, Bishop JE, Laurent GJ (1994) Characterization of fibroblast mitogens and chemoattractants produced by endothelial cells exposed to hypoxia. Am J Respir Cell Mol Biol 10(5):552–559
Dictenberg JB, Zimmermann W, Sparks CA, Young A, Vidair C, Zheng Y, Carrington W, Fay FS, Doxsey SJ (1998) Pericentrin and γ-tubulin form a protein complex organized into a novel lattice at the centrosome. J Cell Biol 141(1):163–174
Ermis A, Oberringer M, Wirbel R, Koschnick M, Mutschler W, Hanselmann RG (1998) Tetraploidization is a physiological enhancer of wound healing. Eur Surg Res 30:385–392
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200(4):500–503
Gahan PB (1977) Increased levels of euploidy as a strategy against rapid aging in diploid mammalian systems: a hypothesis. Exp Gerontol 12:133–136
Galitski T, Saldanha AJ, Styles CA, Lander ES, Fink GR (1999) Ploidy regulation of gene expression. Science 285(9):251–254
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133(4):1710–1715
Graeber TG, Peterson JF, Tsai M, Monica K, Forace AJ, Giaccia AJ (1994) Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low oxygen conditions is independent of p53 status. Mol Cell Biol 14(9):6264–6277
Guidotti JE, Bregerie O, Robert A, Debey P, Brechot C, Desdouets C (2003) Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem 278(21):19095–19101
Hanselmann RG, Oberringer M (2001) Polyploidization: a Janus-faced mechanism. Med Hypoth 56(1):58–64
Hunt TK (1988) The physiology of wound healing. Ann Emerg Med 17:1265–1273
Loffler M (1987) Restimulation of cell cycle progression by hypoxic tumour cells with deoxynucleosides requires ppm oxygen tension. Exp Cell Res 169(1):255–261
Margolis RL, Lohez OD, Andreassen PR (2003) G1 tetraploidy checkpoint and the suppression of tumorigenesis. J Cell Biochem 88(4):673–683
Meek DW (2000) The role of p53 in the response to mitotic spindle damage. Pathol Biol 48(3):246–254
Niinikoski J (1969) The effect of oxygen supply on wound healing and formation of experimental granulation tissue. Acta Physiol Scand 334:1–72
Oberringer M, Lothschütz D, Jennewein M, Koschnick M, Mutschler W, Hanselmann RG (1999) Centrosome-multiplication accompanies a transient clustering of tetraploid cells during tissue repair. Mol Cell Biol Res Commun 2(3):190–196
Oksala T, Therman E (1974) Mitotic abnormalities and cancer. In: German J (ed) Chromosomes and cancer. Wiley, New York, pp 239–263
Owens GK, Schwarz SM (1982) Alterations in vascular smooth muscle mass in the spontaneously hypertensive rat. Role of cellular hypertrophy, hyperploidy, and hyperplasia. Circ Res 51:280–289
Ravid K, Lu J, Zimmet JM, Jones MR (2002) Roads to polyploidy: the megakaryocyte example. J Cell Physiol 190:7–20
Rofstad EK, Johnson NM, Lyng H (1996) Hypoxia-induced tetraploidisation of a diploid melanoma cell line in vitro. Br J Cancer 74(Suppl 27):136–139
Saddiqui A, Galiono RD, Connors D, Gruskin E, Wu L, Mustoe TA (1996) Differential effects of oxygen on human dermal fibroblasts: acute versus chronic hypoxia. Wound Rep Reg 4:211–218
Stempien-Otero A, Karsan A, Cornejo CJ, Xiang H, Eunson T, Morrison RS, Kay M, Winn R, Harlan J (1999) Mechanisms of hypoxia-induced endothelial cell death. Role of p53 in apoptosis. J Cell Biol 274(12):8039–8045
Storchova Z, Pellman D (2004) From polyploidy to aneuploidy, genome instability and cancer. Nature Rev Mol Cell Biol 5:45–54
Therman E, Susman M (1993) Human chromosomes; structure, behavior, effects, 3rd edn. Springer-Verlag, New York, p 149
Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain Z, Rosen N, Seremetiev A, Becker HD, Hunt TK (2003) Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Rep Reg 11:504–509
Vogel C, Kienitz A, Hofmann I, Müller R, Bastians H (2004) Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene 23:6845–6853
Wagner S, Hussain Z, Hunt TK, Bacic B, Becker HD (2004) Stimulation of fibroblast proliferation by lactate-mediated oxidants. Wound Rep Reg 12:368–373
Webster KA, Discher DJ, Kaiser S, Hernandez O, Sato B, Bishopric NH (1999) Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest 104(3):239–252
Acknowledgements
This study was supported by the Saarland ministry for culture and education, grant no. D4-14.2.1.1 LFFP 0204.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oberringer, M., Jennewein, M., Motsch, S.E. et al. Different cell cycle responses of wound healing protagonists to transient in vitro hypoxia. Histochem Cell Biol 123, 595–603 (2005). https://doi.org/10.1007/s00418-005-0782-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0782-5