Abstract
Gluconacetobacter diazotrophicus PAL5 and Azospirillum brasilense REC3 are plant growth promoting bacteria. They are able to produce hydroxamate and catechol type siderophores, respectively, when iron is not available, chelating this metal to facilitate its absorption. Iron is required by plants and is involved in physiological processes as part of many important compounds. The aim of this work was to evaluate the two siderophores producing bacteria in their contribution to iron nutrition for strawberry plants through the growth index, leaf and root area, greenness index, total soluble phenolic compounds and total iron content. Strawberry plants were grown hydroponically with a 16-h photoperiod in Hoagland nutrient solution, modified in iron sources, and inoculated with each bacterium. At day 60, the highest values of growth index, root area, greenness index, and iron content, were obtained for treatments with reduced iron, and the lowest values in treatments without iron addition. Values in treatments with oxidized iron and inoculated with bacteria were similar to those obtained with reduced iron and uninoculated plants. At day 30, phenolic compounds were higher in treatments without iron addition and uninoculated, while they decreased when plants were inoculated. For treatments with reduced iron, phenolic compounds content was low and increased when plants were inoculated. In conclusion, the siderophores produced by G. diazotrophicus PAL5 and A. brasilense REC3 can contribute to the iron nutrition of hydroponically grown strawberry plants. The participation of the hydroxamates was better than that of the catechols in the provision of iron to the plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron is required by plants in small quantities, being involved in physiological processes as part of many important compounds, such as iron-containing proteins (i.e., cytochromes and ferredoxins) and chlorophylls (Hochmuth 2011; Briat et al. 2015). Although iron is present in the environment, it is not always available, often limiting the plant growth (Walker and Connolly 2008). However, the plants have evolved to obtain iron from the environment through different mechanisms, known as Strategy I (in non-grassed monocots and dicotyledon plants by acidification of the rhizosphere, the surrounding area of the root) and Strategy II (in grasses, that solubilize the Fe(III) ions forming an iron-phytosiderophore complex) (Bienfait 1988).
Among the sustainable approaches to enhance the iron supply to plants is the use of biofertilizers containing plant-growth promoting bacteria (PGPB). The PGPB constitute a heterogeneous group of beneficial bacteria that can be found in the rhizosphere, associated with the roots, and that are able to improve the growth of plants and to protect them from diseases and abiotic stress through direct and indirect actions (Bashan and Holguin 1998; Dimkpa et al. 2009; Zhao et al. 2011; Glick 2012). One of the bacterial plant growth promoting mechanism is the siderophores production; they are secondary metabolites with low molecular weight (< 2000 Da) able to chelate iron to facilitate the metal absorption in poor environments (Budzikiewicz 2010; Chu et al. 2010). Siderophores can improve vegetal growth by increasing plant nutrient availability through iron uptake (Bashan and de-Bashan 2005), or by preventing the growth of soil borne pathogens due to iron limitation (Kumar 1999; Gupta et al. 2002; Miethke and Marahiel 2007; Chaiharn et al. 2009; Sayyed and Chincholkar 2009).
Within the PGPB producers of siderophores are Gluconacetobacter diazotrophicus (Logeshwaran et al. 2009) and Azospirillum brasilense (Saxena et al. 1986; Tapia-Hernandez et al. 1990; Shah et al. 1992). G. diazotrophicus, a member of the family Acetobacteraceae, is a N2-fixing bacterium originally associated with sugar cane (Saccharum sp.) and currently used in agriculture (Cavalcante and Döbereiner 1988; Reis and Teixeira 2015; Pedraza 2016). A. brasilense, a member of the family Rodospirillaceae, is one of the most investigated PGPB and extensively applied in agriculture (Bashan and de-Bashan 2010; Baldani et al. 2014). Logeshwaran et al. (2009) observed that G. diazotrophicus PAL5 produces in vitro hydroxamate type siderophores that could be responsible to promote plant growth after inoculation. In addition, it was reported that A. brasilense REC3, a strain isolated from strawberry (Fragaria ananassa, Duch; Pedraza et al. 2007), produces catechol type siderophores with the capacity to control the phytopathological fungus Colletotrichum acutatum in strawberry plants (Tortora et al. 2011). However, the siderophores production capacity of these strains has not been assessed associated with the iron nutrition of plants, including strawberry.
Strawberry is a hybrid species cultivated worldwide for its fruit, grown in crop fields or in hydroponics under greenhouse conditions. In both situations, profitable strawberry production requires careful attention to many cultural practices, including nutrient management (Trejo-Téllez and Gómez-Merino 2014). The iron deficiency in strawberry plants is observed in the younger leaves, starting as internerval chlorosis and then turning the whole leaf yellow-green, yellow-lemon and finally pale yellow. Also, phenolic compounds are frequently reported to be the main components of root exudates in response to iron deficiency (Römheld and Maschner 1986; Curie and Briat 2003; Hell and Stephan 2003; Colombo et al. 2014).
Considering these backgrounds, the working hypothesis was that G. diazotrophicus (hydroxamate producer) and A. brasilense (catechol producer) can contribute to the iron nutrition of strawberry plants through the siderophores production, according to their chemical type. Therefore, the aim of this work was to evaluate the two siderophores producing bacteria to promote the growth of strawberry plants and their contribution to iron nutrition. In particular, the growth index, leaf and root area were assessed as the expression of the plant growth promoting direct mechanism exerted by the siderophores to provide iron to the plants. Also, iron supply was observed through the greenness index, as an indirect way to assess the chlorophyll content, where iron plays an important role.
Materials and methods
Bacteria, growth conditions and inoculum preparation
Pure cultures of Gluconacetobacter diazotrophicus strain PAL5 (ATCC 49037) isolated from sugar cane roots (Cavalcante and Döbereiner 1988) and Azospirillum brasilense strain REC3 isolated from strawberry roots (Pedraza et al. 2007) were grown for 48 h at 30 °C under agitation in an orbital shaker (Vicking model Dubnoff, Argentina) at 120 rpm in LGI-P and NFb liquid media, respectively (Baldani et al. 2014).
LGI-P composition (g/l): crystallized cane sugar, 100; K2HPO4, 0.2; KH2PO4, 0.6; MgSO4·7H2O, 0.2; CaCl2·2H2O, 0.02; Na2MoO4·2H2O, 0.002; bromothymol blue (5 g/l in 0.2 M KOH), 5 ml; FeCl3·6H2O, 0.01. Distilled water to bring total solution to 1000 ml, pH 5.5.
NFb composition (g/l): malic acid, 5.0; K2HPO4, 0.5; MgSO4·7H2O, 0.2; NaCl, 0.1; CaCl2·2H2O, 0.02; micronutrient solution (CuSO4.5H2O, 0.04; ZnSO4·7H2O, 0.12; H3BO3, 1.40; Na2MoO4·2H2O, 1.0; MnSO4·H2O, 1.175. Complete volume to 1,000 ml with distilled water), 2 ml; bromothymol blue (5 g/l in 0.2 N KOH), 2 ml; FeEDTA (solution 16.4 g/l), 4 ml; vitamin solution (biotin, 10 mg; pyridoxal-HCl, 20 mg. Dissolve in hotwater bath. Complete to 100 ml with distilled water), 1 ml; KOH, 4.5 g. Distilled water to bring the final volume to 1000 ml, pH 6.5.
After incubation, the cells were centrifuged at 2000×g for 10 min and washed twice with sterile bi-distilled water pH 7.0 to remove any residual culture medium that may interfere on the plant assay. Subsequently, the cells were resuspended in sterile bi-distilled water. The bacterial concentration for plant inoculation was about 108 CFU/ml (OD560 nm 0.3) according to Delaporte-Quintana et al. (2017).
Plant material
Micropropagated plants of strawberry (Fragaria ananassa, Duch) cv. ‘Pájaro’ were used. They were obtained from the Strawberry Active Germplasm Bank, National University of Tucumán. The plantlets were tested to corroborate the absence of microbes by plating root and leaf macerates in trypticase soy agar medium (TSA) (Difco-BBL, Sparks, MD) and incubating for 120 h at 30 °C. Only the plantlets free of microbes were used for the experiments.
Plant assay
Plants were grown hydroponically in 100 ml glass container covered with aluminum foil to avoid the incidence of light on the roots. All glassware used were previously deferred with hydrochloric acid (10%), washed 3 times with sterile distilled water, and autoclaved for 20 min at 1 atm. The containers were filled with 50% Hoagland nutrient solution modified by Bussler (1972) pH 6.5 and autoclaved for 20 min at 1 atm. Variations were made in the nutrient solution according to the iron source used. When indicated, inoculations were performed with strains G. diazotrophicus PAL5 and A. brasilense REC3 by root immersion during 30 min. In Table 1 are described the 9 treatments applied according to the iron source used in the nutrient solution and their bacterial condition. The plants were maintained in the growing chamber at 24 °C, 70% relative humidity, and at 16-h photoperiod (250 μmol photons/m2/s) during 60 days till the end of the assay. When necessary the plants received sterile distilled water to maintain the nutrient solution level. During this period, the following features were evaluated: growth index, root area, root-hairs proliferation, most probable number (MPN) of diazotrophs, greenness index and total iron content. An additional set of plants received the same treatments and conditions as described above in order to use them for evaluating total soluble phenolic compounds and leaf area at 30 days from the beginning of the assay.
The experiment was performed in a 3 × 3 factorial with a completely randomized design and each treatment included 10 replicates. The bacterial factor was analyzed in 3 levels: plants without bacterial inoculation, plants inoculated with G. diazotrophicus PAL5, and plants inoculated with A. brasilense REC3. The iron factor was also analyzed in 3 levels: nutritive solution without iron, with reduced iron (Fe-EDTA Na2), and with oxidized iron (Fe2(SO4)3). Interactions between factor levels were analyzed and the corresponding assumptions for this experiment were verified.
Growth index (GI)
At 60 days, the plants were removed from the containers and the roots were washed with sterile water and dried using tissue paper. Then, they were dried in oven at 60 °C until constant weight. The GI values were calculated from the total biomass dry weight values of the plants, obtained at the beginning (B1) and at the end (B2) of the assay, according to the formula: GI = (B2 − B1)/B1.
Leaf area
The leaf area was determined at 30 days taken photographs of the central full expanded leaf from each plant of the different treatments and calculated using the digital image processing program “ImageJ 1.52a. Wayne Rasband, National Institute of Health, USA” (Schneider et al. 2012).
Root area and root-hairs proliferation
Root area was determined at 60 days by the gravimetric method described by Carley and Watson (1966). Briefly, it was performed by immersing air-dried roots of each plant in a Ca(NO3)2 saturated solution and recording the weight of Ca(NO3)2 removed from the solution. The results were expressed as g of Ca(NO3)2 adsorbed onto the roots. To observe root-hairs proliferation, root samples were washed with tap water, dried using tissue paper and then stained with 0.2% crystal violet dye for 5 min and washed with tap water. Samples were mounted on slides with 30% glycerol and observed with a microscope at 100 × (Zeiss, Germany).
MPN of diazotrophs
At the end of the assay, samples of roots were collected and processed according to Pedraza et al. (2007) to obtain the MPN in LGI-P and NFb semi-solid medium (Baldani et al. 2014), using the McCrady Table for 3 repetitions.
Total soluble phenolic compounds
Total soluble phenolic compounds were determined at 30 days in roots and shoots by a colorimetric technique according to Swain and Hillis (1959). Briefly, 1 g of fresh weigh (FW) sample was ground using ethanol as solvent for the extrusion of soluble phenolic compounds. It was left at room temperature protected from light for 48 h and after that time it was centrifuged and the supernatant was collected. The sample was prepared by adding distilled water, Folin-Ciocalteu reagent and sodium carbonate; then the absorbance was read in a spectrophotometer (SPECTRUM SP1103, China) at 760 nm of wavelength, using phenol as standard.
Greenness index
Greenness index constitutes an indirect way to assess the chlorophyll content. In strawberry, the internerval chlorosis and albino color in new leaves can express iron-deficiency (Trejo-Téllez and Gómez-Merino 2014). Greenness index was determined with a portable Chlorophyll-meter Minolta SPAD 502 (Minolta, Japan). The evaluations were carried out by triplicate every 10 days starting on the day 30 of the assays. Color was determined on the last central fully expanded leaf of plants from each treatment and the results were expressed as SPAD (Single-Photon Avalanche Diode) values.
Total iron content
Total iron content was determined at 60 days in the entire plant by atomic absorption spectrophotometry after calcination of tissues according to Estefan et al. (2013). This determination was carried out in the Laboratory of Chemistry of Agroindustrial Experimental Station Obispo Colombres, Tucumán, Argentina.
Statistical analysis
The plant assays were performed three times. Each experiment was statistically analyzed separately; however, data were combined since the results were similar. The results were statistically tested using unidirectional analysis of variance (ANOVA) with a level of significance set at α = 0.05. When differences were significant, Fisher's least significant difference (LSD) test (p ≤ 0.05) was applied. Both analyses were performed using InfoStat software version 2015 (Di Rienzo et al. 2015). To compare the simultaneous effect of the different treatments on the plant iron-nutrition, a Principal Component Analysis (PCA) was performed by using the R statistic software (RStudio Team 2015). To identify the Principal Components, Kaiser’s stopping rule and Scree test along with the percentage of variance were used. Also, to identify variables of each principal component, the broken-stick model was applied. Interpretation and evaluation of the PCA was plotted in the variables factor map and individuals factor map by using the FactoMineR package of R statistic software (RStudio Team 2015).
Results
The results were obtained in two periods of time from the beginning of the assay: 30 days (total soluble phenols and leaf area) and 60 days (growth index, root area, root-hair proliferation, total iron-content and greenness index). These last features were evaluated until day 60 as from day 40 most of the phenotypic changes of the plants in relation to iron deficiency were observed. In both cases, 30 and 60 days after applying the treatments and making the determinations, the plants were in a vegetative stage.
Strawberry plant growth promotion
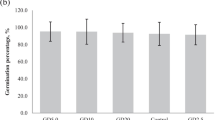
The highest growth index (GI) values were observed when Fe(II) was applied (8.55 ± 0.03 a; p ≤ 0.05) and when plants were inoculated with G. diazotrophicus strain PAL5 (Fe(II) + PAL5; 8.85 ± 0.22 a; p ≤ 0.05) and A. brasilense REC3 (Fe(II) + REC3; 8.78 ± 0.20 a; p ≤ 0.05), and when iron was oxidized but plants were inoculated with strain PAL5 (Fe(III) + PAL5; 8.42 ± 0.10 a; p ≤ 0.05). The lowest GI values were observed when iron was not included in the nutrient solution (WIA; 5.90 ± 0.05 c; p ≤ 0.05) and when the plants were inoculated with strain REC3 (REC3; 5.99 ± 0.11 c; p ≤ 0.05) in the same condition of the nutrient solution (Fig. 1).
Growth index (GI) of strawberry plants grown hydroponically during 60 days. Treatments: WIA nutrient solution without iron addition; PAL5 nutrient solution without iron addition and inoculated with Gluconacetobacter diazotrophicus PAL5; REC3 nutrient solution without iron addition and inoculated with Azospirillum brasilense REC3; Fe(II) complete nutrient solution, including 6.4 mM Fe-EDTA Na2 (reduced Fe); Fe(II) + PAL5 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with PAL5; Fe(II) + REC3 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with REC3; Fe(III) nutrient solution containing 6.4 mM Fe2(SO4)3 (oxidized Fe); Fe(III) + PAL5 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with PAL5; Fe(III) + REC3 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with REC3. Data represent mean values ± SD of three independent experiments. Similar letters did not differ significantly according to the Fisher's least significant difference (LSD) test with a p-value ≤ 0.05
Leaf area
Leaf area is routinely measured in experiments where some physiological phenomenon such as photosynthesis, respiration, plant water consumption or transpiration is being studied. Due to having a shallow root system and high leaf area, strawberry needs a high water supply and is sensitive to water deficit (Hancock 1999). Considering the hydroponic condition in this experiment, where water was not a limitation, leaf area was recorded up to 30 days from the beginning of the assay. As result, the leaf area showed higher values in the treatment with reduced iron and inoculated with A. brasilense strain REC3 (Fe(II) + REC3; 17.96 ± 3.03 a; p ≤ 0.05) than in all the other treatments. For example, without iron addition (WIA; 4.94 ± 3.55 d; p ≤ 0.05) and inoculated with G. diazotrophicus strain PAL5 (PAL5; 5.15 ± 0.48 d; p ≤ 0.05); in treatments with oxidized iron (Fe(III); 6.31 ± 2.12 d; p ≤ 0.05) and inoculated with A. brasilense REC3 (Fe(III) + REC3; 7.39 ± 0.69 d; p ≤ 0.05) (Fig. 2a). Representative examples of the leaves area for each of the treatments applied are shown in Fig. 2b.
Leaf area of strawberry plants grown hydroponically during 30 days. a Leaf area expressed in cm2. Treatments: WIA, nutrient solution without iron addition; PAL5 nutrient solution without iron addition and inoculated with Gluconacetobacter diazotrophicus PAL5; REC3 nutrient solution without iron addition and inoculated with Azospirillum brasilense REC3; Fe(II) complete nutrient solution, including 6.4 mM Fe-EDTA Na2 (reduced Fe); Fe(II) + PAL5 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with PAL5; Fe(II) + REC3 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with REC3; Fe(III) nutrient solution containing 6.4 mM Fe2(SO4)3 (oxidized Fe); Fe(III) + PAL5 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with PAL5; Fe(III) + REC3 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with REC3. Data represent mean values ± SD of three independent experiments. Similar letters did not differ significantly according to the Fisher's least significant difference (LSD) test with a p-value ≤ 0.05. b Photographs of representative leaves from the different treatments. Bar = 1 cm
Root area and root-hairs proliferation
The analysis of the root area by the gravimetric method of Carley and Watson (1966) showed the highest values in plants from treatments with unavailable iron and inoculated with G. diazotrophicus PAL5 by root immersion in 20 ml of a suspension containing about 108 CFU/ml (Fe(III) + PAL5; 0.29 ± 0.02 a; p ≤ 0.05) and in plants from treatments with available iron in nutrient solution; however there was no statistical differences between uninoculated (Fe(II); 0.29 ± 0.02 a; p ≤ 0.05) and inoculated plants (Fe(II) + PAL5; 0.30 ± 0.02 a; Fe(II) + REC3; 0.30 ± 0.02 a; p ≤ 0.05) (Fig. 3a). Uninoculated plants from the treatment with nutrient solution without iron addition showed the lowest root area values (WIA; 0.16 ± 0.01 e; p ≤ 0.05). Nevertheless, when plants were grown without the addition of iron in the nutrient solution, but inoculated (PAL5 and REC3), the root area increased (0.27 ± 0.01 b and 0.23 ± 0.02 d; p ≤ 0.05). This effect was supported by the microscopic observation of the root hairs proliferation, showing that the treatments inoculated with G. diazotrophicus PAL5 and A. brasilense REC3 had a greater proliferation of them (Fig. 3b).
Root area and root hairs proliferation. a Root area assessed by the gravimetric method of Carley and Watson (1966). Treatments: WIA nutrient solution without iron addition; PAL5 nutrient solution without iron addition and inoculated with Gluconacetobacter diazotrophicus PAL5; REC3 nutrient solution without iron addition and inoculated with Azospirillum brasilense REC3; Fe(II) complete nutrient solution, including 6.4 mM Fe-EDTA Na2 (reduced Fe); Fe(II) + PAL5 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with PAL5; Fe(II) + REC3 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with REC3; Fe(III) nutrient solution containing 6.4 mM Fe2(SO4)3 (oxidized Fe); Fe(III) + PAL5 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with PAL5; Fe(III) + REC3 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with REC3. Data represent mean values ± SD of three independent experiments. Similar letters did not differ significantly according to the Fisher's least significant difference (LSD) test with a p-value ≤ 0.05. b Photographs of representative observations (100X) of root hairs proliferation according to the indicated treatments. Bar = 250 µm
MPN of diazotrophs in strawberry plants
The MPN of diazotrophs associated with the roots at the end of the assay are shown in Fig. 4. When iron was not added to the nutrient solution, the MPN of diazotrophs corresponded to log 6.56/g fresh root in plants inoculated with G. diazotrophicus PAL5 (PAL5) and log 7.39/g fresh root in plants inoculated with A. brasilense REC3 (REC3). No diazotroph was found in plants without bacterial inoculation (WIA). In reduced iron condition, the MPN of diazotrophs were log 7.09/g fresh root in plants inoculated with G. diazotrophicus PAL5 (Fe(II) + PAL5), and log 5.57/g fresh root in plants inoculated with A. brasilense REC3 (Fe(II) + REC3), while no diazotroph was found in plants without bacterial inoculation (Fe(II)). Finally, in oxidized iron condition, the MPN of diazotrophs were log 5.87/g fresh root in plants inoculated with G. diazotrophicus PAL5 (Fe(III) + PAL5), and log 7.01/g fresh root in plants inoculated with A. brasilense REC3 (Fe(III) + REC3), while no diazotroph was found in plants without bacterial inoculation (Fe(III)).
Most probable number of diazotrophs from strawberry roots obtained by maceration of fresh material. Treatments: WIA nutrient solution without iron addition; PAL5 nutrient solution without iron addition and inoculated with Gluconacetobacter diazotrophicus PAL5; REC3 nutrient solution without iron addition and inoculated with Azospirillum brasilense REC3; Fe(II) complete nutrient solution, including 6.4 mM Fe-EDTA Na2 (reduced Fe); Fe(II) + PAL5 complete nutrient solution, including 6.4 mM of Fe-EDTA Na2 and inoculated with PAL5; Fe(II) + REC3 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with REC3; Fe(III) nutrient solution containing 6.4 mM Fe2(SO4)3 (oxidized Fe); Fe(III) + PAL5 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with PAL5; Fe(III) + REC3 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with REC3
Total soluble phenolic compounds
Total soluble phenols content was evaluated up to 30 days after the beginning of the assay according to previous determinations in strawberry by Tortora et al. (2011); they observed differences in the content during the first week of inoculation with A. brasilense REC3. However, this period was also considered based on results obtained by Jin et al. (2007) and by El-Baz et al. (2004) who evaluated the content of total soluble phenols in red clover and cucumber plants, respectively, in a period of about 25 days.
In this work, the total soluble phenolic compounds showed higher values in roots than in shoots (Fig. 5). Values in roots were higher in uninoculated plants when iron was absent in the nutrient solution (WIA; 33.22 ± 6.43 A; p ≤ 0.05) and in the plants grown with oxidized iron (Fe(III); 27.05 ± 2.69 B; p ≤ 0.05) than in plants grown in nutrient solution without iron addition and inoculated with A. brasilense REC3 and G. diazotrophicus PAL5 (REC3; 5.38 ± 1.75 EF; PAL5; 3.89 ± 1.15 F; p ≤ 0.05).
Total soluble phenolic compounds (Phenols µmol/g FW) assessed in root and shoot of strawberry plants grown hydroponically during 30 days. Treatments: WIA nutrient solution without iron addition; PAL5, nutrient solution without iron addition and inoculated with Gluconacetobacter diazotrophicus PAL5; REC3 nutrient solution without iron addition and inoculated with Azospirillum brasilense REC3; Fe(II) complete nutrient solution, including 6.4 mM of Fe-EDTA Na2 (reduced Fe); Fe(II) + PAL5 complete nutrient solution, including 6.4 mM of Fe-EDTA Na2 and inoculated with PAL5; Fe(II) + REC3 complete nutrient solution, including 6.4 mM of Fe-EDTA Na2 and inoculated with REC3; Fe(III) nutrient solution containing 6.4 mM Fe2(SO4)3 (oxidized Fe); Fe(III) + PAL5 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with PAL5; Fe(III) + REC3 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with REC3. Data represent mean values ± SD of three independent experiments. Similar letters did not differ significantly according to the Fisher's least significant difference (LSD) test with a p-value ≤ 0.05
The total soluble phenolic compounds in shoots were higher in uninoculated plants grown with oxidized iron (Fe(III); 15.61 ± 3.29 a; p ≤ 0.05) and grown without the addition of iron (WIA; 9.85 ± 0.81 b; p ≤ 0.05) than in uninoculated plants grown with reduced iron (Fe(II); 4.83 ± 1.52 d; p ≤ 0.05), in plants inoculated with G. diazotrophicus PAL5 grown with reduced iron (Fe(II) + PAL5; 5.25 ± 0.72 d; p ≤ 0.05), in plants inoculated with G. diazotrophicus PAL5 grown with oxidized iron (Fe(III) + PAL5; 4.71 ± 1 d; p ≤ 0.05), and in plants inoculated with A. brasilense REC3 grown with oxidized iron (Fe(III) + REC3; 5.81 ± 1.94 d; p ≤ 0.05).
Greenness index and iron-content in strawberry plants
The greenness index (expressed as SPAD values) was recorded after 30, 40, 50 and 60 days from the beginning of the assay. At 30 days, the SPAD values were similar among the treatments and did not show significant differences. At 60 days, the differences of SPAD values depended on the iron condition in the nutrient solution and whether the plants were inoculated or not (Fig. 6). The lowest values were obtained in leaves from plants grown without iron addition (WIA; 19.48 ± 3.53 I; p ≤ 0.05), corresponding to a yellow-green color due to the internerval chlorosis as observed from the day 40 of growth. However, the SPAD values were higher in plants grown without iron addition inoculated with G. diazotrophicus PAL5 or A. brasilense REC3 than in uninoculated plants (PAL5; 31.28 ± 4.64 G; REC3; 27.49 ± 0.72 H; p ≤ 0.05). A similar situation was observed in reduced and oxidized iron conditions. Nevertheless, when oxidized iron was applied (Fe(III)), the SPAD values were higher than those observed in plants without receiving iron (WIA), and closer than those from reduced iron condition (Fe(II)). At 40 and 50 days, the greenness index values were similar to the ones obtained on day 60 (data not shown).
Greenness index of strawberry plants grown hydroponically during 60 days. It was recorded after 30, 40, 50 and 60 days from the beginning of the assay (40 and 50 days not shown). Treatments: WIA nutrient solution without iron addition; PAL5 nutrient solution without iron addition and inoculated with Gluconacetobacter diazotrophicus PAL5; REC3 nutrient solution without iron addition and inoculated with Azospirillum brasilense REC3; Fe(II) complete nutrient solution, including 6.4 mM Fe-EDTA Na2 (reduced Fe); Fe(II) + PAL5, complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with PAL5; Fe(II) + REC3, complete nutrient solution, including 6.4 mM of Fe-EDTA Na2 and inoculated with REC3; Fe(III) nutrient solution containing 6.4 mM Fe2(SO4)3 (oxidized Fe); Fe(III) + PAL5 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with PAL5; Fe(III) + REC3 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with REC3. Data represent mean values ± SD of three independent experiments. Similar letters did not differ significantly according to the Fisher's least significant difference (LSD) test with a p-value ≤ 0.05
At the end of the assay (60 days) the total iron content, including roots and shoots, was assessed. The highest values were observed in reduced iron condition (Fe(II)), especially when plants were inoculated with the strains PAL5 and REC3 (Fe(II) + PAL5 and Fe(II) + REC3) (Table 2). On the contrary, the lowest values were found when no iron was added (WIA). Even when iron was not added to the nutrient solution, plants had more iron in tissues when inoculated with bacteria (PAL5 or REC3). The iron content increase (%) due to inoculation with PAL5 or REC3 was higher in treatments without iron (41.10 and 60.57%, respectively) than in treatments with reduced iron (9.80 and 10.58%) or with oxidized iron (2.28 and 4.38%).
Principal component analysis
The principal component analysis (PCA) showed that, following Kaiser’s stopping rule and Scree test, the first principal component (PC1) explained 90.68% of the total variability and the second principal component (PC2) explained 5.99%. The broken-stick model applied considered variables with equal or greater values than 2/3 of the highest variable value within each principal component. Therefore, PC1 included data on growth index, root area, total iron-content and greenness index, assessed at the end of the assay (60 days), shown in the variables factor map (Fig. 7a). In this analysis, all evaluated variables increases towards the positive values of PC1 (X-axis).
Principal component analysis (PCA). a PCA according to the variables factor map of growth index, root area, greenness index and iron content. Treatments: WIA, nutrient solution without iron addition; PAL5 nutrient solution without iron addition and inoculated with Gluconacetobacter diazotrophicus PAL5; REC3 nutrient solution without iron addition and inoculated with Azospirillum brasilense REC3; Fe(II) complete nutrient solution, including 6.4 mM of Fe-EDTA Na2 (reduced Fe); Fe(II) + PAL5 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with PAL5; Fe(II) + REC3 complete nutrient solution, including 6.4 mM Fe-EDTA Na2 and inoculated with REC3; Fe(III), nutrient solution containing 6.4 mM Fe2(SO4)3 (oxidized Fe); Fe(III) + PAL5 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with PAL5; Fe(III) + REC3 nutrient solution containing 6.4 mM Fe2(SO4)3 and inoculated with REC3. b Simultaneous PCA of the different treatments, indicated above, according to the individual factor map. The black arrow indicates the way of increasing of the parameters showed in the right corner. PC principal component
When comparing simultaneously through PCA the effect of the different treatments on the plants´ iron-nutrition, two groups were observed in the individuals factor map (Fig. 7b). This was plotted from PCA, according to the coordinates of the variables by using the FactoMineR package of R statistic software, and the two groups were formed by the proximity between individuals (treatments) considering the PC1 (X-axis) as follows: Low component values (red triangle spot shape), including plants grown in nutrient solution without iron addition inoculated and control without inoculation, and high component values (green circle spot shape), for plants receiving reduced and oxidized iron, inoculated and controls without bacterial inoculation. The total soluble phenolic compounds and leaf area were not included in the PCA because those parameters were measured at 30 days.
Discussion
Rhizosphere microorganisms, especially PGPB, play a critical role in providing plants with appropriate amounts of micronutrients, such as iron (de-Santiago et al. 2013; Pii et al. 2015) by mean of their siderophores as a mechanism for plant growth promotion (Kloepper et al. 1980; Gamalero and Glick 2011; Siebner-Freibach et al. 2003; Verma et al. 2011). In this work we used two PGPB able to produce siderophores: the wild type strain G. diazotrophicus PAL5 (Logeshwaran et al. 2009) and the strain A. brasilense REC3 (Pedraza et al. 2007) to test whether they were able to increase the iron content of the strawberry plants and to improve the total plant growth. In this approach different features were determined, such as the growth index, the root hairs proliferation, the greenness index and the iron content. This was also performed by considering the role of the chemical group of siderophores.
Growth index of strawberry plants hydroponically grown
In terms of iron-availability, the growth index (GI) values were improved in plants receiving reduced iron (Fe-EDTA Na2) and in plants receiving oxidized iron (Fe2(SO4)3) and inoculated with strain PAL5. Although the data did not show statistically significant differences between uninoculated and inoculated plants grown with reduced iron, the GI values were higher when strain PAL5 and REC3 were associated with them. This fact is possible due to the enhanced availability of iron in the nutrient solution to support the plant growth, but without the participation of siderophores as they are produced only when iron is limited (Miethke and Marahiel 2007; Neilands 1995). However, some other PGPB mechanisms may have participated in the association. In the case of plants receiving oxidized iron, the inoculation with strains PAL5 and REC3 improved GI values compared to uninoculated plants. This positive effect may be due to the presence of the siderophores produced by both bacteria to capture the oxidized iron (Fe(III)). Furthermore, plants treated with PAL5 showed similar GI values to the uninoculated plants receiving reduced iron. This is supported by the chemical group, considering that G. diazotrophicus PAL5 produces hydroxamate-type siderophores, which are hydrophilic (Logeshwaran et al. 2009; Crosa and Walsh 2002), while A. brasilense REC3 produces catechol-type siderophores (Tortora et al. 2011), that are generally hydrophobic, remain associated with the cell surface, are highly unstable and easily oxidized (Neilands 1973). The results observed herein may respond to the characteristics of these two chemical groups of siderophores, being more efficient the hydroxamate-type in iron chelation to support strawberry plant nutrition under hydroponic growth conditions.
The lowest GI values were obtained when iron was not added to the nutrient solution. However, it is possible to note that plants inoculated with strains PAL5 or REC3 had higher values than those without bacterial inoculation. This may be due to the accumulated iron in plant tissues during the in vitro growth period and to the siderophores production, stimulated by low quantities of iron present in the nutrient solution. These iron traces were included in several chemical reagents (indicated on their labels) used in the formulation of the Hoagland nutrient solution and were less than 10 ppm. Additionally, the fact that the values of GI of the plants grown in the presence of oxidized iron and without inoculation (Fe(III)) overcame the values of the control plants grown in nutrient solution without iron addition (WIA), could also be linked to the rhizosphere acidification as a mechanism that releases protons and helps to reduce the iron present in the solution by strawberry plants (Pii et al. 2016; Delaporte-Quintana et al. 2017). The reduction of the rhizosphere’s pH is an important adaptation mechanism adopted by the dicotyledonous and non-grassed monocotyledons (plants of Strategy I) to solubilize the scarcely available sources of iron (Colombo et al. 2014). Considering that strawberry belongs to this group, it is worth mentioning that other plants such as red clover and cucumber plants also respond to iron deficiency by inducing the ferric chelate reductase anchored in the plasma membrane of the epidermal cell and also through the stimulation of the proton pump of this membrane to increase the exudation of protons (Robinson et al. 1999; Pii et al. 2016). Even so, the values of the treatment with oxidized iron, without inoculation, were slightly lower than those of the treatment with reduced iron.
Leaf area, root area and MPN of diazotrophs
The leaf area assessed in the assay revealed that the plants treated with reduced iron in the nutrient solution had larger leaves than plants exposed to other treatments. This may be due to the role of iron in chlorophyll production and other vital functions of the plant (Abadía and Abadía 1993). Similar results were obtained by Valentinuzzi et al. (2015) when comparing leaves from strawberry plants grown with reduced iron and without iron addition. In the present work there was not found a direct relationship between the production of biomass, expressed as GI and the leaf area, with respect to iron intake. Perhaps, the foliar area is not a good parameter that reflects the contribution of iron via siderophores. Although iron, as a micronutrient, participates in many biochemical processes, it is not a structural element of plants (Hopkins 1999).
When evaluating the root area, no significant differences were observed among the treatments with reduced iron in the nutrient solution (Fe(II); Fe(II) + PAL5; and Fe(II) + REC3). Probably this effect was due to the hydroponic condition used in the bioassay, which allows the roots to be in direct contact with all the nutrients in solution, and the plants do not require the root expansion to look for nutrients.
In the nutrient solution not supplemented with iron, the values in plants inoculated with bacteria exceeded those of plants without inoculation, being PAL5 greater than REC3. This allowed us to infer the contribution of iron via siderophores, especially in PAL5, stimulated by iron traces of the nutrient solution, in addition to the possible acidification effect of the rhizosphere. Actually, when oxidized iron was included in the nutrient solution, high root surface values were observed only in plants inoculated with PAL5 (hydroxamates producing bacterium). In a qualitative form, a greater proliferation of root hairs was also observed in the treatments with bacterial inoculation. This would be associated with the capacity to produce auxins by G. diazotrophicus PAL5 and A. brasilense REC3 (Fuentes-Ramírez et al. 1993; Pedraza et al. 2004, 2010). These observations are supported by the MPN of diazotrophs determined at the end of the assay (positive only in inoculated plants) that allowed us to infer that both bacterial strains were associated with the roots of strawberry plants in hydroponics. The bacterial association with strawberry roots in hydroponics was also observed by scanning electron microscopy by Grillo-Puertas et al. (2018) for strain PAL5 and by Guerrero-Molina et al. (2015) for strain REC3. In the treatments where the effect of siderophores was detected (WIA and Fe(III)), the MPN was higher in the plants inoculated with REC3 than in those inoculated with PAL5. Possibly the catechols would stimulate the greater growth of REC3, since they are hydrophobic and remain associated with bacteria, while the hydrophilic hydroxamates would diffuse more in the nutrient solution. This would also be supported by the fact that the role of siderophores is primarily to chelate iron in the environment and make them available to microbial cells (Bellenger et al. 2008; Braud et al. 2009a, b).
Total soluble phenolic compounds as response to iron deficiency
The total soluble phenolic compounds, measured at 30 days from the beginning of the assay, showed more quantity of them in the treatment without iron addition and uninoculated plants. This was sustained by the fact that under biotic and abiotic stress, an increase in the content of phenolics in plant tissues and in root exudates can occur, as observed by Cesco et al. (2010) in maize, soybean, alfalfa, white lupin, and tomato, among others. However, in plants grown without iron supplementation in the nutrient solution, but inoculated with G. diazotrophicus PAL5 or A. brasilense REC3, the phenolic content decreased. It has been reported that the nutritional stress for iron could be reduced by the siderophores secreted by the bacteria used (Logeshwaran et al. 2009; Tortora et al. 2011) that provide them with the iron from the traces present in the Hoagland solution. When plants were supplemented with oxidized iron, the phenolic content was similar than those when iron was not added to the nutrient solution. This may be due to the unavailable iron source, as also observed in several plant species that release phenolics from roots under iron deficiency (Mimmo et al. 2014). However, when plants were inoculated with bacteria in the presence of oxidized iron, the phenolics decreased due to the participation of siderophores. In treatments with reduced iron, an available form of this micronutrient, the release of phenolic compounds was reduced. Even though, when plants were inoculated with the bacteria, the total soluble phenolic compounds increased. This is probably due to the biotic interaction established between strawberry roots and bacteria, which can increase the amount of phenolic compounds, as was also observed by Cesco et al. (2010). Furthermore, this increase was reported by Tortora et al. (2012) in strawberry plants inoculated with A. brasilense REC3 and grown without iron limitation; therefore, without the participation of siderophores. It has been suggested that phenolics could contribute to the plant iron acquisition by (a) mediating the mobilization of root apoplastic iron; (b) by improving iron solubility in the rhizosphere, mainly due to their reducing and chelating properties; and (c) by their allelopathic activity influencing the rhizosphere microbial communities to produce siderophores and auxins (Jin et al. 2007).
Greenness index, iron content and principal component analysis
To assess the symptoms of iron deficiency and iron sufficiency in strawberry plants, the greenness index was recorded after 30, 40, 50 and 60 days from the beginning of the assay. At 60 days the lowest values were observed in plants where iron was not applied (WIA). They showed a pale yellow color on the leaves, indicating a symptom of iron deficiency. A marked internerval chlorosis in young leaves of strawberry was also observed by Kirschbaum and Borquez (2006). It is known that the scarcity of iron causes a decrease in the photosynthetic pigments (chlorophyll and some carotenoids) on the leaves (Abadía and Abadía 1993). This metal deficiency was reflected in all the evaluated parameters herein. Symptoms of iron deficiency in strawberry plants appeared at 40 days from the beginning of the assay, and are similar to those reported by Pestana et al. (2012) and Valentinuzzi et al. (2015). The highest greenness values were observed in plants from treatments with reduced iron an inoculated with bacteria (Fe(II) + PAL5 and Fe(II) + REC3), and with oxidized iron and inoculated with strain PAL5 (Fe(III) + PAL5). These values indicate that there was no iron deficiency in these plant growing conditions.
The iron content in the plant tissues determined at the end of the assay resulted to be in line with those of the greenness index. The values of iron taken as reference for strawberry plants to indicate deficiency of this element are between 5 and 40 ppm; conversely 50–3000 ppm indicates iron sufficiency (Ulrich et al. 1980). Values below 50 ppm were observed in the treatment without the addition of iron (WIA), corresponding to the initial amount of iron in the plants; but they were above 50 ppm when plants were inoculated with G. diazotrophicus PAL5 and A. brasilense REC3. In these last cases, the participation of the siderophores was implicated. When reduced and oxidized iron was included in the nutrient solution, values ranged from 72 to 81 ppm, being higher when plants were inoculated. However, it can be assumed that the participation of siderophores was possible only in the presence of oxidized iron in the nutrient solution, since when there is supplementation with reduced iron, an easily available form of iron, the siderophores are not produced (Neilands 1995). Besides, according to the iron values of plants inoculated with bacteria and where the participation of siderophores occurred (without iron and with oxidized iron in the nutrient solution), the hydroxamates (produced by G. diazotrophicus PAL5) contributed with a greater amount of iron than catechols (produced by A. brasilense REC3). The latter could be possible as catechols are highly unstable and easily oxidized (Neilands 1973). Besides the contribution of both strains in the iron nutrition of strawberry plants, mediated by the siderophores, the participation of other processes can also be considered, such as the acidification of the rhizosphere, as previously observed for phosphorus nutrition in strawberry plants by Delaporte-Quintana et al. (2017), and the secretion of phenolic compounds (Jin et al. 2007). Additionally, the siderophores effect exerted by G. diazotrophicus PAL5 and A. brasilense REC3 was not only observed on the iron amount in strawberry plant tissues, but also in the biomass production (growth index), root area, and greenness index (SPAD values) after the PCA.
Conclusions
In this work we show that the siderophores produced by the plant growth promoting bacteria G. diazotrophicus PAL5 and A. brasilense REC3 can contribute to the iron nutrition in strawberry plants grown in hydroponics. This was reflected by the growth index, root area, greenness index, and iron content in the plant tissues. It was also observed that the participation of the hydroxamates was better than that of the catechols in the provision of iron to the plants.
In spite of the present results were obtained under controlled environmental conditions, in agriculture, the acquisition of iron by PGPB’ siderophores will be influenced by soil properties (i.e., organic matter content, pH, and texture). It is known that the iron content of many soils is much higher than the amount needed by crops; however, the problem lies in the poor solubility of the ionic forms of iron. Thus, additional studies should be carried out in field conditions to evaluate whether G. diazotrophicus and A. brasilense contribute, not only to the availability of iron, but also to the development of strawberry roots to improve the uptake of water and others soil nutrients.
References
Abadía J, Abadía A (1993) Iron and plant pigments. In: Barton LL, Hemming BC (eds) Iron chelation in plants and soil microorganisms. Academic Press, New York, pp 327–343
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Bashan Y, de Bashan LE (2005) Plant growth-promoting. In: Hillel D (ed) Encyclopedia of soils in the environment, 1st edn. Elsevier, Oxford, pp 103–115
Bashan Y, de Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. In: Sparks DL (ed) Advances in agronomy. Elsevier, San Diego, pp 77–136
Bashan Y, Holguin G (1998) Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol Biochem 30:1225–1228
Bellenger JP, Wichard T, Kustka AB, Kraepiel AML (2008) Uptake of molybdenum and vanadium by a nitrogen-fixing soil bacterium using siderophores. Nat Geosci 1:243
Bienfait HF (1988) Mechanisms in Fe-efficiency reactions of higher plants. J Plant Nutr 11(6–11):605–629. https://doi.org/10.1038/ngeo161
Braud A, Hoegy F, Jezequel K, Lebeau T, Schalk IJ (2009a) New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environ Microbiol 11(5):1079–1091. https://doi.org/10.1111/j.1462-2920.2008.01838.x
Braud A, Jézéquel K, Bazot S, Lebeau T (2009b) Enhanced phytoextraction of an agricultural Cr-and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74(2):280–286. https://doi.org/10.1016/j.chemosphere.2008.09.013
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20(1):33–40. https://doi.org/10.1016/j.tplants.2014.07.005
Budzikiewicz H (2010) Microbial siderophores. In: Kinghorn A, Falk H, Kobayashi J (eds) Fortschritte der Chemie organischer Naturstoffe (Progress in the chemistry of organic natural products). Springer, Vienna, pp 1–75
Bussler W, Epstein E (1972) Mineral nutrition of plants: principles and perspectives. J Plant Nutr Soil Sci 132:158–159. https://doi.org/10.1002/jpln.19721320211
Carley HE, Watson RD (1966) A new gravimetric method for estimating root-surface areas. Soil Sci 102(5):289–291
Cavalcante VA, Döbereiner J (1988) A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil 108(1):23–31. https://doi.org/10.1007/BF02370096
Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L (2010) Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329(1–2):1–25. https://doi.org/10.1007/s11104-009-0266-9
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biotechnol 25(11):1919–1928. https://doi.org/10.1007/s11274-009-0090-7
Chu BC, Garcia-Herrero A, Johanson TH, Krewulak KD, Lau CK, Peacock RS, Vogel HJ (2010) Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 23:601–611. https://doi.org/10.1007/s10534-010-9361-x
Colombo C, Palumbo G, He JZ, Pinton R, Cesco S (2014) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments 14(3):538–548. https://doi.org/10.1007/s11368-013-0814-z
Crosa JH, Walsh CT (2002) Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol R 66(2):223–249. https://doi.org/10.1128/MMBR.66.2.223-249.2002
Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54(1):183–206
Delaporte-Quintana P, Grillo-Puertas M, Lovaisa NC, Teixeira KR, Rapisarda VA, Pedraza RO (2017) Contribution of Gluconacetobacter diazotrophicus to phosphorus nutrition in strawberry plants. Plant Soil 419(1–2):335–347
de Santiago A, García-López AM, Quintero JM, Avilés M, Delgado A (2013) Effect of Trichoderma asperellum strain T34 and glucose addition on iron nutrition in cucumber grown on calcareous soils. Soil Biol Biochem 57:598–605. https://doi.org/10.1016/j.soilbio.2012.06.02020
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2015) InfoStat versión 2015. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. https://www.infostat.com.ar. Accessed 26 April 2019
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32(12):1682–1694. https://doi.org/10.1111/j.1365-3040.2009.02028.x
El-Baz FK, Mohamed AA, Aboul-Enein AM, Salama ZA (2004) Alteration in root exudates level during Fe-deficiency in two cucumber cultivars. Int J Agric Biol 6:45–48
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. In: A manual for the West Asia and North Africa region, pp 170–176
Fuentes-Ramírez L, Jimenez-Salgado T, Abarca-Ocampo IR, Caballero-Mellado J (1993) Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil 154(2):145–150. https://doi.org/10.1007/BF00012519
Gamalero E, Glick BR (2011) Mechanisms used by plant growth-promoting bacteria. Bacteria in agrobiology: plant nutrient management. Springer, Berlin, Heidelberg, pp 17–46
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica. https://doi.org/10.6064/2012/963401
Grillo-Puertas M, Delaporte-Quintana P, Pedraza RO, Rapisarda VA (2018) Intracellular polyphosphate levels in Gluconacetobacter diazotrophicus affect tolerance to abiotic stressors and biofilm formation. Microbes Environ 33(4):440–445
Guerrero-Molina MF, Lovaisa NC, Salazar SM, Martínez-Zamora MG, Díaz-Ricci JC, Pedraza RO (2015) Physiological, structural and molecular traits activated in strawberry plants after inoculation with the plant growth-promoting bacterium Azospirillum brasilense REC 3. Plant Biol 17(3):766–773. https://doi.org/10.1111/plb.12270
Gupta C, Durbey R, Maheshwari D (2002) Plant growth enhancement and suppression of Macrophomina phaseolina causing charcoal rot of peanut by fluorescent Pseudomonas. Biol Fert Soils 35(6):399–405
Hancock JF (1999) Strawberries. CABI Publishing, New York, p 237
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216(4):541–551. https://doi.org/10.1007/s00425-002-0920-4
Hochmuth G (2011) Iron (Fe) nutrition of plants. Technical report, IFAS Extension Service, University of Florida, pp 1–7
Hopkins WG (1999) Introduction to plant physiology, 2nd edn. Wiley, New York, pp 61–76
Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144(1):278–285
Kirschbaum DS, Borquez AM (2006) Nutrición mineral de la frutilla (Fragaria x ananassa Duch.). III simpósio nacional de morango. II encontro sobre pequenas frutas e frutas nativas do Mercosur. Palestras 3:118–127
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286(5776):885
Kumar BD (1999) Fusarial wilt suppression and crop improvement through two rhizobacterial strains in chick pea growing in soils infested with Fusarium oxysporum f. sp. ciceris. Biol Fert Soils 29(1):87–91
Logeshwaran P, Thangaraju M, Rajasundari K (2009) Hydroxamate siderophores of endophytic bacteria Gluconacetobacter diazotrophicus isolated from sugarcane roots. Aust J Basic Appl Sci 3:3564–3567
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol R 71(3):413–451
Mimmo T, Del Buono D, Terzano R, Tomasi N, Vigani G, Crecchio C, Cesco S (2014) Rhizospheric organic compounds in the soil–microorganism–plant system: their role in iron availability. Eur J Soil Sci 65(5):629–642
Neilands JB (1973) Microbial iron transport compounds (siderochromes). In: Eichorn GL (ed) Inorganic biochemistry, vol 1. Elsevier Scientific Publishing Co., Amsterdam, pp 167–202
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270(45):26723–26726
Pedraza RO (2016) Acetic acid bacteria as plant growth promoters. In: Matsushita K, Toyama H, Tonouchi N, Okamoto-Kainuma A (eds) Acetic acid bacteria. Springer, Tokyo, pp 101–120
Pedraza RO, Ramírez-Mata A, Xiqui ML, Baca BE (2004) Aromatic amino acid aminotransferase activity and indole-3-acetic acid production by associative nitrogen-fixing bacteria. FEMS Microbiol Lett 233(1):15–21
Pedraza RO, Motok J, Tortora ML, Salazar SM, Díaz-Ricci JC (2007) Natural occurrence of Azospirillum brasilense in strawberry plants. Plant Soil 295(1–2):169–178
Pedraza RO, Motok J, Salazar SM, Ragout AL, Mentel MI, Tortora ML, Díaz-Ricci JC (2010) Growth-promotion of strawberry plants inoculated with Azospirillum brasilense. World J Microbiol Biotechnol 26(2):265–272
Pestana M, Correia PJ, Saavedra T, Gama F, Abadía A, de Varennes A (2012) Development and recovery of iron deficiency by iron resupply to roots or leaves of strawberry plants. Plant Physiol Biochem 53:1–5
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. Rev Biol Fert Soils 51(4):403–415
Pii Y, Borruso L, Brusetti L, Crecchio C, Cesco S, Mimmo T (2016) The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol Biochem 99:39–48
Reis VM, Teixeira KRdS (2015) Nitrogen fixing bacteria in the family Acetobacteraceae and their role in agriculture. J Basic Microbiol 55(8):931–949
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397(6721):694
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80(1):175–180
RStudio Team (2015) Integrated development for R. RStudio, Inc. Boston, MA. https://www.rstudio.com. Accessed 26 April 2019
Saxena B, Modi M, Modi VV (1986) Isolation and characterization of siderophores from Azospirillum lipoferum D-2. Microbiology 132(8):2219–2224
Sayyed RZ, Chincholkar SB (2009) Siderophore-producing Alcaligenes feacalis exhibited more biocontrol potential Vis-à-Vis chemical fungicide. Curr Microbiol 58(1):47–51
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671
Shah S, Karkhanis V, Desai A (1992) Isolation and characterization of siderophore, with antimicrobial activity, from Azospirillum lipoferum M. Curr Microbiol 25(6):347–351
Siebner-Freibach H, Hadar Y, Chen Y (2003) Siderophores sorbed on Ca-montmorillonite as an iron source for plants. Plant Soil 251(1):115–124
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric 10(1):63–68
Tapia-Hernandez A, Mascarua-Esparza MA, Caballero-Mellado J (1990) Production of bacteriocins and siderophore-like activity by Azospirillum brasilense. Microbios 64(259):73–83
Tortora ML, Díaz-Ricci JC, Pedraza RO (2011) Azospirillum brasilense siderophores with antifungal activity against Colletotrichum acutatum. Arch Microbiol 193(4):275–286
Tortora ML, Díaz-Ricci JC, Pedraza RO (2012) Protection of strawberry plants (Fragaria ananassa Duch.) against anthracnose disease induced by Azospirillum brasilense. Plant Soil 356(1–2):279–290
Trejo-Téllez LI, Gómez-Merino FC (2014) Nutrient management in strawberry: effects on yield, quality and plant health. In: Malone N (ed) Strawberries. Nova Science Publishers, Hauppauge, pp 239–267
Ulrich A, Mostafa MAE, Allen WW, Davis PA (1980) Strawberry deficiency symptoms: a visual and plant analysis guide to fertilization. University of California Agriculture Sci Publication, pp 30–31
Valentinuzzi F, Pii Y, Vigani G, Lehmann M, Cesco S, Mimmo T (2015) Phosphorus and iron deficiencies induce a metabolic reprogramming and affect the exudation traits of the woody plant Fragaria× ananassa. J Exp Bot 66(20):6483–6495
Verma VC, Singh SK, Prakash S (2011) Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss J Basic Microbiol 51(5):550–556
Walker EL, Connolly EL (2008) Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr Opin Plant Biol 11(5):530–535
Zhao Q, Shen Q, Ran W, Xiao T, Xu D, Xu Y (2011) Inoculation of soil by Bacillus subtillis Y-IVI improves plant growth and colonization of the rhizosphere and interior tissues of muskmelon (Cucumis melo L.). Biol Fert Soils 47(5):507–514
Acknowledgements
This paper is dedicated to the memory of Prof. Yoav Bashan. We thank Dr. Roque Interdonato for his help in the determination of phenolic compounds. This work was supported by Secretaría de Ciencia, Arte y Tecnología, Universidad Nacional de Tucumán (Program A621). PDQ is fellow of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delaporte-Quintana, P., Lovaisa, N.C., Rapisarda, V.A. et al. The plant growth promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. Plant Growth Regul 91, 185–199 (2020). https://doi.org/10.1007/s10725-020-00598-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-020-00598-0