Abstract
Silicon is one of the most abundant elements found in nature and also abundant in the earth’s crust. Although it is not considered one of the essential elements, it has several fruitful effects on plant evolution, expansion and amelioration of biotic and abiotic stress factors. In present time of climate change, silicon supplementation can be an environment friendly and inexpensive way towards improved crop yield. Major food crops of world including rice, barley and sugarcane are accumulators of silicon. Silicon accumulation which varies among the species is dependent on abundance and expression of Si transporters. The accumulation of silicon not only acts as a mechanical barrier for pathogen and pest infestation into the plant tissue but also modulates phytohormone balance and maintains redox homeostasis to counteract stress. The present review discusses in detail the mechanism of silicon uptake, interaction with different phytohormones and transport on molecular level. Also, it provides comprehensive vision of the molecular mechanism regarding stress tolerance induced by silicon application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
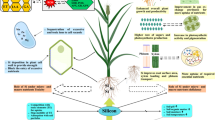
Silicon is numbered as second utmost prevalent element on the earth’s crust following oxygen and a crucial micronutrient for various plants (Raza et al. 2023). It is mostly obtained in combination with oxygen as silica (SiO2), or with other elements to form silicates like CaSiO3, MgSiO3 and K2SiO3. Silicate minerals are also renowned as one of the biggest rock forming minerals present on earth. The content of silicon (Si) in soil, depends on the type of the parent rock that ranges from 1 to 45% (Sommer et al. 2006). Silicon is easily available to plants in the form of monosilicic acid in soil. Silicon has an important role as a beneficial macronutrient that is widespread in monocot and dicot plants. In many plant species, particularly monocots the uptake of Si from soil even surpasses the uptake of essential nutrients (Epstein 1994), still Si is not considered an essential element due to the lack of conclusive indication on direct involvement of Si in plant metabolism. Also, most of the plants complete their life cycle even in the absence of Si, which is one of the criteria to classify an element as essential (Arnon and Stout 1939). Si stimulates defense-related enzymes, encourages the formation of antimicrobial compounds, controls signalling pathways, and induces the expression of defense-related genes to activate defence mechanism, hormone regulation and gene expression patterns (Shanmugaiah et al. 2023).
Several investigations have been made on effect of Si on plant growth and development. Despite this, several studies show that silicon has a positive impact on plant development, agricultural productivity, and the reduction of biotic and abiotic stressors. The current review highlights recent advances in silicon absorption and transport mechanisms, as well as their significance in biotic and abiotic stress tolerance.
Uptake and transport of silicon
Silicon can be found in soil in liquid or solid or adsorbed phase fractions. Poorly crystalline and microcrystalline, crystalline, and amorphous forms of silicon make the solid Si phase. Primary and secondary silicates, as well as other silica materials, include the biggest fraction of crystalline form. Amorphous phase of silicon is either derived from silicon complexes with Al, Fe, heavy metals and soil organic matter or as biogenic form that is originating from plant residues and microorganisms (Matichencov and Bocharnikova 2001; Sauer et al. 2006). Monosilicic acid (H4SiO4) and polysilicic acids make both the liquid and adsorption phase fractions of Si. Weathering of silicate minerals as well as biogenic silica contribute to Si in soil solution and adsorbed phase (Fraysse et al. 2006). The type of silicon taken by plant roots is monosilicic acid (H4SiO4). The pH of soil and amount of clay, minerals, organic matter, and Fe/Al oxides/hydroxides in the soil solution determine the concentration of H4SiO4 in the soil solution (Tubana and Hackman 2015).
At pH greater than 9, monosilicic acid is converted into ionic silicates (Imtiaz et al. 2016). Plants have been classed as Si accumulators (10–15% of shoot dry weight) or intermediates (1–3% of shoot dry weight) including dry land grasses, and excluders or non-accumulators (< 1% of shoot dry weight) based on their silicon accumulation (Jones and Handreck 1967). However, it is also found that many non-accumulators, such as tomatoes, accumulate more Si in their roots than in their shoots (Huang et al. 2011). The H4SiO4 is absorbed by the root cells and is translocated via xylem to the leaf epidermal cells. Through transpiration, the accumulated leaf H4SiO4 is further condensed and polymerized into hard silica gel (SiO2.nH2O). Phytolith is a hard, immobile type of silica that is accumulated in shoots and cannot be translocated to fresh emerging leaves (Jones and Handreck 1967; Raven 1983).
In higher plants, three types of uptake of Si in relation to water uptake was proposed by Takahashi et al. (1990) that is, passive (uptake of Si is analogous to the uptake of water), active (uptake of Si is faster compared to that of water) and rejective (Si uptake is even slower than water). However, these modes of Si uptake are based upon the measurement of relative Si content and transpiration rates and here transpiration rate was assumed to be 500 and Si content of the soil to be 35 mM (Kathryn et al. 2003; Ma et al. 2001).
Active Si uptake involves specific silicon transporters. Several silicon transporters have been discovered in plants of various species (Mitani and Ma 2005). In plants, there are two types of Si transporters: channel-type (Lsi1) and efflux transporters (Lsi2). Lsi1 transfers silicon into plant cells from the surrounding environment whereas, efflux transporters are involved in the movement of Si from plant cells to the xylem (Fig. 1). Using a rice mutant (Lsi1, low silicon 1) that was deficient in Si uptake, the first Si transporter (OsLsi1) in higher plants was found and cloned (Ma et al. 2006, 2007). Lsi1 is a member of the Nod26-like major intrinsic protein (NIP) aquaporin-like protein subfamily. In the projected amino acid sequence of 298 residues, there are six transmembrane domains and two NPA motifs that are highly conserved in the aquaporin water channel family. Rice’s efflux transporter (Lsi2) was also discovered and cloned using a rice mutant that was deficient in Si uptake. Lsi2 is a hypothetical anion transporter that is not related to Lsi1. Lsi1 takes up the Si form distal side of exodermis and its deprotonated form is passed by Lsi2 in aerenchyma at the proximal side of cell. Unloading of silicon from xylem and its distribution to different aerial parts is accomplished by Si transporter Lsi6 (Yamaji et al. 2008). Lsi6 is located mostly in xylem transfer cells and the enlarged vascular bundles’ outer margins. When the Lsi6 gene is knocked out, the panicles accumulate less Si, whereas the flag leaf accumulates more Si (Ma 2010). Lsi6 appears to be a carrier betrothed in silicon transfer from major vascular bundles of roots to diffuse vascular bundles linked to the panicles (Yamaji and Ma 2009). Along with Lsi2 and Lsi6, another Si transporter, Lsi3 also distributes Si in plant tissues.
Si deposition in the tissues was affected in Lsi2, Lsi3 and Lsi6 knockout rice mutants, but there was no influence on Si uptake from the environment, which is predominantly controlled by Lsi1. Si transporters found in other crops such as maize and barley perform comparable activities to those found in rice, but their localization and expression patterns differ (Mitani et al. 2009). The functional analysis of tomato Si-influx and Si-efflux transporter-encoding genes, SiLsi1 and SiLsi2, revealed that low Si accumulation in tomato leaves was caused by non-functioning state of SiLsi2 (Sun et al. 2020). The overexpression of a cucumber Lsi 2 gene in tomato led in the accumulation of Si in the shoots of tomato (Sun et al. 2020).
Silicon in biotic stress tolerance
Living organisms, for instance fungal, bacterial, and viral pathogens, mycoplasma, oomycetes, nematodes, insects, birds, weeds and parasitic plants cause biotic stress that is the major concern worldwide for agricultural production. Application of Si to plants help in alleviating various biotic stresses including disease incidence and pest attack. Plants having high Si content in shoots or roots resist pest attack than those devoid of Si. The main consequence, however, is that it aids in the prevention of soil-borne or foliar disease in major crops that are attacked by various biotrophic, hemi-biotrophic, or necrotrophic pathogens (Cai et al. 2008). It also reduced the development, reproductive period, longevity, and fecundity of insect pests by prolonging incubation and latent periods, lowering conidial production, and reducing numerous characteristics of fungal, bacterial, and viral lesions (expansion rate, size, and number) (Wiese et al. 2005). Disease development and insect preference rates dropped considerably as a result, and susceptible cultivar’s resistance was enhanced nearly equal to the cultivars with full or partial resistance in several circumstances. Silicon enhanced resistance helps in avoiding the penetration of pathogen with the help of structural reinforcement (Epstein 2001) or biochemical modification. To successfully infect the host plant, the virus must cross physical barriers such as wax, cuticles, and cell walls. The mechanical strength of the plants is aided by density of silica in long and short epidermal cells, the thick layer of silica under the cuticle (the double cuticular layer), strengthened Si-cellulose membrane, papilla development, and complexes created with organic compounds in epidermal cell walls. Physical barriers serve to limit pathogen penetration and make plant cells less susceptible to enzymatic damage caused by fungal pathogens. Plant defence against disease and insect pest attack can also be induced by (1) increased activity of enzymes that are responsible for activating defense mechanism in leaves that is phenylalanine ammonia-lyase, polyphenol oxidase, peroxidase and glucanase (Liang et al. 2015), (2) increased production of anti-disease and anti-insect compounds and defensive chemicals in plants, such as phenolic metabolism product (lignin), flavonoids, phytoalexins and pathogenesis-related proteins (Sakr 2018). Antimicrobial compounds help to prevent the disease occurring in plants. The main enzyme for oxidation of phenolic substance that is polyphenol oxidase (PPO) is present in free form in the cytoplasm or are bound in chloroplast, mitochondria as well as other subcellular organelles (Quarta et al. 2013). It has been linked to be involved in lignin synthesis there by increasing antibacterial resistance of host plants (Song et al. 2016). Si is utilised to induce the accumulation of antimicrobial compounds like phenols, flavonoids, and phytoalexins after pathogen infection (Rodrigues et al. 2004; Remus et al. 2005). Peroxidase (POD) and chitinase (CHT), the enzymes involved in host-pathogen interactions, are boosted by silicon. POD is linked to cell-wall reinforcement and cell-wall protein cross-linking (Brisson et al. 1994).
ET, JA and SA also play a vital role in providing immunity to plants to regulate defense response by plants (Clarke et al. 2000). Silicon exhibits a defense mechanism called systemic acquired resistance that triggers when pathogen attacks the plant. Silicon triggers the local defence by activating SA signalling, plant organs after receiving the signals, stimulate SAR for further defence. The uptake of silica in root and leaves lowers the accumulation of ROS and also lipid peroxidation in the membrane. Deposition of silica in the leaf tissue, on the other hand, improves protection of plant against various diseases (Mathur and Roy 2020).
Insect pest tolerance
Silicon increases plant tolerance to insect manifestation mainly by deposition of Si in leaves as phytoliths which results in weakening of insect mouth parts and poor digestion. Its effect on the decrease of pathogens and insect pest attack is also attributed to the chemical protection by enzyme synthesis. The reduced digestibility of feeding on silicon enriched tissue reduces digestibility of food and reduces insect growth. In fact, plant resistance to herbivory is largely due to silicon accumulation, which causes changes in plant nutritional quality and lowers herbivore performance (Frew et al. 2019).
Stress related hormones such as jasmonic acid also acts as a master regulator in resistance against arthropod herbivores and pathogen (Erb et al. 2012). Jasmonic acid regulates defense mechanism for tissue chewing herbivore while salicylic acid and jasmonic acid pathway together activates the defense mechanism against fluid feeding herbivores (Zust and Aggarwal 2016). Different forms of silica were used against several insects (Table 1) such as in case of cucumber for protection from white fly Bemisia tabaci, calcium silicate was used which reduced the oviposition and mortality of nymph (Correa et al. 2005), in sweet cherry, sodium metasilicate was used where spore germination and germ tube extension of Penicillium expansum and Monilinia fructicola were suppressed (Qin and Tian 2005). In sugarcane insect population of spittlebug was affected by applying Si in plant defense. There was an increase in nymphal mortality as well as decreased longevity of males and females was observed (Korndorfer et al. 2011). Ebrahimi et al. (2012) noticed the effect of sodium silicate where it inhibited the mycelial growth in apple whereas calcium silicate was used to protect sugarcane plants from stalk borer (Keeping et al. 2013). Significance of silicon in rice plants was detected against rice leaf folder both at lower and higher rates. Silicon modification at higher rate lowered third-instar weight gain and pupal weight. The larval development was prolonged at both low and high levels of Si content, and the larval survival rate and pupation rate was also suppressed. As a result, resistance to rice leaf folder was developed in the susceptible rice variety (Han et al. 2015). Similarly, fly ash was used in rice plants to avoid the adverse effects by ear head bug and increase in yield was also observed (Peera and Khanam 2020). In maize, foliar application and soil drenching techniques were used to treat silicon dioxide (SiO2) and potassium silicate (K2SiO3) in plants. The findings revealed that foliar treatments of SiO2 and K2SiO3 increased mortality percentage and developmental period in autumn armyworms but decreased larval and pupal biomass (P 0.05). Likewise, both Si sources significantly (P 0.05) reduced lipase activity of larvae and adult fecundity, but the adults had a longer life expectancy (Haq et al. 2021).
Silicon is also involved in induction of plant defense responses in phytophagous insects. It also induces biochemical responses such as increased antioxidant enzyme activity and synthesis of secondary metabolites which reduce insect infestation. Silicon amendment to rice plant enhanced silicification of leaf sheaths. Thus, affecting the working of brown spot hopper. Compared to non-amended plants, silicon amended plants infested with brown plant hopper had higher catalase and superoxide dismutase (SOD) activity. Also, higher activation of polyphenol oxidase, β1, 3- glucanase and phenylalanine was observed (Yang et al. 2017). Potassium silicate and sodium meta-silicate offered protection against Stromatinia cepivora in onion and garlic by activating defense-related genes (Elshahawy et al. 2021).
Disease resistance
The interaction between disease resistance and plant pathogens is a complex process mediated by pathogen and plant derived chemicals. Silicon application also triggers immune response against various pathogens. The first interaction between a plant and a pathogen occurs at the apoplast, when membrane-localized receptors called Pattern Recognition Receptor (PRR) recognise microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs), triggering the first line of defence known as PAMP triggered immunity (PTI) (Bigeard et al. 2014). Further, in response to PTI, pathogen secrete various effectors recognised by R genes and induces second line of defence called ETI (Effector Triggered Immunity). Mechanical barrier posed by Si deposition in the apoplast prevents secretion of effectors by pathogen. It also prevents the pathogen from suppressing the immune response by preventing the effectors from reaching these target locations (Vivancos et al. 2015). Physical barrier was formed by applying slag-based Si fertilizer in rice where immobile Si deposited in cell wall and papillae sites of host plant protects them from brown spot disease (Ning et al. 2014). Si- deficient plants showed a poor defence response than Si- treated plants, according to a transcriptomic analysis of the effect of Si on Phytophthora sojae-infected soybean plants. On Si-treated plants, P. sojae had substantially lower diversity and intensity of effector transcripts. These findings back up the theory that Si disrupts the signalling mechanism between a plant and a pathogen, resulting in an incompatible connection (Rasoolizadeh et al. 2018). Another well studied aspect of transcriptome analysis in tomato against Ralstonia solanacearum revealed that Si primes the defense capacity of plants by enhancing expression of jasmonic acid/ethylene genes. They overexpressed the genes JERF3, TSRF1and ACCO, the oxidative stress markers (FD-I and POD) and the basal defense marker (AGP-1 g) thereby alleviating stress imposed by the pathogen (Ghareeb et al. 2011). Furthermore, Si treatment may improve tomato resistance to R. solanacearum infection in three ways: by activating PTI-related responses; by influencing multiple hormone (e.g., SA, JA, ET, and auxin) signalling pathways; and by alleviating adverse effects (e.g., senescence, water deficiency, and oxidative stress) caused by infection (Jiang and Zhang 2021). Silicon is proposed to improve disease resistance through increasing the generation of secondary metabolites and antioxidant/defense related enzymes, as well as scavenging ROS. Effect of silicon against bacterial wilt in sweet pepper was recorded where Si directly affected colonization of the pathogen, increased Ca+ 2 absorption, and signalled to produce plant defence enzymes (Alves et al. 2015). Potassium silicate was used in case of bitter gourd to enhance the activity of all the defense related enzymes and pathogenesis-related proteins providing protection against powdery mildew (Ratnayake et al. 2016). Silicon also effects metabolism of defence hormones like ethylene, jasmonic acid and salicylic acid. In rice, silicon induces brown spot resistance by interfering with the action of fungal ethylene (Bockhaven et al. 2015). Applications of silicon has shown to reduce disease incidence in various plant species (Table 2). In Chinese cantaloupe, sodium silicate inhibited growth of pathogen that was responsible for postharvest rotting (Guo et al. 2007). Potassium silicate was used in case of soybean against soybean rust and reduced intensity of soybean rust was detected after applying potassium silicate in the field (Rodrigues et al. 2009).
Role of silicon in abiotic stress tolerance
Being sessile organism plants are continuously being challenged by adverse environmental conditions such as heat, cold, drought, frost, heavy metal toxicity. These conditions negatively affect plant growth as well as reduce the crop yield. During environmental stress, ROS gets accumulated and its production is an obvious result of abiotic stresses and it is gaining importance not only due to its widespread production and subsequent damage to plants, but also for its diverse roles in signalling cascades, that affect other biomolecules, hormones involved in growth, development, and stress tolerance regulation. Superoxide (O2), hydroxyl (OH), per hydroxy (HO2), and alkoxy (RO) group are examples of free radicals, while hydrogen peroxide (H2O2) and singlet oxygen are examples of non-radicals that are covered by ROS. Their production and reactivity are well understood. When ROS levels exceed a cell’s antioxidative capabilities, oxidative stress occurs, which can cause the cell to malfunction and eventually the cells die. Most biomolecules in the cell react easily with singlet oxygen, superoxide, hydroxyl and hydrogen peroxide, causing their breakdown and death, contributing to cellular stress. ROS are generated in plant cells as a result of environmental changes and developmental transitions such as seed germination. Plant cells are equipped with antioxidative machinery (Fig. 2), which includes both enzymatic and non-enzymatic components, to combat oxidative stress and excess ROS (Yadav and Sharma 2016). As a result, maintaining a favourable balance between ROS generation and antioxidant defence becomes necessary so that it preserves photosynthetic machinery, membrane integrity and nucleic acid and protein degradation (Hasanuzzaman et al. 2020). ROS must be effectively controlled and eliminated in order for organisms to survive. A powerful antioxidant defence system is responsible for that and includes antioxidant enzymes such as SOD, CAT, POD, APX and GR as well as low molecular weight antioxidants that is ascorbic acid, GSH or lipid like tocopherol, carotenoids, quinines and also some polyphenols. Metalloenzyme SOD helps in mediating the disproportionation of O2¯ into H2O2 and O2 and is present in all aerobic organism and subcellular components that are vulnerable to oxidative stress (Kim et al. 2017). Other antioxidant enzyme catalase removes H2O2 generated in peroxisome and controls peroxisomal H2O2 synthesis whereas peroxidase is specific for GSH and uses H2O2 to oxidise the other substrate. MDHAR is also found in chloroplast and cytosol that regenerates the reduced ascorbate and was typically found in drought stressed conditions in rice seedlings (Yadav and Sharma 2016). Silicon has shown to reduced ROS accumulation by increasing the activity of these enzymes (Mostafa et al. 2021) thus providing tolerance to various abiotic stresses (Table 3).
In salt stressed tomato plants, application of sodium silicate along with NaCl enhanced the activity of SOD and CAT enzyme (Al-aghabary et al. 2005). A decrease in H2O2 level and MDA concentration showed that silicon reduces oxidative stress caused by salinity. Wheat growth under salt stress was improved using calcium silicate which lowered the sodium absorption and increased potassium uptake (Ali et al. 2009; Liang 1999) noted that Si has a protective effect against salt stress by allowing for the selective absorption and transport of potassium and sodium. Both sodium and potassium silicate were used to check sugarcane’s resilience to drought stress. Regardless of the water conditions, it was discovered that Si leads to lower C concentrations by causing the stomatal closer for longer periods of time throughout the day. Additionally, the type of Si also had an impact on the concentrations of nitrogen and phosphorus (Teixeira et al. 2020). In sorghum under drought stress, silica enhanced the leaf area index, chlorophyll content, leaf dry weight, specific leaf weight, shoot dry weight, total dry weight, and root dry weight of the plant (Ahmed et al. 2011). At low temperatures the impact of Sodium silicate was tested in barley where it was observed that biochemical properties of leaf apoplasm was modified, which further mitigated the cold stress (Joudmand and Hajiboland 2019). Similarly, in maize, silica showed cold-protective effects with enhanced superoxide dismutase (SOD) activity in shoot and roots as well as an increase in concentrations of proline, phenolics, and antioxidants and reduced levels of H2O2 in the tissues (Moradtalab et al. 2018).
In sorghum, foliar spray of calcium silicate increased the forage dry yield and water use efficiency (Niyazi et al. 2018), while in leaves of oil palm seedlings, enhanced proline concentration, nitrate reductase activity (NRA), stomatal closure and chlorophyll content was observed. Amanah et al. (2019) also tried Na2SiO3 in oil palm against drought stress and observed that the oil palm seedlings showed tolerance against drought. Foliar application of silicon in sweet pepper helped to defend salt stress by enhancing the activity of antioxidant enzymes (Abdelaal et al. 2020). In sugarcane, silicon (Si) supplementation mitigated the damage caused due to water deficiency by improving the C:N:P balance, increasing C, N, and P use efficiencies and the biomass conversion, and finally enhancing the yield (Teixeira et al. 2020, 2022). Si treatment increased rice submergence tolerance and decreased yield loss by reducing the unfavourable impacts of reactive oxygen species and quiescence strategy. Through, the synergistic regulation of endogenous hormones ethylene (ET), gibberellic acid (GA), and jasmonic acid (JA), Si dramatically suppressed elongation and internode length in wild type rice under submergence (Pan et al. 2022). However, the addition of Si had no effect on the expression of the SUB1A gene, which is responsible for submergence tolerance features of rice (Xu et al. 2006; Pan et al. 2022).
Silicon contributes in the alleviation of heavy metal stress caused by cadmium, copper, aluminium, and other metals (Table 4). Si application alters soil physical qualities such as pH, electrical conductivity, and organic matter, as well as soil microorganisms, causing nutrient release that competes with heavy metals for uptake and translocation to plant roots to be expedited. Furthermore, Si significantly reduced heavy metal uptake through chelation or arresting heavy metals in the soil. Heavy metal bioavailability is reduced which as a result limits their transmission from root to shoot. Also, Si reduces adverse effects of heavy metal toxicity in the cells by stimulating the antioxidant enzyme activity, complex formation with metal ions and compartmentalization of metal -silicon complex and exerting change in the cell wall, thus controlling the transport of heavy metals (Tubana and Heckman 2015). Application of Si in cadmium stressed rapeseed, reduced MDA content and H2O2. There was an increase in AsA and GSH pools as well as activities of glyoxalase system (Gly I and Gly II) enzymes and CAT, leading to an improved antioxidant defense (Hasanuzzaman et al. 2017). Rice showed the positive effect of Si against cadmium stress by reducing the accumulation of heavy metals in rice roots (Kim et al. 2014). The combined stress of Cd and Pb in quinoa led to 11-fold increase in H2O2 generation and a 13-fold increase in TBARS synthesis, as well as a decrease in membrane stability (59%). Exogenous injection of K and Si in combination reduced oxidative stress caused by metals by eight times. SOD, CAT, APX, and POD activities increased 9-fold, 7-fold, and 11-fold, respectively (Algharby et al. 2022).
Interaction of silicon with other phytohormones for growth, development and stress alleviation
Silicon (Si) plays a crucial role to enhance the nutritional state of crop plants for the promotion of development and productivity. Under biotic and abiotic stresses, the exogenous application of Si activates plant defense and phytohormone signalling mechanisms (Khan et al. 2022) (Fig. 3). Plant biological processes are frequently influenced by phytohormones that also control cellular signalling in response to salt, drought, extremely high temperatures and nutrient shortage stress (Salvi et al. 2021). In order to better understand the mechanism, regulation and interaction of silicon with various phytohormones, the findings from diverse investigations have been discussed.
In rice plants, it was observed that under arsenate stress, the expression of auxin biosynthesis genes (OsYUCCA1 and OsTAA1) dropped along with the decrease in root biomass. However, the presence of Si significantly boosted the expression of OsYUCCA1 and OsTAA1, which ultimately leads to an increase in the number of roots and biomass. It was suggested that Si-driven root development under arsenate stress in rice plants is mediated by auxin biosynthesis genes (Tripathi et al. 2021). The addition of IAA to the AgNPs + Si mixture was also effective in reducing the harmful effects of the silver nanoparticles in Brassica juncea. Increased length of shoots and roots as well as reduced nitric oxide levels under AgNPs stress was observed in Brassica juncea (Vishwakarma et al. 2019). In maize, phytohormones (auxin IAA, gibberellins GA and abscisic acid ABA) and antioxidant enzyme activity (superoxide dismutase SOD, ascorbic peroxidase APX, and catalase CAT) were used to determine if plants were resistant to drought stress. Stressed plants without silicon treatment showed enhanced SOD and CAT activity along with a decrease in IAA. Plants grown on non-tillage soils applied with silicon treatment showed increase in IAA concentration with decreased SOD and CAT activity (Merhij et al. 2019).
Silicon supplementation along with NAA indicated that silica might have contributed in polar transport of auxin as an elicitor of ABA biosynthesis leading to initiation of lateral root growth of cowpea (Hu et al. 2020). However, exogenous GA3 and potassium silicate proved to be extremely successful in reducing salt-induced damages in okra cultivars. Along with that, fresh weight and dry weight of roots and shoots, plant height, root and shoot length were also increased (Ayub et al. 2018). In date palm, exogenous application of Si and GA3 activated the genes associated with the heat shock factor. Moreover, the combined treatment of Si and GA3 dramatically reduced the transcript accumulation of genes involved in ABA signalling (PYL4, PYL8, and PYR1). These results imply that the combined application of Si and GA3 promotes plant growth and metabolic regulation and confers stress resilience (Khan et al. 2020). Cytokinin is also a major plant growth hormone which is responsible for cell division and shoot proliferation in plants. Si treatment enhanced zeatin concentration in the root and shoot tissues of maize which finally lead to protect plants from cold-stress (Moradtalab et al. 2018).
Si also encourages the production of phenylalanine ammonia-lyase, which is a precursor to SA biosynthesis (Souri et al. 2020). Silicon in combination with SA promotes arsenic toxicity tolerance in wheat by inducing plant morphological characters, minimise electrolyte leakage and decreased ROS lipid peroxidation (Maghsoudi et al. 2020, Arif et al. 2021). The physiological effects of methyl jasmonate (MeJA) (0.5 mM) and silicon nanoparticles (2 mM) on some salinity-related genes (DREB, cAPX, Mn-SOD, and GST) in strawberry cv. Paros was noticed under saline (50 mM NaCl) and non-saline conditions. The use of MeJA and Si nanoparticles boosted the transcription of the DREB, cAPX, MnSOD, and GST genes which ultimately leads to enhance salinity tolerance in strawberry cultivars. The strawberry plants responded better to salt stress by improving enzymatic and non-enzymatic antioxidants’ physiological defence systems and by upregulating the transcription of salinity-related genes in response to MeJA and Si application (Moradi et al. 2022).
Si increased the levels of endogenous hormones in G. uralensis seedlings. Si had an increasing impact on IAA and GA3 concentrations after 10 days of application. Whereas, at a concentration of 2 mM, Si had also a greater impact on ABA biosynthesis after 20 days. Hence, Si can control endogenous hormone concentrations and thereby enhance the growth of G. uralensis seedlings under salt stress conditions. This study showed that Si had a concentration and time-dependent positive effect and might also reduce the negative effects of salt stress by controlling the levels of endogenous hormones (IAA, GA3, and ABA) (Lang et al. 2019).
Future perspectives and conclusion
Silicon is a major component of soil and has a proven role in plant growth, development, and biotic and abiotic stresses resilience. It is commonly known that silicon has positive impacts on agriculture production and productivity. Si has been shown to positively modulate the physiological characteristics of agricultural plants, aiding in the fight against abiotic stress situations. Si’s ability to upregulate phytohormones and the signalling cascades that they use to survive abiotic stressors is essential to its function. Surprisingly, Si is said to increase the resilience to abiotic stress conditions by overexpressing the genes that control the production of phytohormones. Through signal transduction pathways, Si-induced phytohormones mediate stress tolerance, which is predominantly controlled by these mechanisms. The molecular mechanisms underpinning Si absorption, transport, and accumulation in various crops are still poorly understood. It needs to be understand whether manipulation of Si transporters and further accumulation of Si in non-accumulators can improve stress tolerance.
An in-depth understanding of Si’s crucial function in overcoming stress tolerance is still lacking, largely because the molecular mechanism is very little understood. Furthermore, considerable scientific research into how Si and phytohormones work together to improve plants’ ability to tolerate drought is still in its early stages. To confirm the significance of Si as the key component in fertilisers for fortification and sustainable agricultural development, it is also necessary to uncover other genes related to physiological functions. Detailed investigation of the crosstalk between Si and phytohormones can be improved by cutting-edge omics technologies including transcriptomic, proteomic, and metabolomic research in order to elucidate new signalling molecules responsible for alleviating the stress tolerance in plants. Additionally, Si’s function in controlling signal transduction pathways under a variety of abiotic stress conditions requires specific attention. Silicon has a strong impact on crop quality, strength, and yield and therefore silicon supplementation holds a great potential as a plant protectant and growth stimulant for sustainable resilient agriculture.
Change history
10 August 2023
”The original online version of this article was revised”: The communicated note has been revised.
Abbreviations
- Si:
-
Silicon
- CaSiO3− :
-
Calcium silicate
- MgSiO3 :
-
Magnesium silicate
- K2SiO3 :
-
Potassium silicate
- Al:
-
Aluminium
- Fe:
-
Iron
- SiO2:
-
Silica
- H4SiO4 :
-
Monosilicic acid
- SiO2.nH2O:
-
Hard silica gel
- Lsi1, Lsi2:
-
Silicon transporters
- NIP:
-
Nod26-like major intrinsic protein
- PPO:
-
Polyphenol oxidase
- POD:
-
Peroxidase
- CHT:
-
Chitinase
- ET:
-
Ethylene
- JA:
-
Jasmonic acid
- SA:
-
Salicylic acid
- PRR:
-
Pattern recognition receptor
- MAMPs or PAMPs:
-
Pathogen-Associated Molecular Patterns
- PTI:
-
PAMP triggered immunity
- ETI:
-
Effector Triggered Immunity
- NRA:
-
Nitrate reductase activity
- O2 :
-
Superoxide
- OH:
-
Hydroxyl
- HO2 :
-
Per hydroxy
- RO:
-
Alkoxy
- H2O2 :
-
hydrogen peroxide
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- APX:
-
Ascorbate peroxidase
References
Abdelaal KA, Mazrou YS, Hafez YM (2020) Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 9.
Ahmed M, Hassen FU, Qadeer U, Aslam MA (2011) Silicon application and drought tolerance mechanism of sorghum. Afr Jour Agric Res 6:594–607
Al-aghabary K, Zhujun Zhu Z, Shi Q (2005) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. Jour Plant Nut 27:2101–2115
Alharby HF, Al-Zahrani HS, Abbas G (2022) Potassium and silicon synergistically increase cadmium and lead tolerance and phytostabilization by quinoa through modulation of physiological and biochemical attributes. Toxics 10(4):169. https://doi.org/10.3390/toxics10040169
Ali SMA, Basra R, Ahmad A, Wahid (2009) Optimizing silicon application to improve salinity tolerance in wheat. Soil Env 28:1–9
Alves AO, Santos MM, Souza LJ, Souza EB, Mariano RL (2015) Use of silicon for reducing the severity of bacterial wilt of sweet pepper. Jour Plant Pathol 97:419–429
Amanah DM, Harris N, Santi LP (2019) Physiological responses of bio-silica-treated oil palm seedlings to drought stress. Menara Perk 87:20–30
Arif Y, Singh P, Bajguz A, Alam P, Hayat S (2021) Silicon mediated abiotic stress tolerance in plants using physio-biochemical, omic approach and cross-talk with phytohormones. Plant Physiol Biochem 166:278–289. https://doi.org/10.1016/j.plaphy.2021.06.002
Arnon DI, Stout PR (1939) The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol 14:371–375
Ayub Q, Khan SM, Khan A, Hussain I, Ahmad Z, Khan MA (2018) Effect of gibberellic acid and potassium silicate on physiological growth of Okra (Abelmoschus esculentus L.) under salinity stress. Pure Appl Biol 7. https://doi.org/10.19045/bspab.2018.70002
Bigeard J, Rayapuram N, Pflieger D, Hirt H (2014) Phosphorylation-dependent regulation of plant chromatin and chromatin-associated proteins. Proteomics 14:2127–2140
Bockhaven JV, Spichal L, Novak O, Strnad M, Asano T, Kikuchi S, Hofte M, Vleesschauwer DD (2015) Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol 206:761–773
Brisson LF, Tenhaken R, Lamb C (1994) Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6:1703–1712
Cai K, Gao D, Luo S, Zeng R, Yang J, Zhu X (2008) Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol Plant 134:324–333
Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X (2000) Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12:2175–2190
Correa RSB, Moraes JC, Alexander M, Auad E, Carvalho GA (2005) Silicon and acibenzolar-S-Methyl as resistance inducers in cucumber, against the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Biotype B. Neotro Ento 34:429–433
Ebrahimi L, Aminian H, Etebarian HR, Sahebani N (2012) Control of apple blue mould disease with Torulaspora delbrueckii in combination with Silicon. Arch Phytopathol Plant Prot 45:2057–2065
Elshahawy IE, Osman SA, Abd-El-Kareem F (2021) Protective effects of silicon and silicate salts against white rot disease of onion and garlic, caused by Stromatinia cepivora. Jour Plant Pathol 103:27–43
Epstein E (1994) The anomaly of silicon in plant biology. PNAS 91:11–17
Epstein E (2001) Silicon in Plants: Facts vs Concepts. Amsterdam: Elsevier Science 8:1–15
Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259
Flora C, Khandekar S, Boldt J, Leisner S (2019) Silicon alleviates long-term copper toxicity and influences gene expression in Nicotiana tabacum. Jour Plant Nutr 42:864–878
Fraysse F, Pokrovsky OS, Schott J, Meunier JD (2006) Surface properties, solubility and dissolution kinetics of bamboo phytoliths. Geochim et Cosmo Acta 70:1939–1951
Frew A, Weston LA, Gurr GM (2019) Silicon reduces herbivore performance via different mechanisms, depending on host-plant species. Austral Ecol 44:1092–1097
Ghareeb H, Bozso Z, Ott PG, Repenning C, Stahl F, Wydra K (2011) Transcriptome of silicon-induced resistance against ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol 75:83–89. https://doi.org/10.1016/j.pmpp.2010.11.004
Guo Y, Liu L, Zhao J, Bi Y (2007) Use of silicon oxide and sodium silicate for controlling Trichothecium roseum postharvest rot in chinese cantaloupe (Cucumis melo L). Intern Jour Food Sci Technol 42:1012–1018
Han Y, Lei W, Wen L, Hou M (2015) Silicon-Mediated resistance in a Susceptible Rice Variety to the Rice Leaf Folder, Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae).Plos one1–13
Haq IDIU, Khurshid A, Inayat R, Kexin Z, Changzhong L, Ali S, Zuan ATK, Al-Hashimi A, Abbasi AM (2021) Silicon-based induced resistance in maize against fall armyworm [Spodoptera frugiperda (Lepidoptera: Noctuidae)]. Plos one 1–16
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front Plant Sci 8:1–9
Hasanuzzaman M, Bhuyan MHMB, Parvin K, Bhuiyan TF, Anee TI, Nahar K, Hossen MS, Zulfiqar F, Alam MM, Fujita M (2020) Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. Inter Jour Mol Sci 21:8695
Hu J, Li Y, Jeong BR (2020) Silicon promotes root development by modulating polar transport of auxin during cutting propagation of poinsettia. Acta Hortic 1291:269–276. https://doi.org/10.17660/ActaHortic.2020.1291.32
Huang CH, Roberts PD, Datnoff LE (2011) Silicon suppresses fusarium crown and root rot of tomato. Jour Phytopathol 159:546–554
Imtiaz M, Rizwan MS, Mushtaq MA, Ashraf M, Shahzad SM, Yousaf B, Saeed DA, Rizwan M, Nawaz MA, Mehmood S, Tu S (2016) Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: a review.Jour Environ Manage1–9
Jiang N H, Zhang SH (2021) Effects of combined application of potassium silicate and salicylic acid on the defense response of hydroponically grown tomato plants to ralstonia solanacearum infection. Sustainability 13:3750. https://doi.org/10.3390/su13073750
Jones LHP, Handreck KA (1967) Silica in soils, plants, and animals. Adv Agro 19:107–149
Joudmand A, Hajiboland R (2019) Silicon mitigates cold stress in barley plants via modifying the activity of apoplasmic enzymes and concentration of metabolites. Acta Physiol Plant 41:1–13
Kathryn E, Richmond M Sussman (2003) Got silicon the non-essential beneficial plant nutrient. Cur Opi Plant Biol 6:268–272
Keeping MG, Meyer JH, Sewpersad C (2013) Soil silicon amendments increase resistance of sugarcane to stalk borer Eldana saccharina Walker (Lepidoptera: Pyralidae) under field conditions. Plant Soil 363:297–318
Khan A, Bilal S, Khan AL, Imran M, Shahzad R, Al-Harrasi A, Al-Rawahi A, Al-Azhri M, Mohanta TK, Lee IJ (2020) Silicon and Gibberellins: synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L). Plants 9:620. https://doi.org/10.3390/plants9050620
Khan I, Awan SA, Rizwan M, Brestic M, Xie W (2022) Silicon: an essential element for plant nutrition and phytohormones signaling mechanism under stressful conditions. Plant Growth Regul. https://doi.org/10.1007/s10725-022-00872-3
Kim YH, Khan AL, Kim DH (2014) Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol 14:13
Kim YH, Khan AL, Waqas M, Lee IJ (2017) Silicon regulates antioxidant activities of crop plants under abiotic-Induced oxidative stress: a review. Front Plant Sci 8:1–7
Korndörfer AP, Grisoto E, Vendramim JD (2011) Induction of insect plant resistance to the spittlebug Mahanarva fimbriolata Stal (Hemiptera: Cercopidae) in sugarcane by silicon application. Neotrop Entomol 40:387–392
Lang DY, Fei PX, Cao GY, Jia XX, Li YT, Zhang XH (2019) Silicon promotes seedling growth and alters endogenous IAA, GA3 and ABA concentrations in Glycyrrhiza uralensis under 100 mM NaCl stress. J Hortucul Sci Biotechnol 94:87–93
Liang Y (1999) Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 209:217
Liang YC, Chen Q, Liu Q, Zhang WH, Ding RX (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L). Jour Plant Physiol 160:1157–1164
Liang Y, Nikolic M, Belanger R, Gong H, Song A (2015) Silicon and insect pest resistance. Silicon in Agriculture; Springer, Berlin, Germany, pp 197–207
Ma JF (2010) Silicon transporters in higher plants. Advances in experimental medicine and biology. Springer 679:99–109
Ma JF, Miyake Y, Takahashi E (2001) Silicon as a beneficial element for crop plants. Silicon in Agriculture. Edited by Datonoff L, Korndorfer G, Synder G. New York: Elsevier Science. 17–39
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448:209–212
Maghsoudi K, Arvin MJ, Ashraf M (2020) Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide and silicon. Jour Soil Sci Plant Nut 20:577–588
Mathur P, Roy S (2020) Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol Biochem 157:114–127
Matichencov VV, Bocharnikova EA (2001) The relationship between silicon and soil physical and chemical properties. In: Snyder GH, Korndörfer GH (eds) Silicon in Agriculture, Studies in Plant Science, vol 8. Amsterdam, The Netherlands: Elsevier, Datnoff L. E, pp 209–219
Merhij EI, Abo Al Timmen WM, Jasim AH (2019) The effect of silicon, tillage and the interaction between them on some antioxidants and phytohormones during drought stress of maize (Zea mays L.) plants. Plant Arc 19:66–74
Mitani N, Chiba Y, Yamaji, N, Ma JF (2009) Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 21(7):2133–2142.
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. Jour Exper Bot 56:1255–1261
Moradi P, Vafaee Y, Mozafari AA, Tahir NA (2022) Silicon nanoparticles and methyl jasmonate improve physiological response and increase expression of stress-related genes in Strawberry cv. Paros under salinity stress. Silicon 14:10559–10569. https://doi.org/10.1007/s12633-022-01791-8
Moradtalab N, Weinmann M, Walker F, Hoglinger B, Ludewig U, Neumann G (2018) Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front Plant Sci 9:420. https://doi.org/10.3389/fpls.2018.00420
Mostafa MG, Rahman MM, Ansary MMU, Keya SS, Abdelrahman M, Miah MG, Tran LSp (2021) Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit Reviews Biotech 41:918–934
Najihah NI, Hanafi MM, Idris AS, Hakim MA (2015) Silicon treatment in oil palms confers resistance to basal stem rot disease caused by Ganoderma boninense. Crop Protec 67:151–159
Ning D, Song A, Fan F, Li Z, Liang Y (2014) Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance. PLoS ONE 9:1–9
Niyazi BAM (2018) Forage production and water use efficiency (WUE) of sorghum (Sorghum Bicolor L.) under drought stress as affected by silicon (SI) treatments. Inter Jour Engineer Res Technol 7:32–38
Pan T, Wang L, Peng Z, Tian J, Cai K (2022) Silicon enhances the submergence tolerance of rice by regulating quiescence strategy and alleviating oxidative damage. Plant Physiol Biochem 182:124–132.
Peera SKPG, Khanam R (2020) Fly ash as a source of silicon and potassium for mitigating ear head bug (Leptocorisa acuta) in rice under different abiotic stress condition. Jour Pharmaco Phytochem 9:1662–1665
Qin GZ, Tian SP (2005) Enhancement of biocontrol activity of Cryptococcus laurentii by silicon and the possible mechanisms involved. Phytopathol 95:69–75
Quarta A, Mita G, Durante M, Arlorio M, Paolis AD (2013) Isolation of a polyphenol oxidase (PPO) cDNA from artichoke and expression analysis in wounded artichoke heads. Plant Physiol Biochem 68:52–60
Rasoolizadeh A, Labbe C, Sonah H, Deshmukh RK, Belzile F, Menzies JG, Bélanger RR (2018) Silicon protects soybean plants against Phytophthora sojae by interfering with effector–receptor expression. BMC Plant Biol 18:97
Ratnayake RMRNK, Daundasekera WAM, Ariyarathne HM, Ganehenege MY (2016) Some biochemical defense responses enhanced by soluble silicon in bitter gourd-powdery mildew pathosystem. Australasian Plant Pathol 45:425–433
Raven JA (1983) The transport and function of silicon in plants. Biol Rev 58:179–207
Raza T, Abbas M, Amna, Imran S, Khan MY, Rebi A, RafieRad Z, Eash NS (2023) Impact of silicon on plant nutrition and significance of silicon mobilizing bacteria in agronomic practices. https://doi.org/10.1007/s12633-023-02302-z. Silicon
Remus Borel W, Menzies JG, Belanger RR (2005) Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol Mol Plant Pathol 66:108–115
Rodrigues FA, Mcnally DJ, Datnoff LE, Jones JB, Labbé C, Benhamou N (2004) Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathol 94:177–183
Rodrigues FA, Duarte HSS, Domiciano GP (2009) Foliar application of potassium silicate reduces the intensity of soybean rust. Australasian Plant Pathol 38:366–372
Sakr N (2018) Silicon-enhanced resistance of plants to biotic stresses Review article. Acta Phytopathol Entom Hungarica 53:125–142
Salvi P, Manna M, Kaur H, Thakur T, Gandass N, Bhatt D, Muthamilarasan M (2021) Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep 40:1305–1329. https://doi.org/10.1007/s00299-021-02683-8
Sauer D, Burghardt W (2006) The occurrence and distribution of various forms of silica and zeolites in soils developed from wast.es of iron production. CATENA 65:247–257
Shanmugaiah V, Gauba A, Hari SK, Prasad R, Ramamoorthy V, Sharma MP (2023) Effect of silicon micronutrient on plant’s cellular signaling cascades in stimulating plant growth by mitigating the environmental stressors. Plant Growth Regul. https://doi.org/10.1007/s10725-023-00982-6
Singh VP, Tripathi DK, Kumar D (2011) Influence of exogenous Silicon Addition on Aluminium Tolerance in Rice Seedlings. Biol Trace Element Res 144:1260–1274
Sommer M, Kaczorek D, Kuzyakov Y, B.reuer J (2006) Silicon pools and fluxes in soils and landscapes—a review. Jour Plant Nutr Soil Sci 169:310–329
Song A, Xue G, Cui P, Fan F, Liu H, Chang Y (2016) The role of silicon in enhancing resistance to bacterial blight of hydroponic- and soil-cultured rice. Sci Rep 6:24640
Souri Z, Khanna K, Karimi N, Ahmad P (2020) Silicon and plants: current knowledge and future prospects. Jour Plant Growth Reg 40:906–925
Sun H, Duan Y, Mitani-Ueno N, Che J, Jia J, Liu J, Guo J, Ma JF, Gong H (2020) Tomato roots have a functional silicon influx transporter but not a functional silicon efflux transporter. Plant Cell Environ 43:732–744
Takahashi E, Ma JF, Miyake Y (1990) The possibility of silicon as an essential element for higher plants. Agricul Food Chem 2:99–102
Teixeira GCM, Prado RM, Rocha AMS, Piccolo MC (2020) Root- and foliar-applied silicon modifies C: N: P ratio and increases the nutritional efficiency of pre-sprouted sugarcane seedlings under water deficit.Plos one1–24
Teixeira GCM, de Mello Prado R, Rocha AMS, de Cássia Piccolo M (2022) Silicon as a sustainable option to increase biomass with less water by inducing Carbon:Nitrogen:Phosphorus stoichiometric homeostasis in sugarcane and energy cane. Front Plant Sci 1213:826512. https://doi.org/10.3389/fpls.2022.826512
Tripathi DK, Rai P, Guerriero G, Sharma S, Corpas FJ, Singh VP (2021) Silicon induces adventitious root formation in rice under arsenate stress with involvement of nitric oxide and indole-3-acetic acid. Jour Exp Bot 72(12):4457–4471. https://doi.org/10.1093/jxb/eraa488
Tubaña BS, Heckman JR (2015) Silicon in soils and plants. In: Rodrigues F., Datnoff L. (eds) Silicon and Plant Diseases. Springer. Cham 7–51
Vaculik M, Pavlovic A, Lux A (2015) Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxic Environ Safety 120:66–73
Vishwakarma K, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S (2019) Silicon and plant growth promoting rhizobacteria differentially regulate AgNP-induced toxicity in Brassica juncea: implication of nitric oxide. Jour Hazard Mat 121806. https://doi.org/10.1016/j.jhazmat.2019.121806
Vivancos J, Labbe C, Menzies JG, Bélanger RR (2015) Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol Plant Pathol 16:572–582
Wiese J, Wiese H, Schwartz J, Schubert S (2005) Osmotic stress and silicon act additively in enhancing pathogen resistance in barley against barley powdery mildew. Plant Nut Soil Sci 168:269–274
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442(7103):705–708
Yadav NV, Sharma S (2016) Reactive oxygen species, oxidative stress and ROS scavenging system in plants. Jour Chem Pharma Res 8:595–604
Yamaji N, Ma JF (2009) A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21:2878–2883
Yamaji N, Mitani N, Ma JF (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20:1381–1389
Yang L, Han Y, Li P, Wen L, Hou M (2017) Silicon amendment to rice plants impairs sucking behaviors and population growth in the phloem feeder Nilaparvata lugens (Hemiptera: Delphacidae). Sci Rep 7:1101. https://doi.org/10.1038/s41598-017-01060-4
Zust T, Agrawal AA (2016) Mechanisms and evolution of plant resistance to aphids. Nat Plants 2:15206
Acknowledgements
Authors are grateful to Department of Biotechnology, Dr Y S P University of Horticulture and Forestry, Solan, India for providing the infrastructure and support for the study.
Author information
Authors and Affiliations
Contributions
Conceptualization: AS, Search strategy, article outline, tables and figures: AT (Aayushee Thakur) and AS and VS, Writing of rough draft: AT (Aayushee Thakur) and AT (Anchal Tandon), Article editing: AS and VS, Final review: corresponding author. Before submitting the manuscript, a final revision and approval of manuscript has been performed by all the authors.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing interest.
Additional information
Communicated by Pramod Kumar Nagar
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“The original online version of this article was revised”: The communicated note
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thakur, A., Singh, A., Tandon, A. et al. Insights into the molecular mechanisms of uptake, phytohormone interactions and stress alleviation by silicon: a beneficial but non-essential nutrient for plants. Plant Growth Regul 101, 1–13 (2023). https://doi.org/10.1007/s10725-023-01002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01002-3