Abstract
Microbial-assisted biofortification has emerged as a sustainable approach to improve food security by enhancing the nutrient content as well as yield of agricultural crops. In the present study, we have examined the potential of siderophore-producing plant growth-promoting endophytic bacterial strain in improving nutrient content and vegetative growth of Spinacia oleracea (spinach) under iron-deficit conditions. A total of 234 isolates, 13 from the root, 15 from the stem, and 17 from the leaves were isolated and tested positive for siderophore production. In vitro, results related to plant growth-promoting (PGP) traits have revealed the endophyte Enterobacter quasihormaechei (NBRI FY32) isolated from Fe-deficit spinach plant exhibited multifarious PGP traits with the highest siderophore-producing ability and the only bacterial strain possessing phytase activity as well as oxalate degradation ability. Furthermore, the results from the greenhouse experiment demonstrated that, under both Fe-sufficient and -deficit conditions, inoculation with NBRI FY32 significantly improved the overall plant vegetative parameters compared to the corresponding uninoculated control. Also, NBRI FY32 inoculation has significantly enhanced nitrogen (N), phosphorus (P), potassium (K), sodium (Na), calcium (Ca), zinc (Zn), manganese (Mn), and Iron (Fe) content in plants under Fe-sufficient and -deficit conditions. In addition, NBRI FY32 showed maximum colonizing and survivability in spinach root and shoot under Fe-sufficient and -deficit conditions. Thus, the findings of this study demonstrate for the first time that siderophore-producing endophyte E. quasihormaechei (NBRI FY32) having multiple PGP attributes along with high competence and colonization can fortify spinach plant by enhancing nutrient levels in the host plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofortification of agricultural crops is one of the sustainable ways to tackle food security problems by enhancing the nutrient level in crop plants (Huang et al. 2020). In addition, biofortified crops have demonstrated a significant positive effect on human health (Praharaj et al. 2021). Among various biofortification approaches, microbe-mediated solubilization and mobilization of nutrients in the plant is recognized as one of the efficient ways to ameliorate nutrient deficiency in the human diet (Singh et al. 2017). In the past few decades, rhizospheric microorganisms have been considered to enhance the accumulation of micronutrients in different crops (Abaid-Ullah et al. 2015; Prasanna et al. 2015). However, in recent years, the focus has switched to the endophytes, which are part of the interior microbiome. Endophytic microbes reside inside the host plant tissue without causing any injury and increase the growth of plants through indirect and direct mechanisms (Rana et al. 2021). The direct mechanisms include phytohormone production such as auxin, cytokinin, and gibberellin, ACC deaminase activity and nutrient solubilization such as phosphate, nitrogen fixation, and sequestration of iron by bacterial siderophores (Adeleke et al. 2021; Lipková et al. 2021; Rana et al. 2021). The indirect mechanisms involve the prevention of the negative effects of phytopathogenic microbes by the production of siderophores, cell wall degrading enzymes, antibiotics, hydrogen cyanide, induced systemic resistance, and quorum quenching (Macedo-Raygoza et al. 2019; Lipková et al. 2021). The exploitation of siderophore-producing plant growth-promoting bacterial endophytes could prove to be an important strategy for enhancing plant growth and nutrient content in agricultural crops. Some of the bacterial genera namely Enterobacter, Bacillus, Acidovorax, Burkholderia, Variovorax, Curtobacterium, Pantoea, Pseudomonas, Agrobacterium, and Klebsiella have been reported as plant growth-promoting endophytes (Rana et al. 2021). However, the scientific community is continuously searching for new PGP and biocontrol endophytic bacterial strains in an effort to increase plant growth and lower the necessity for agrochemicals. The exploitation of PGP endophytes capable of improving plant health and enhancing nutrient content under nutrient-deficient conditions will definitely present a major path toward sustainable agriculture. Recently, several reports have well established the function of endophytic bacterial strains toward the biofortification of different crop plants (Makar et al. 2021; Verma et al. 2021; Vishwakarma et al. 2021). In addition, bacterial endophytes enhance other phyto-beneficial attributes including photosynthetic pigments, vegetative growth, osmotic regulation, modification of root architecture, and improved mineral absorption (Cochard et al. 2022; Sundram et al. 2022). Furthermore, bacterial endophytes also play a crucial role in increasing the bioactive constituents in host plants (Li et al. 2023). Furthermore, in comparison to plant growth promoting rhizobacteria (PGPR), endophytes exhibited improved adaptations against abiotic stresses, that results in enhanced plant growth (Pillay and Nowak 1997). However, certain endophytes proved to be unsuccessful under normal environmental conditions because of their inability to colonize the host system (Bagheri et al. 2021). Therefore, for the successful application of bacterial endophytes under normal as well as stressed conditions, it is a prerequisite that bacterial endophytes should demonstrate effective colonization inside the host plant (Tharek et al. 2021).
The present study aimed to isolate and characterize PGP attributes of endophytic bacteria from spinach plants under Fe-sufficient and Fe-deficit conditions. Furthermore, there are no data available on the endophyte-mediated biofortification of spinach plants under Fe-deficit conditions. Therefore, we further aimed to compare the potential application of two endophytic bacterial strains having different siderophore-producing abilities for enhancing plant growth and nutrient content under Fe-sufficient and Fe-deficit conditions. To the best of our knowledge, this is the first report illustrating multiple PGP attributes of Enterobacter quasihormaechei as an endophyte for conferring plant fitness and biofortification under Fe-deficit soil conditions.
Materials and Methods
Plant Material Preparation
A loose loam soil having a pH of 7.2 and 0.9 mg Fe kg−1 soil of DPTA-extractable Fe was used for the pot experiments. After air drying, the soil was powdered, and chemical fertilizers were applied at the recommended rate. Five Spinacia oleracea L. (Spinach) seeds were sowed per pot and soil moisture was maintained at 15% (wt/wt) with deionized water. After 16 d growth, three uniform-sized seedlings were thinned per pot. The pots were divided into two groups after 30 days of development in a growth chamber. In one pot set, the leaves were sprayed with 100 mM FeEDTA solution every other day (Fe-sufficient), whereas in the other, deionized water was used (called Fe-deficit).
Sampling and Isolation of Siderophore-Producing Bacteria
After 60 d of growth, plant material (leaf, stem, and root) was sampled from each pot, washed with tap water, and surface sterilized with 5% sodium hypochlorite for 5 min followed by washing 4–5 times with sterile distilled water. The sterile samples were diluted serially in 0.85% saline solution and the final suspension was spread on agar plates with 20 mM FeCl3 or 1% (v/v) CAS (chromeazurol S) indicator and incubated at 26–28 °C (Schwyn and Neilands 1987). The microbial colonies with an orange halo having a diameter > 5 mm were selected as siderophore secretors. Pure cultures of the endophytic bacterial isolates were maintained in 30% glycerol at − 80 °C.
Quantitative Estimation of PGP Attributes of Selected Isolates
Auxin Production
Auxin (IAA) production by selected isolates was determined according to Bric et al. (1991). 24 h old grown cultures were centrifuged at 10,000 rpm for 10 min followed by the addition of 100 μl of orthophosphoric acid (10 mM) and 4 ml of Salkowski’s reagent in 2 ml of bacterial supernatant and incubated for 30 min at room temperature. Absorbance was recorded at 530 nm.
Phosphate Solubilization
Phosphate solubilization was quantified by using NBRIP medium for the selected isolates as per the protocol of Nautiyal (1999). Briefly, 50 µl of bacterial culture (overnight grown in nutrient broth) was inoculated in test tubes containing 5 ml NBRI-BPB medium followed by incubation at 28 ± 2 °C on an incubator shaker (180 rpm) for 48 h. Finally, the cells were harvested at 10,000 rpm for 10 min by centrifugation and the supernatant was used to estimate the amount of solubilized phosphate spectrophotometrically at 600 nm (Nautiyal 1999).
Siderophore Production
Siderophore quantification was performed by cultivating overnight grown bacterial strains on sodium succinate medium (SSM) emended with 20 µM Fe at 26–28 °C in an incubator shaker and absorbance was measured at 400 nm (Meyer and Abdallah 1978; Sah and Singh 2016).
Biofilm Formation
Biofilm production was measured using a microtiter plate assay, as described by Srivastava et al. (2008). Briefly, bacterial cultures were grown in a microtiter plate, and the supernatant was removed. The plate was then washed with phosphate-buffered saline and dried. Next, the plate was stained with crystal violet, and the excess stain was removed with ethanol. The biofilm was then quantified by measuring the absorbance of the crystal violet at 590 nm.
Determination of Oxalate Degradation Activity
For estimation of oxalate degradation activity, selected strains were inoculated onto MRS (Hi Media, India) agar plates containing 50 mmol L−1 of calcium oxalate. After incubation at 28 °C for 48 h, the presence of clear areas around bacterial colonies was considered as an indication of oxalate degradation activity by the bacterial strains.
Determination of Phytase Activity
The selected bacterial endophytes were streaked on a phytase screening medium (PSM) and incubated at 28 °C for 24 h to evaluate phytase production ability. The translucent area of the plate will give a visual indication of extracellular phytase production by the bacterial strains (Kerovuo et al. 1998).
Selection and Identification of Bacterial Endophytes on the Basis of 16S rRNA Sequencing Analysis
Selected high siderophore-producing endophytic bacteria were identified based on the 16S rRNA gene sequencing analysis. For the isolation of genomic DNA, GenElute™ Bacterial Genomic DNA Kit was used. PCR was carried out in 50 μl vol containing 1X PCR buffer, 1.0 U Taq polymerase, 200 mM of each dNTP, 1.50 mM MgCl2, 10 μM of each primer (27F: 5′-AGA GTT TGA TCC TGG CTC AG-3′ and 1492R: 5′-GGT TAC CTT GTT ACG ACT T-3′), and 20‒30 ng of bacterial genomic DNA. The PCR product was purified using a QIAquick® PCR purification kit (Qiagen, Germany) followed by sequencing (3730XL DNA analyzer, Applied Biosystems, USA) (Misra et al. 2017).
Evaluation of Selected Bacterial Strains for Enhancing Vegetative Parameters and Bioavailable Content of Nutrients in Spinach Under Greenhouse Condition
Plant vegetative Parameters
Following the characterization of selected bacterial strains based on their phytase-producing and oxalate degradation ability, endophytic bacterial strains differing in siderophore producibility were evaluated for their capability of plant growth promotion using spinach as a host plant under greenhouse conditions. The experiments were carried out at the CSIR-National Botanical Research Institute, Lucknow, India (latitude/longitude 11°24'N/79°44'E), with 18 plant replicates evenly distributed in 06 pots (03 plant replicates per pot) containing 2.0 mm sieved field soil (2.0 kg soil per pot). Spinach seeds were surface sterilized using 0.1% HgCl2, followed by thorough washing with sterile distilled water and bacterized as described by Nautiyal (1997). The following were the bacterial strain-specific treatments for each host plant: T1 = full Hoagland solution (positive control); T2 = iron-free Hoagland solution (negative control); T3 = bacteria (siderophore positive) + Fe; T4 = bacteria (siderophore positive) without Fe; T5 = bacteria (siderophore negative) + Fe; T6 = bacteria (siderophore negative) without Fe. Pots were treated with standard Hoagland's solution once per week; for the remaining growth period of plants, sterile water was used. Standard Hoagland’s solution supplemented with 20 μM Fe will be used to irrigate the pots having Fe as a treatment. Soil moisture was maintained at 20% with Hoagland solution (supplemented with Fe and without Fe) in control treatments and 48 h grown bacterial culture (~ 108‒9 CFU ml−1) in treatments receiving bacterial inoculation. Plants in all the treatments were simultaneously grown and harvested at the same time. After 30 days, each treatment was used to measure shoot and root length, leaf number, and plant fresh and dry weight.
Plant Nutrient Content
Subsequently, only shoot samples of each condition were subjected to air drying for their further nutrient analysis, while due to less quantity of root samples, they could not be considered for estimation of their nutrient content. The air-dried shoot samples were ground to powder. K, P, Ca, and Na contents in the shoot were estimated using the tri-acid mixture (H2SO4: HClO4:HNO3; 1:10:4 ratio) by wet-digestion at 200 °C. Subsequently, the vanadomolybdophosphoric acid colorimetric (Evolution 201; Thermo Scientific, USA) method (Tandon 1993) was used to determine the shoot P content. Whereas, K, Na, and Ca contents in the shoot were estimated with the help of a flame photometer (FP114; Thermo Scientific, USA) using the ammonium acetate method (Hanway and Heidel 1952). N content in the shoot was quantified using Kjeldahl method (Jackson 1973) with the help of an automatic nitrogen estimation apparatus (KelPlus Classic DX VA; Pelican Equipments, Chennai, India). To measure, Fe, Mn, and Zn air-dried shoot samples (0.1 g) were finely ground and digested in 3 ml HNO3 at 120 °C for 6 h (Dixit et al. 2016). Following this, the digested samples were analyzed using an inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7500 cx, USA). Rhodium (Rh) and Stannous (Sn) were used as internal calibration standards in ICP-MS.
Root Colonization Activity of Endophytic Bacterial Strains
In order to evaluate the colonization ability of selected endophytic bacterial strains in plant root and shoot cultivated in sterilized soil, a spontaneous strain of NBRI FY32 and NBRI DS5 having rifampicin resistance (RifR) was isolated on nutrient agar (NA) plates containing 250 µg rifampicin ml−1 (Nautiyal 1997). One gram of plant tissue (root and shoot) was cleaned under tap for 2 min to eliminate loosely adhered soil particles followed by surface sterilization using 5% sodium hypochlorite for 5 min and washing 4–5 times with autoclaved distilled water. The surface sterilized samples were crushed in 5 ml of 0.85% saline solution and serially diluted, then spread onto NA plates. NA plates were used to recover a mixed population of endospheric bacteria, and NA plates were supplemented with 50 µg of rifampicin ml−1 for NBRI FY32 and NBRI DS5. The average colonization of NBRI FY32 and NBRI DS5 (Log10CFU/g root/shoot dry weight) was estimated from three plant samples. When root and shoot homogenates of uninoculated controls were plated from sterilized soils, no naturally occurring RifR bacteria were observed.
Statistical Analysis
Means were tested for variance homogeneity to assess the variation among obtained values. These means were then compared using ANOVA, followed by the Duncan test to determine significance (p ≤ 0.05).
Results
Isolation and Screening of Endophytic Bacteria Based on Siderophore Production
In the present study, isolation of siderophore-producing endophytic bacteria was performed from iron sufficient as well as deficit conditions (Fig. 1). Out of total, 234 isolates, 13 from the root, 15 strains from the stem, and 17 strains from leaves were tested positive for siderophore production (Fig. 2). Further, these selected endophytic bacteria were characterized for different plant growth promoting attributes such as indole acetic acid (IAA) production, biofilm formation, phosphate (P) solubilization, and siderophore production.
Quantification of PGP Attributes
IAA production by selected bacterial isolates was exhibited in the range of 10.73 µg ml−1 to 23.22 µg ml−1 (Table 1). However, NBRI BR5 (23.22 μg ml−1) and NBRI EY30 (18.67 μg ml−1) were noted as the highest IAA producers under Fe-sufficient and Fe-deficit conditions, respectively. NBRI AN3 (12.30 μg ml−1) and NBRI DK24 (10.73 μg ml−1) were considered to be the lowest IAA producers under Fe-sufficient and Fe-deficit conditions, respectively (Table 1).
Regarding Phosphate solubilization, 45 selected bacterial isolates exhibited in the range of 21.71 to 48.79 μg ml−1 (Table 1). The most effective phosphate solubilizer bacterial isolate under Fe-sufficient and Fe-deficit conditions was found to be NBRI CK1 (42.71 μg ml−1) and NBRI EY26 (48.79 μg ml−1), respectively (Table 1). NBRI CS2 (24.22 μg ml−1) and NBRI DL3 (21.71 μg ml−1) demonstrated the least ability for the same under Fe-sufficient and Fe-deficit conditions, respectively (Table 1).
Regarding siderophore production, the selected 45 bacterial isolates demonstrated siderophore production in the range of 28.11 μM to 118.05 μM. NBRI AN3 (112.03 μM) and NBRI FY32 (118.05 μM) exhibited the highest quantitative value for siderophore production under Fe-sufficient and Fe-deficit conditions, respectively. The lowest siderophore-producing ability was exhibited by NBRI CR8 (29.78 μM) and NBRI DL3 (28.11 μM) under Fe-sufficient and Fe-deficit conditions, respectively, among selected 45 isolates (Table 1).
In the matter of biofilm formation, NBRI BR8 and NBRI DS8 demonstrated maximum ability from Fe-sufficient and Fe-deficit conditions, respectively, whereas, NBRI AK1 and NBRI BR5 demonstrated the lowest biofilm formation capability under Fe-sufficient condition, and NBRI EY30 under Fe-deficit condition (Table 1).
Estimation of Phytase-Producing and Oxalate Degradation Activity of Selected Endophytic Bacterial Strains
Further, we have selected 09 high siderophore-producing endophytic bacterial strains having multifarious PGP attributes and evaluated them for having phytase-producing and oxalate degradation activity. In the current study, the selected 09 bacterial strains were initially evaluated for qualitative estimation of phytase-producing and oxalate degradation activity. It was found that out of nine bacterial strains, only NBRI FY32 has the capability to produce phytase and degrade oxalate by forming a halo zone in the medium (Fig. 3a and b). Moreover, eight bacterial strains i.e., NBRI AK1, NBRI AN3, NBRI DK24, NBRI EL3, NBRI EN27, and NBRI EK29 were unable to demonstrate phytase-producing as well as oxalate degradation activity (Table 2). However, NBRI EY30 and NBRI DL4 exhibited only phytase-producing ability (Table 2).
16S rRNA-Based Identification of Selected Endophytic Bacterial Strains
All nine selected potent siderophore-producing endophytes were identified through partial 16S rRNA gene sequencing (~ 1400 bp amplicon) which revealed that most of the endophytic isolates belonged to the Bacillaceae family. Among the Bacillaceae family, one of the isolates belonged to each B. zanthoxyli, B. massilionigeriensis, and P. flexa, while two belonged to each P. megaterium and P. aryabhattai, respectively. Apart from Bacillus species, the rest of the endophytic bacterial isolates were affirmed to Lysinibacillus fusiformis (01) and Enterobacter quasihormaechei (01) (Fig. 4). The sequences of these nine endophytic bacterial isolates were submitted to the database of NCBI (GenBank) along with their accession numbers (Table 2). For phylogenetic reconstruction analysis, the Neighbor-joining statistical method with the Jukes-Cantor model was used.
Inoculation Effect of Endophytes in Plant Growth and Nutrient Enhancement Under Greenhouse Conditions
Effect of Endophyte Inoculation on Spinach Vegetative Growth
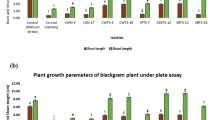
The spinach (Spinacia oleracea L.) plant was used as a model plant for determining growth promotion by using two endophytic bacterial strains under both Fe-sufficient and Fe-deficit conditions. In this study, NBRI FY32 was selected as a high-siderophore-producing endophyte with phytase-producing and oxalate degradation ability, whereas NBRI DS5 was selected as a non-siderophore-forming endophyte. Greenhouse results demonstrated that inoculation with NBRI FY32 has significantly enhanced the overall plant vegetative growth in comparison to respective uninoculated controls under both Fe-sufficient and Fe-deficit conditions (Table 3 and Fig. 5). However, plants under Fe-deficit control condition recorded significantly least values for all the concerned vegetative parameters among all the treatments (Table 3). NBRI DS5 only resulted in significantly high values for root length and fresh and dry plant weight as compared to control plants under Fe-sufficient conditions (Table 3). NBRI FY32 has demonstrated significant enhancement in root and shoot length, number of leaves, and plant fresh and dry weight by 3.38%, 21.07%, 16.34%, 19.36%, and 11.53%, respectively, under Fe-sufficient conditions, while 33.46%, 46.12%, 26.10%, 38.82%, and 27.14% under Fe-deficit condition, respectively (Table 3).
Representation of bacterial strains inoculation on spinach under Fe-sufficient and Fe-deficit condition in greenhouse. In this figure, T1 = full Hoagland solution (positive control); T2 = iron-free Hoagland solution (negative control); T3 = bacteria (siderophore positive) + Fe; T4 = bacteria (siderophore positive) without Fe; T5 = bacteria (siderophore negative) + Fe; T6 = bacteria (siderophore negative) without Fe
Effect of Endophyte Inoculation on Spinach Nutrient Content
In the present study, the phyto-beneficial impact of endophytic bacterial strains on spinach was verified under Fe-sufficient and Fe-deficit conditions by evaluating the nutrient content level. Almost all the nutrients in the spinach shoot were significantly enhanced upon inoculation of NBRI FY32 under both Fe-sufficient and Fe-deficit conditions (Table 4). Nitrogen (N), Potassium (K), Phosphorus (P), Sodium (Na), Calcium (Ca), Manganese (Mn), and Zinc (Zn) contents were found to be significantly higher in plants inoculated with NBRI FY32 under Fe-sufficient condition, while plants without any bacterial inoculation recorded significantly minimum value for the same under Fe-deficit condition (Table 4). Iron (Fe) content was observed to be significantly higher in plants inoculated with NBRI FY32 under Fe-deficit conditions, whereas plants receiving no bacteria demonstrated significantly minimum Fe content under Fe-deficit conditions (Table 4). In the present study, K, N, P, Na, Ca, Mn, and Zn contents were increased by 24.36%, 22.28%, 32.23%, 6.60%, 13.15%, 2.66%, and 7.71%, respectively, in plants inoculated with NBRI FY32 as compared to control plants under Fe-sufficient condition. However, under Fe-deficit conditions, K, N, P, Na, Ca, and Zn contents were enhanced by 24.95%, 29.35%, 21.04%, 8.80%, 6.01%, and 20.17%, respectively, in plants treated with NBRI FY32.
Colonization of Endophytic Bacterial Strains in Spinach
Simultaneously, we observed the colonization ability of endophytic bacterial strains along with the heterogeneous bacterial population in spinach shoots under Fe-sufficient and Fe-deficit conditions. NBRI FY32 showed maximum colonizing and survival ability in spinach root and shoot with 2.06 Log10 CFU g−1 and 1.71 Log10 CFU g−1 under Fe-sufficient conditions (Table 5). Although, under Fe-deficit condition NBRI DS5 demonstrated maximum colonization in root with 1.88 Log10 CFU g−1 while NBRI FY32 was observed to have maximum colonization in shoot with 1.33 Log10 CFU g−1 (Table 5). Also, a heterogeneous endophytic bacterial population was observed higher in both the root and shoot of plants inoculated with NBRI FY32 under Fe-sufficient conditions (Table 5). Although under Fe-deficit conditions, the maximum heterogeneous population was found in plant shoot inoculated with NBRI DS5 with 2.61 Log10 CFU g−1, while plant inoculated with NBRI FY32 demonstrated maximum count in root with 4.35 Log10 CFU g−1 (Table 5). However, no natural population of RifR bacterial endophytes was detected in roots well as in the shoot of plants receiving no bacterial treatment under Fe-sufficient and Fe- deficit conditions.
Discussion
Exploration of microbes possessing multifarious plant growth-promoting attributes and biofortification ability is indispensable to tackling the food security problem. Earlier studies have illustrated the crucial role of rhizobacterial strains in plant growth and nutrient enhancement under normal as well as stressed conditions (Bisht et al. 2019; Misra et al. 2019). However, recent reports have established a close association of endophytes with their host plants in comparison to microbes isolated from soil or other plants (Maggini et al. 2019). Researchers have well reported the important role of endophytes in the fortification of nutrients under normal conditions but limited reports are available under stress conditions (Zhang et al. 2019; Kaur et al. 2020; Rana et al. 2021; Sun et al. 2021). Although, earlier studies have reported the isolation of endophytic bacterial strains from different host crops under non-stress conditions (Lipková et al. 2021; Dubey et al. 2021). However, in the present study, bacterial endophytes were isolated from spinach plants under Fe-sufficient and Fe-deficit conditions.
Isolation and characterization of microbes for nutrient enhancement in host crops have successfully ameliorated nutrient deficiency in the human diet (Singh et al. 2017). Enhancing micronutrients in crop plants through conventional breeding, mineral fertilizers, and transgenic methods is reported but with less consistency (Murgia et al. 2012). Several endophytic bacteria have been reported to fortify the iron contents in food crops besides improving crop growth through siderophore production (Zhang et al. 2019; Kaur et al. 2020; Rana et al. 2021). Recently, Sultana et al. (2021) showed that plant growth promoting Bacillus aryabhattai MS3 exhibited the highest siderophore production under salt stress and iron-limited condition.
Among different PGP attributes, the biofilm-forming abilities of microbes allow them to endure harsh environmental conditions by conferring tolerance against various abiotic stresses (Kamjumphol et al. 2013). In the context of biofilm formation, our result demonstrates that NBRI BR8 and NBRI DS8 from Fe-sufficient and Fe-deficit conditions represented maximum ability. The ability of Phyto-beneficial bacterial strains to form biofilms aids plants in defending themselves against various pathogenic diseases (Deng et al. 2011; Chen et al. 2012, 2013). This ability has provided bacteria the advantage of being isolated from harsh habitats including deserts and animal digestive systems (Matulová et al. 2011; Earl et al. 2012; Schyns et al. 2013). For adaptation, biofilm formation by endophytic bacterial strains is among the processes that need to be primarily modulated for getting access to plant internal tissues (Taulé et al. 2021; Pinski et al. 2019; Levy et al. 2018; Bogino et al. 2013). Recently, Jeong et al. (2021) reported that Kosakonia cowanii, a seed endophyte from a xerophytic invasive plant, Lactuca serriola, demonstrated drought tolerance to the host by producing a high concentration of biofilm constituents. Also, biofilm formation is required for successful colonization by an efficient endophyte to deliver its plant growth-promoting effect inside the host plant (Tharek et al. 2021).
Considering auxin production in the present study, we have reported IAA production by endophytic bacterial strains under control and iron-deficit conditions. Recently, several researchers have reported auxin production by bacterial endophytes under normal conditions (Lipková et al. 2021; Sun et al. 2021). However, several studies have demonstrated a reduction in auxin-producing ability by bacterial strains other than endophytes under stressed conditions (Tank and Saraf 2010; Soleimani et al. 2017; Sarkar et al. 2018).
Phosphorus is another essential micronutrient that is commonly immobilized and inaccessible to plants. In the present study, endophytic bacterial strains have demonstrated a wide range of P-solubilizing abilities. Earlier studies have demonstrated that P-solubilizing endophytic bacterial strains are able to promote plant growth under normal conditions (Dubey et al. 2021; Lipková et al. 2021). Moreover, endophytic bacteria with P-solubilization activity directly improved plant responses to abiotic stressors (Rajini et al. 2020).
Simultaneously, PGP endophytic bacteria demonstrate another important trait of siderophore formation, which allows bacteria to chelate the accessible form of iron (Fe3+) in the rhizosphere, keeping it unavailable to undesirable microbes and limiting their proliferation (Zhang et al. 2019). In the present study, several endophytic bacterial strains demonstrated siderophore-producing ability which was found to be in accordance with previous reports (Jin et al. 2010; Tripathi et al. 2020). Recent reports have suggested the phyto-beneficial role of siderophore-producing endophytic bacterial strains by significantly promoting plant growth in terms of vegetative parameters and photosynthetic pigments (Yadav 2019; Lobo and dos Santos 2019; Rana et al. 2021). Besides enhancing plant growth, endophytic bacterial strains play a crucial role in fortifying the iron contents in plants through siderophore production (Kaur et al. 2020). The iron-chelated complex was translocated inside the plant by integral transporter proteins present on the plasma membrane of the root (Boukhalfa and Crumbliss 2002). Inoculations of maize and wheat plants with Pantoea agglomerans and Arthrobacter sulfonivorans, respectively, the siderophores-producing endophytes, have assisted in higher translocation of iron in shoots and roots (Singh et al. 2018; Rana et al. 2021). In addition, Sultana et al. (2021) have reported that Siderophore-producing bacterial strains other than endophytes have enhanced plant growth by ameliorating salt stress and Fe limitation concurrently.
Essential minerals including calcium, magnesium, iron, and zinc are chelated by phytic and oxalic acid under normal physiological conditions. Moreover, they also bind to proteins and amino acids and block digestion enzymes (Martins et al. 2017). Thus, phytic and oxalic acid should be hydrolyzed by enzymes for improved nutrient bioavailability since they are an anti-nutritive factor for food derived from plants. Endophytic bacteria possessing phytic and oxalic acid degrading abilities may convert, mobilize, and solubilize nutrients. In the present study, among the 09 selected PGP bacterial endophytes, only NBRI FY32 Enterobacter quasihormaechei demonstrated the presence of both phytic and oxalic acid degrading activities. Our study found to be in agreement with the findings of a recent study describing that an endophyte SSP2 Enterobacter hormaechei isolated from Chinese fir with high phosphate solubilizing activity has significantly promoted the vegetative growth and nutrient content of Chinese fir (Chen et al. 2021). Recently, Mei et al. (2021) reported that bacterial endophyte Pantoea agglomerans IALR1325 containing phytase activity has promoted phosphate solubilization and therefore, significantly enhanced pepper and tomato growth under controlled conditions.
Kost et al. (2014) explored the role of oxalate-degrading plant-beneficial species of the Burkholderia genus for successful plant colonization as an endophyte. In addition, this study suggested oxalate degradation by endophytic bacteria aided in heavy metal tolerance and biocontrol of plants. In the present study, we have also reported that NBRI FY32 Enterobacter quasihormaechei is an oxalate-degrading endophyte. However, Kumar and Belur (2018) studied that endophytes usually colonize plant tissues by producing the necessary enzymes for the colonization of plant tissues.
Further, the present study demonstrated growth promotion in terms of vegetative parameters and nutrient content by the inoculation of siderophore-producing PGP endophytes under controlled conditions. Recent studies have demonstrated that endophytic bacterial strains can enhance plant growth directly or indirectly through several mechanisms (Mahmood and Kataoka 2020; Rana et al. 2021; Salehin et al. 2021). However, there are several reports demonstrating the role of bacterial endophytes in the growth promotion of plants under different abiotic stress conditions (Jiménez-Gómez et al. 2020; Kushwaha et al. 2020) but their role under Fe-deficit conditions is still scanty. In the present study, the results of the greenhouse revealed that plants inoculated with NBRI FY32 demonstrated significant enhancement in vegetative parameters and high nutrient content under both Fe-sufficient and Fe-deficit conditions.
Similar to our findings, several reports have also demonstrated that siderophore-producing endophytes positively influence plant growth by providing available iron to plants but these studies were restricted to non-stressed conditions (Zhang et al. 2019; Rana et al. 2021). Moreover, the effect of seed inoculation with endophytic bacterial strains on enhanced vegetative parameters of the plant was reported earlier by Sah et al. (2017) by performing a greenhouse experiment using RSP5 (high siderophore producer) and RSP8 (low siderophore producer). In the present study, NBRI FY32 was selected as a high siderophore-producing bacteria, while NBRI DS5 was non-siderophore-producing bacteria. Similar to our findings, Sah et al. (2017) reported that the lack of RSP5 and RSP8 in the control plant resulted in reduced iron transportation and plant growth. Microbes play a vital role in facilitating plant iron (Fe) absorption under Fe-limiting conditions (Jin et al. 2010). Correspondingly, the siderophore produced by NBRI FY32 in the current study was able to solubilize the insoluble forms of iron in the soil, making it accessible to plants. Nevertheless, the prior study revealed that the microbial community in the rhizosphere, which influences the composition of siderophore-secreting microorganisms in the rhizosphere, has a significant impact on Fe acquisition (Jin et al. 2010; Camejo et al. 2013).
The lack of nutrient availability may be a severe barrier to plant growth in many places across the globe, particularly in the tropics where soils are very deficient in nutrients. Plants get the majority of their mineral nutrients through the rhizosphere. The present study revealed that plants inoculated with NBRI FY32 resulted in enhanced nutrient content under Fe-sufficient and Fe-deficit conditions. Our results corroborate with the findings of earlier reports that have established the role of endophytic bacterial strains in increasing nutrient content in strawberries, radishes, tomatoes, and lettuce (Makar et al. 2021; Verma et al. 2021; Vishwakarma et al. 2021). In a metagenomic analysis of the structure and functions of the bacterial endophyte community in Panax ginseng at various ages, Hong et al. (2019) found significant putative genes that may be involved in iron acquisition and siderophore formation and may contribute to bacterial activities in plants.
Furthermore, Rana et al. (2021) explained the ecological significance of endophytic bacterial strains by demonstrating their interaction more strongly with plant hosts than rhizospheric microbes through active colonization of microniches within the plant tissues. In the present work, spinach plants inoculated with NBRI FY32 supported the higher heterogeneous endophytic bacterial count. Since NBRI FY32 has exhibited oxalate-degrading activity in the present study, therefore it might be the rationale behind the active colonization of plant internal tissues. Verma et al. (2019) evaluated the endophytic bacterial count from wheat tissues (root and stem), and the results of the endophytic bacterial count from those tissues were found to be compatible with the present findings. Recently, Tharek et al. (2021) has analyzed the genome of E. coli USML2 and revealed genes essential for endophytic bacterial colonization such as flagella biosynthesis, chemotaxis, EPS, and biofilm formation which further proved the plant growth-promoting potentials of E. coli USML2 as an endophyte. Furthermore, as compared to other plant tissues, the count of bacterial endophytes in plant roots grown in non-sterile soil was reported to be higher (Sun et al. 2008). The enhanced colonization of bacterial endophytes in the apical zone of the root is due to various mechanisms of colonization and interaction with the host plant (Botta et al. 2013).
Conclusion
The role of siderophore-producing endophytic bacterial strains having multifaceted PGP attributes is essential for the confrontation of problems associated with the low nutrient content of spinach plants under nutrient-deficient conditions. The present study will assist in outlining the significant functions of siderophore-producing bacterial endophyte E. quasihormaechei (NBRI FY32) in Fe-sufficient as well as Fe-deficit conditions. The improvement of nutrient content in spinach plants under Fe-deficit conditions has evidenced the biofortification of host plants with certitude and in a safe way. Therefore, the present study will provide an advantage in screening out potential endophytic isolates with enhanced colonization for sustainable food crop development under nutrient-deprived conditions.
References
Abaid-Ullah M, Hassan MN, Jamil M, Brader G, Shah MKN, Sessitsch A, Hafeez FY (2015) Plant growth promoting rhizobacteria: an alternate way to improve yield and quality of wheat (Triticum aestivum). Int J Agric Biol 17:51–60
Adeleke BS, Babalola OO, Glick BR (2021) Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 20:100433
Bagheri A, Askari Seyahooei M, Fathipour Y (2021) The Endophytes. In: Omkar (ed.), Microbial approaches for insect pest management. 151, 215, Springer, Cham
Bisht N, Tiwari S, Singh PC, Niranja A, Chauhan PS (2019) A multifaceted rhizobacterium Paenibacillus lentimorbus alleviates nutrient deficiency-induced stress in Cicer arietinum L. Microbiol Res 223:110–119
Bogino PC, Oliva MD, Sorroche FG, Giordano W (2013) The role of bacterial biofilms and surface components in plant-bacterial associations. Int J Mol Sci 14:15838–15859
Botta AL, Santacecilia A, Ercole C, Cacchio P, Del Gallo M (2013) In vitro and in vivo inoculation of four endophytic bacteria on Lycopersicon esculentum. N Biotechno 30:666–674
Boukhalfa H, Crumbliss AL (2002) Chemical aspects of siderophore mediated iron transport. Biometals 15:325–339. https://doi.org/10.1023/A:1020218608266
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Camejo D, Marti MC, Martinez-Alcala I, Medina-Bellver JI, Marques S, Jimenez A (2013) Effect of Fe deficiency on alfalfa plants grown in the presence of Pseudomonas. J Agric Sci 152:731–740
Chen Y, Cao S, Chai Y, Clardy J, Kolter R, Guo JH, Losick R (2012) A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol 85:418–430
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo J (2013) Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15:848–864
Chen J, Zhao G, Wei Y, Dong Y, Hou L, Jiao R (2021) Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Sci Rep 111:9081
Cochard B, Giroud B, Crovadore J, Chablais R, Arminjon L, Lefort F (2022) Endophytic PGPR from tomato roots: isolation, in vitro characterization and in vivo evaluation of treated tomatoes (Solanum lycopersicum L.). Microorganisms 10:765
Deng Y, Zhu Y, Wang P, Zhu L, Zheng J, Li R, Ruan L, Peng D, Sun M (2011) Complete genome sequence of Bacillus subtilis BSn5: an endophytic bacterium of Amorphophallus konjac with antimicrobial activity for the plant pathogen Erwinia carotovora subsp. Carotovora J Bacteriol 2070:2071
Dixit G, Singh AP, Kumar A, Mishra S, Dwivedi S, Kumar S, Trivedi PK, Pandey V, Tripathi RD (2016) Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol Biochem 99:86–96
Dubey A, Saiyam D, Kumar A, Hashem A (2021) Bacterial root endophytes: characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress. Int J Environ Res 18:931
Earl AM, Eppinger M, Fricke WF, Rosovitz MJ, Rasko DA, Daugherty S, Losick R, Kolter R, Rovel J (2012) Whole genome sequences of Bacillus subtilis and close relatives. J Bacteriol 2378–2379
Hanway JJ, Heidel H (1952) Soil analysis methods as used in Iowa state college. Soil testing laboratory pp. 131
Hong CE, Kim JU, Lee JW, Bang KH, Jo IH (2019) Metagenomic analysis of bacterial endophyte community structure and functions in Panax ginseng at different ages. 3 Biotech 9:1–8
Huang S, Wang P, Yamaji N, Ma JF (2020) Plant nutrition for human nutrition: hints from rice research and future perspectives. Mol Plant 13:825–835
Jackson ML (1973) Soil chemical analysis (II Edition). Prentice Hall of India Private Limited, New Delhi, India
Jeong S, Kim TM, Choi B, Kim Y, Kim E (2021) Invasive Lactuca serriola seeds contain endophytic bacteria that contribute to drought tolerance. Sci Rep 11:1–12
Jiménez-Gómez A, Saati-Santamaría Z, Kostovcik M, Rivas R, Velázquez E, Mateos PF, Menéndez E, García-Fraile P (2020) Selection of the root endophyte Pseudomonas brassicacearum CDVBN10 as plant growth promoter for Brassica napus L. crops. Agronomy 10:1788
Jin CW, Li GX, Yu XH, Zheng SJ (2010) Plant Fe status affects the composition of siderophore-secreting microbes in the rhizosphere. Ann Bot 105:835–841
Kamjumphol W, Chareonsudjai S, Chareonsudjai P, Wongratanacheewin S, Taweechaisupapong S (2013) Environmental factors affecting Burkholderia pseudomallei biofilm formation. Southeast Asian J Trop Med Public Health 44P:72–81
Kaur T, Rana KL, Kour D, Sheikh I, Yadav N, Kumar V, Yadav AN, Dhaliwal HS, Saxena AK (2020) Microbe-mediated biofortification for micronutrients: present status and future challenges. In: Rastegari AA, Yadav AN, Yadav N (eds) New and future developments in microbial biotechnology and bioengineering. Elsevier, London, pp 1–17
Kerovuo J, Lauraeus M, Nurminen P, Kalkkinen N, Apajalahti J (1998) Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl Environ Microbiol 64:2079–2085
Kost T, Stopnisek N, Agnoli K, Eberl L, Weisskopf L (2014) Oxalotrophy, a widespread trait of plant-associated Burkholderia species, is involved in successful root colonization of lupin and maize by Burkholderia phytofirmans. Front Microbiol 4:421
Kumar K, Belur PD (2018) Production of oxalate oxidase from endophytic Ochrobactrum intermedium CL6. J Pure Appl Microbiol 12:2327–2336
Kushwaha P, Kashyap PL, Bhardwaj AK, Kuppusamy P, Srivastava AK, Tiwari RK (2020) Bacterial endophyte mediated plant tolerance to salinity: growth responses and mechanisms of action. World J Microbiol Biotechnol 36:1–6
Levy A, Conway JM, Dangl JL, Woyke T (2018) Elucidating bacterial gene functions in the plant microbiome. Cell Host Microbe 24:475–485
Li Y, Jin J, Li P, Wang Q, Xu L, Wei G, Li Z (2023) Regional variations and plant compartments shape the community structures of the endophytic microbiome and secondary metabolites of Astragalus mongholicus. Ind Crops Prod 192:116037
Lipková N, Medo J, Artimová R, Maková J, Petrová J, Javoreková S, Michalko J (2021) Growth promotion of rapeseed (Brassica napus L.) and blackleg disease (Leptosphaeria maculans) suppression mediated by endophytic bacteria. Agronomy 11:1966
Lobo LLB, dos Santos RM, Rigobelo EC (2019) Promotion of maize growth using endophytic bacteria under greenhouse and field conditions. Aust J Crop Sci 13:2067–2074
Macedo-Raygoza GM, Valdez-Salas B, Prado FM, Prieto KR, Yamaguchi LF, Kato MJ, Canto-Canché BB, Carrillo-Beltrán M, Di Mascio P, White JF, Beltrán-García MJ (2019) Enterobacter cloacae, an Endophyte that establishes a nutrient transfer symbiosis with banana plants and protects against the black sigatoka pathogen. Front Microbiol 10:804
Maggini V, Mengoni A, Gallo ER, Biffi S, Fani R, Firenzuoli F, Bogani P (2019) Tissue specificity and differential effects on in vitro plant growth of single bacterial endophytes isolated from the roots, leaves and rhizospheric soil of Echinacea purpurea. BMC Plant Biol 19:1–9
Mahmood A, Kataoka R (2020) Metabolite profiling reveals a complex response of plants to application of plant growth-promoting endophytic bacteria. Microbiol Res 234:126421
Makar O, Kuźniar A, Patsula O, Kavulych Y, Kozlovskyy V, Wolińska A, Skórzyńska-Polit E, Vatamaniuk O, Terek O, Romanyuk N (2021) Bacterial endophytes of spring wheat grains and the potential to acquire Fe, Cu, and Zn under their low soil bioavailability. Biology 10:409
Martins ZE, Pinto E, Almeida AA, Pinho O, Ferreira IM (2017) Fibre fortification of wheat bread: impact on mineral composition and bioaccessibility. Food Funct 8:1979–1987
Matulová M, Husárová S, Capek P, Sancelme M, Delort AM (2011) NMR structural study of fructans produced by Bacillus sp. 3B6: bacterium isolated in cloud water. Carbohydr Res 346:501–507
Mei C, Chretien RL, Amaradasa BS, He Y, Turner A, Lowman S (2021) Characterization of phosphate solubilizing bacterial endophytes and plant growth promotion in vitro and in greenhouse. Microorganisms 9:1935
Meyer JA, Abdallah MA (1978) The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification, and physicochemical properties. Microbiology 107:319–328
Misra S, Dixit VK, Khan MH, Mishra SK, Dviwedi G, Yadav S, Lehri A, Chauhan PS (2017) Exploitation of agro-climatic environment for selection of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt-tolerant indigenous plant growth promoting rhizobacteria. Microbiol Res 205:25–34
Misra S, Dixit VK, Mishra SK, Chauhan PS (2019) Demonstrating the potential of abiotic stress-tolerant Jeotgalicoccus huakuii NBRI 13E for plant growth promotion and salt stress amelioration. Ann Microbiol 69:419–434
Murgia I, Arosio P, Tarantino D, Soave C (2012) Biofortification for combating ‘hidden hunger’ for iron. Trends Plant Sci 17:47–55
Nautiyal CS (1997) A method for selection and characterization of rhizosphere-competent bacteria of chickpea. Curr Microbiol 34:12–17
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Pillay VK, Nowak J (1997) Inoculum density, temperature, and genotype effects on in vitro growth promotion and epiphytic and endophytic colonization of tomato (Lycopersicon esculentum L.) seedlings inoculated with a pseudomonad bacterium. Can J Microbiol 43:354–361
Pinski A, Betekhtin A, Hupert-Kocurek K, Mur LA, Hasterok R (2019) Defining the genetic basis of plant–endophytic bacteria interactions. Int J of Mol Sci 20:1947
Praharaj S, Skalicky M, Maitra S, Bhadra P, Shankar T, Brestic M, Hejnak V, Vachova P, Hossain A (2021) Zinc biofortification in food crops could alleviate the zinc malnutrition in human health. Molecules 26:3509
Prasanna R, Bidyarani N, Babu S, Hossain F, Shivay YS, Nain L (2015) Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent Food Agric 1:995807
Rajini SB, Nandhini M, Udayashankar AC, Niranjana SR, Lund OS, Prakash HS (2020) Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of Sorghum bicolor. Plant Pathol 69:642–654
Rana KL, Kour D, Kaur T, Devi R, Yadav A, Yadav AN (2021) Bioprospecting of endophytic bacteria from the Indian Himalayas and their role in plant growth promotion of maize (Zea mays L). J App Bio Biotechnol 9:41–50
Sah S, Singh R (2016) Effect of Pseudomonas on micronutrient status of forest and agricultural soil of Uttarakhand. Ecol Env Conserv 22:S279–S284
Sah S, Sahgal M, Singh R (2017) Concomitant ability of siderophore against iron paucity and fusarium wilt in Lycopersicon esculentum. Biosci Biotechnol Res Asia 14:319–328
Salehin A, Puri RR, Hafiz MH, Itoh K (2021) Effect of Co-inoculation of Bacillus sp. strain with bacterial endophytes on plant growth and colonization in tomato plant (Solanum lycopersicum). Microbiol Res 12:480–490
Sarkar A, Ghosh PK, Pramanik K, Mitra S, Soren T, Pandey S, Mondal MH, Maiti TK (2017) A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res Microbiol 169:20–32
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Schyns G, Serra CR, Lapointe T, Pereira-Leal JB, Potot S, Fickers P, Perkins JB, Wyss M, Henriques AO (2013) Genome of a gut strain of Bacillus subtilis. Genome Announc 1:e00184-e1112
Singh D, Rajawat MV, Kaushik R, Prasanna R, Saxena AK (2017) Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 416:107–116
Singh D, Geat N, Rajawat MSV, Mahajan MM, Prasanna R, Singh S (2018) Deciphering the mechanisms of endophyte-mediated biofortification of Fe and Zn in wheat. J Plant Growth Regul 37:174–182
Soleimani R, Alikhani HA, Towfighi H, Khavazi K, Pourbabaee AA (2017) Isolated bacteria from saline-sodic soils alter the response of wheat under high adsorbed sodium and salt stress. Int J Environ Sci Technol 14:143–150
Srivastava S, Yadav A, Seem K, Mishra S, Chaudhary V, Nautiyal CS (2008) Effect of high temperature on Pseudomonas putida NBRI0987 biofilm formation and expression of stress sigma factor RpoS. Curr Microbiol 56:453–457
Sultana S, Alam S, Karim MM (2021) Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J Agri Food Res 4:100150
Sun Z, Yue Z, Liu H, Ma K, Li C (2021) Microbial-assisted wheat iron biofortification using endophytic Bacillus altitudinis WR10. Front Nutr 8:704030
Sundram S, Othman R, Idris AS, Angel LP, Meon S (2022) Improved growth performance of Elaeis guineensis Jacq. Through the applications of arbuscular mycorrhizal (AM) fungi and endophytic bacteria. Curr Microbiol 79:155
Tandon HLS (1993) Methods of analysis of soils, plants, waters and fertilizers. Fertilizer development and consultation organization, New Delhi
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5:51–58
Taule C, Vaz-Jauri P, Battistoni F (2021) Insights into the early stages of plant–endophytic bacteria interaction. J Microbiol Biotechnol 37:1–9
Tharek M, Khairuddin D, Najimudin N, Ghazali AH (2021) Plant growth promoting potentials of beneficial endophytic Escherichia coli USML2 in association with rice seedlings. Trop Life Sci Res 32:119–143
Tripathi A, Awasthi A, Singh S, Sah K, Maji D, Patel VK, Kalra A (2020) Enhancing artemisinin yields through an ecologically functional community of endophytes in Artemisia annua. Ind Crops Prod 150:112375
Verma P, Yadav AN, Khannam KS, Mishra S, Kumar S, Saxena AK, Suman A (2019) Appraisal of diversity and functional attributes of thermotolerant wheat associated bacteria from the peninsular zone of India. Saudi J Biol Sci 26:1882–1895
Verma SK, Sahu PK, Kumar K, Pal G, Gond SK, Kharwar RN, White JF (2021) Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J Appl Microbiol 13:2161–2177
Vishwakarma K, Kumar N, Shandilya C, Varma A (2021) Unravelling the role of endophytes in micronutrient uptake and enhanced crop productivity. In: Shrivastava N, Mahajan S, Varma A (eds) Symbiotic soil microorganisms soil biology. Springer, Cham, pp 63–85
Yadav AN (2019) Microbiomes of wheat (Triticum aestivum L.) endowed with multifunctional plant growth promoting attributes. EC Microbiol 15:1–6
Yu X, Zhang W, Lang D, Zhang X, Cui G, Zhang X (2019) Interactions between endophytes and plants: beneficial effect of endophytes to ameliorate biotic and abiotic stresses in plants. J Plant Biol 62:1–13
Zhang XZ, Zhang D, Sun W, Wang T (2019) The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int J Mol Sci 20:2424
Acknowledgements
This work was supported by the Indian Council of Medical Research [File No. (3/1/2/167/2019-(Nut)] New Delhi, India and CSIR Network Project MLP0048. The authors are thankful to the Director, CSIR-NBRI, Lucknow for providing the necessary resources to conduct this study. CSIR-NBRI allotted the Manuscript Number is CSIR-NBRI_MS/2023/05/10.
Author information
Authors and Affiliations
Contributions
Sankalp Misra: Methodology, Writing – original draft, Investigation. Pradeep Semwal: Formal analysis. Deen Dayal Pandey: Data curation. Shashank Kumar Mishra: Validation, Visualization. Puneet Singh Chauhan: Conceptualization, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Conflicts of interest
All authors have no conflict of interest.
Ethics Approval
Not applicable.
Additional information
Handling Editor: Mikihisa Umehara.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Misra, S., Semwal, P., Pandey, D.D. et al. Siderophore-Producing Spinacia Oleracea Bacterial Endophytes Enhance Nutrient Status and Vegetative Growth Under Iron-Deficit Conditions. J Plant Growth Regul 43, 1317–1330 (2024). https://doi.org/10.1007/s00344-023-11185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11185-8