Abstract
The auxin treatment in tomato plants during anthesis has been extensively used for setting fruits in adverse climatic conditions (e.g., low temperatures and inadequate light), which is well known that reduces pollen availability and fertility. Since auxin application may affect fruit composition and quality, we examined l-ascorbic acid metabolism in seeded fruit (set by natural pollination) and parthenocarpic fruit (set by auxin) in cherry tomato cv. Conchita. Specifically, we studied the oxidized and total ascorbic acid contents, the expression of all characterized genes of l-ascorbic acid metabolism, the activity of ascorbate peroxidase and dehydroascorbate reductase and the immunolocalization of ascorbate peroxidase. Differences were detected between seeded and parthenocarpic fruits, in the expression of some of the genes of ascorbic acid metabolism. However, strong presence of l-ascorbic acid peroxidase protein was detected on the developing seeds. Our data indicate that induced parthenocarpy in auxin treated plants has a significant influence in ascorbic acid metabolism comparing to seeded tomato fruits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that in plants, l-ascorbic acid (AsA) protects cells against oxidative damage (Ishikawa and Shigeoka 2008) caused by reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), which are generated during photosynthesis, oxidative metabolism and under biotic and abiotic stress (Gill and Tuteja 2010). In removing the ROS, AsA is oxidized to dehydroascorbic acid (DHA), which is subsequently restored to AsA via a four-step biochemical pathway known as the l-ascorbate–glutathione cycle or the Foyer, Halliwell, Ashada cycle (Foyer and Noctor 2011) (Fig. 1).
l-Ascorbic acid–glutathione/Foyer–Halliwell–Asada cycle. Ascorbate peroxidase (APX EC 1.11.1.11) catalyzes the deactivation of H2O2 with concomitant oxidation of AsA to monodehydroascorbate (MDHA). MDHA can either dis-proportionate spontaneously to AsA and dehydroascorbate (DHA) or it can be enzymatically reduced to AsA by MDHA reductace (MDHAR EC 1.6.5.4), using NADH (or rarely NADPH) as a reducing agent. Dehydroascorbate (DHA) is the substrate of DHA reductase (DHAR EC 1.8.5.1) that reduces DHA to AsA, using glutathione as a reducing agent. Glutathione disulfide (GSSG) (oxidized form of glutathione) is produced from the activity of DHAR. GSSG is reduced back to glutathione (GSH) by Glutathione reductase (GR EC 1.8.1.7) at the expense of NADP+ (Badejo et al. 2012; Smirnoff 2011)
In humans, AsA similarly protects cells against ROS, and a high daily intake of vitamin C (AsA) is beneficial to health. Tomatoes are a particularly good source of AsA and this, together with their desirable organoleptic qualities, has led to an all year round demand for fresh tomatoes in the market (di Matteo et al. 2010; Hazra and Dutta 2010). The increasing market need for fresh tomato fruits directed the farmers to an extension of growing season and consequently, to cultivate tomato plants during periods of year when weather conditions are less favorable for satisfactory production. Growing season extension has a significant impact on plants especially because pollination and fertilization are greatly affected by low or high temperatures resulting poor fruit set (Hazra and Dutta 2010). To overcome these limitations growers resort to treat tomato plants with synthetic auxin compounds to produce seedless-parthenocarpic fruits. This technique consists a very popular agricultural practice followed in certain parts of the world, ensuring satisfactory early or belated crops and it is a practical way to minimize heating costs in greenhouses and tunnels (de Jong et al. 2009). Apart from the financial aspect, parthenocarpic fruits are considered to have useful properties for the processing industry, such as increased soluble content which results in lower processing costs due to the absence of seeds (Pandolfini et al. 2002). Although the agricultural techniques and the financial importance of parthenocarpic fruits production have been establish, the impact of auxin application (parthenocarpy) on vitamin C concentrations, within the fruit, remains unknown.
Recently, our research group reported that developing seeded and parthenocarpic fruits of cherry tomato exhibited similar levels of tAsA (Tsaniklidis et al. 2012), although an inconsistency between the expression of certain genes with a pivotal role in AsA biosynthesis and AsA levels in both seeded large-fruiting (Badejo et al. 2012) and seeded and parthenocarpic cherry tomatoes (Tsaniklidis et al. 2012) was observed, suggesting multi-level control of the antioxidant system during fruit development.
In the present study, we examine the accumulation levels of DHA, DHA/free AsA and total AsA, the expression of all currently characterized gene isoforms involved in the Foyer, Halliwell, Ashada (l-ascorbic acid-glutathione) cycle, the ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR) enzyme activities as well as the cellular localization of cytosolic APX within seeded and seedless (parthenocarpic) cherry tomatoes. Our objectives are to elucidate the pattern of AsA metabolism in tomato fruit during development and maturation, and to determine possible differences in the AsA metabolism of parthenocarpic fruit set by auxin and seeded fruit derived from natural pollination.
Materials and methods
Plant material and growth conditions
Cherry tomatoes (Solanum lycopersicum L. var. cerasiforme cv. Conchita F1; de Ruiter seeds, Melbourne Australia), were grown in a glasshouse of the Agricultural University of Athens, Greece between December and May. Mean minimum and maximum temperatures in the greenhouse were 12.9 ± 1.9 and 23.9 ± 4.4 °C, respectively, in winter (Dec–Feb) and 15.7 ± 2.0 and 26.6 ± 4.3 °C, respectively, in spring (March–May). Solar radiation varied between 700–1,350 μmol m−2 s−1 PAR (winter) and 700–1,400 μmol m−2 s−1 PAR (spring). Parthenocarpic fruits were produced by the emasculation of closed flowers and subsequent spraying (once immediately after emasculation) with Ortomone, a commercial synthetic auxin preparation (Spirou, Athens Greece), containing 50 ppm 2-naphthoxyacetic acid. The hormone solution used was 0.3 ml per flower. Individual flowers were tagged after treatment. Fruits were harvested in the morning between 10 and 11 am at the following stages of development and maturation: immature green with a diameter of 15 mm at 25 days after flowering DAF (ImG), mature green at 38 DAF (Mg), breaker stage with <10 % red colour at 44 DAF (Br) and red ripe at 52 DAF (RR). Three harvests per stage were performed (in the same day and time for all stages), thus creating 3 replicates. Samples were immediately frozen in liquid nitrogen, homogenized using a pestle and mortar and then stored at −80 °C.
qPCR analysis
Real time experiments were conducted as previously described by Delis et al. (2011). Samples for qPCR analysis were prepared by mixing 1 g from each sample. Total RNA was isolated using an RNeasy extraction kit (Qiagen, Hilden Germany). The quality of total RNA was examined spectrophotometrically by measuring the absorbance at 260 nm and the absorbance ratio of 260/280 nm in Nanodrop (Thermo, Wilmington USA) and on 1.5 % w/v agarose gel. To eliminate genomic DNA, samples were treated with DNase I (Takara, Otsu Shiga Japan) at 37 °C for 30 min. Ubiquitin (UBQ) primers were used on PCR to test for complete DNA removal using S. lycopersicum genomic DNA as the positive control.
First strand cDNA was reverse transcribed with Affinity Script™ Multi Temperature (Stratagene, Santa Clara, USA) from 2 μg of DNase-treated total RNA at 42 °C for 5 min and 55 °C for 60 min using oligo(dT) primer according to the manufacturer’s instructions. The resulting first-strand cDNA was normalized for the expression of the house-keeping gene of UBQ. Gene specific primers for APX (Najami et al. 2008; Aoki et al. 2010), MDHAR (Grantz et al. 1995; Li et al. 2010a), DHAR (Zou et al. 2006; Chen and Gallie 2006), GR (Shu et al. 2011; Henmi et al. 2005) and UBQ genes were designed with Beacon designer v 7.01 software (Premier biosoft, Palo Alto, USA).
qPCR reactions were performed on the MX-3005P system (Stratagene, Santa Clara, USA) using Kapa Probe Fast Universal 2× qPCR Master Mix (Kapa, Woburn, USA) and gene-specific primers following the manufacturer’s instructions. Denaturation was performed at 95 °C for 3 s, annealing was performed at 60 °C for 45 s and elongation was performed at 72 °C for 11 s. The primer specificity and the formation of primer-dimers were monitored by dissociation curve analysis and agarose gel electrophoresis on a 3 % (w/v) gel. In all samples a single amplicon was detected.
The expression levels of a S. lycopersicum UBQ gene were used as internal standards to normalize small differences in the amounts of cDNA template. For the relative quantification of gene expression, a modification of the comparative threshold cycle method was used. The relative abundance of all transcripts amplified was normalized to the constitutive expression level of UBQ mRNA. Relative transcript levels in different samples for the gene of interest were calculated as a ratio against the UBQ gene transcripts. Data were analyzed as previously described (Tsaniklidis et al. 2012). PCR efficiency (E) for each amplicon was calculated employing linear regression of the log (fluorescence) per cycle number data, using LinRegPCR software. For all samples, the qPCR reaction was performed in triplicate for each gene.
Ascorbic acid determination
The AsA and DHA contents of tomatoes were determined according to the method of Davey (2003). Briefly, fruits were harvested, homogenized in liquid nitrogen and total AsA was extracted from 500 mg homogenate inside a pre-chilled Eppendorf with the addition of 1 ml of 6 % cold metaphosphoric acid (Panreac, Barcelona Spain), 2 mM EDTA (Sigma-Aldrich, St. Louis, USA) and 1 % insoluble PVP (MP Biochemicals, Eschwege Germany). The samples were centrifuged at 13,000g at 4 °C for 15 min. The supernatants were removed and stored on ice; 500 μl of the extraction medium was added to the precipitate and a second centrifugation followed. Extracts were pooled and used for AsA and DHA quantitation by HPLC.
HPLC analyses were conducted using a Prominence HPLC system (Shimadzu, Tokyo Japan) equipped with a DGU-20A5 degasser, an SPD-M20A UV–Vis photodiode array detector and a CTO-20A thermo-stated column compartment. A 20 μl aliquot of extract was injected into a Zorbax Stablebond-C18 column, 5 μm, 250 × 4.6 mm (Agilent Technologies, Palo Alto, USA). Sample analytes were separated according to Davey (2003) and quantitative determination was based on a reference curve at 243 nm. For DHA quantitation, to 500 μl of fruit extract, 250 μl of 200 mM DTT in 250 μl 400 mM Tris base was added and incubated for 15 min at room temperature; the reaction was stopped with 250 μl 8 % o-phosphoric acid (Carlo Erba, Rodano, Italy) and the total l-AsA was quantified by HPLC. The amount of DHA was determined as the difference between the reference and the above measurement. Pure DHA (Sigma-Aldrich) was used for the reference curve.
Immunohistolocalization of APX
Polyclonal antibodies coded AS06 180 (Agrisera, Vannas, Sweden) were directed against cytosolic APX protein. Sliced equatorial tomato fruits at each stage of maturity were fixed in 4 % (v/v) paraformaldehyde and 0.25 % (v/v) glutaraldehyde (Sigma-Aldrich) in 10 mM phosphate buffer (pH 7) (Merck-Darmstadt, Germany) at 4 °C, overnight. Embedding in paraffin (Gurr, Poole, UK) and sectioning was carried out as previously described (Tsaniklidis et al. 2012). The slices were block-stained with 0.5 % (w/v) saffranin (Gurr) Sections (12 μm for smaller fruits or 20 μm for larger fruits) were blocked for 1 h in TBS (10 mM Tris–HCl {Sigma-Aldrich}, 100 mM NaCl, pH 8, containing 3 % (w/v) BSA {Pan biotech, Aidenbach, Germany} and 0.05 % (w/v) Tween-20 {Atlas chemicals, Amman, Jordan}) at room temperature, and incubated overnight with an antiserum raised against APX. Pre-immune serum and immune serum were used at a dilution of 1:1,500. Alkaline phosphatase-conjugated anti-rabbit IgGs (Promega, Madison, USA) diluted 1:5,000 in TBS containing 1 % (w/v) BSA and 0.05 % (v/v) Tween-20 were used as secondary antibodies. Signal detection was performed using the alkaline phosphatase substrate, 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Promega). No signal over background was observed using the pre-immune serum as a control.
The sections were examined using a Zeiss Axiolab (Carl Zeiss, Jena, Germany) microscope and photographed with a Powershot A620 digital camera (Canon, Tokyo, Japan).
APX assay
Total APX activity was assessed using the method proposed by Panchuk et al. (2002) with slight modifications. Frozen tissue (400 mg) was ground in liquid nitrogen and mixed with 2 mL extraction buffer containing 50 mM Sodium-phosphate (pH 7.0), 0.25 mM EDTA (Sigma-Aldrich), 2 % soluble polyvinylpyrrolidone—PVP-40 (MP Biochemicals), 10 % (w/v) glycerol (Sigma-Aldrich), and 4 mM l-AsA and centrifuged at 13,000g for 10 min at 2 °C. The supernatant was collected, filtered and immediately used for the estimation of APX activity with a reaction solution containing 25 mM Sodium-phosphate (pH 7.0), 0.1 mM EDTA, 0.2 mM H2O2 and 4 mM l-AsA. 200 μL protein extract with 850 μL of the reaction solution were used to perform the assay. The oxidation rate of l-AsA was assayed spectrophotometrically at 265 nm in a Shimadzu (Tokyo, Japan) UV 160A spectrophotometer using UV cuvettes (Ratiolab, Dreieich, Germany). The assay was made at 10 min intervals at room temperature. Predefined concentrations of AsA were used for the reference curve.
DHAR enzyme assay
Dehydroascorbate reductase activity was assessed using a modified Chen and Gallie (2006) protocol. Tissue (200 mg) was ground in liquid nitrogen and mixed with 2 mL of extraction buffer containing 50 mM Tris HCl (pH 7.4), 100 mM NaCl (Sigma-Aldrich), 2 mM EDTA, 1 mM MgCl2 (MP Biochemicals) and 0.5 % soluble PVP and centrifuged at 13,000g for 10 min at 2 °C. The supernatant was collected, filtered and used for the estimation of DHAR activity with a reaction mixture that contained 50 mM MES-NaOH (pH 6.3) (MP Biochemicals), 2 mM DHA and 5 mM reduced glutathione (Sigma-Aldrich). 850 μL of the reaction mixture were used along with 150 μL protein extract in a total volume of 1 mL. The reduction rate of AsA was assayed spectrophotometrically at 265 nm at 10 min intervals in a Shimadzu UV 160A spectrophotometer using UV cuvettes at room temperature. Predefined concentrations of AsA were used for the reference curve.
Statistical analysis
Statistics were performed using Statgraphics Centurion (Statpoint Technologies, Warrenton, USA). Significant differences between treatments were determined by two-way ANOVA and post hoc comparisons by least significant difference (p < 5 %).
Results
DHA and total ascorbate (tAsA) concentration in fruits
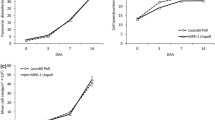
Total ascorbate concentration within the tomato fruits ranged from 1.1 to 1.7 μmol/g FW irrespective of the presence or absence of seeds. At the immature green stage (ImG), ascorbate was present mainly in the oxidized (DHA) form, whereas as ripening proceeded the concentration of reduced AsA increased and DHA decreased (Fig. 2).
Oxidized AsA (DHA) and total AsA (reduced + oxidized AsA) concentration in developing seeded and parthenocarpic cherry tomato fruits of Conchita hybrid. ImG immature green-15 mm diameter (25 DAF), Mg mature green (38 DAF), Br breaker (44 DAF), RR red ripe (52 DAF). Bars represent means (±SE) of three biological replications
The expression of genes involved in the Foyer–Halliwell–Asada cycle
APX expression
Cytosolic localized APX1, APX2 and APX4 in both seeded and parthenocarpic fruits, revealed a similar pattern of expression with comparable transcript accumulation levels among the genes (Fig. 3). In each case, expression was highest at the ImG stage and decreased with ripening. The expression of APX1 was higher in seeded fruit than in parthenocarpic fruit from stages ImG to Br, but not at full ripeness (RR), whereas the expression of APX2 was higher in seeded fruit than in parthenocarpic fruit at all stages of ripeness. On the other hand, the expression of APX4 was similar in both types of fruit at all stages except Mg, where expression was higher in the seeded fruit. In contrast, the expression of APX3 followed a different pattern of expression with the levels of transcripts progressively accumulating from stage ImG to Br in seeded fruit and from ImG to RR in parthenocarpic fruit. Expression of APX3 was higher in seeded fruit at stages Mg and Br and in parthenocarpic fruit at stage ImG, whereas at stage RR no differences between the two types of fruit were observed. The expression of the cytosolic APX5 gene, as well as that of the plastidial APX6 and APX7 genes, was very low. Transcript accumulation of APX5 was higher at stages ImG and Mg (i.e. prior to the climacteric) and decreased significantly during the post-climacteric stages (Br and RR). APX5 expression was also significantly higher in seeded fruit than in parthenocarpic fruit from stage ImG to stage Br. Apart from a progressive increase in the relative expression of APX7 throughout the maturation and ripening of seeded fruit, no other consistent patterns of transcript accumulation was noted in APX7 (pathenocarpic fruit) or APX6 (both fruit types) (Fig. 3).
Accumulation of APX isoform gene transcripts in developing seeded and parthenocarpic cherry tomato fruits of conchita hybrid. Total RNA was isolated from tomato fruits of the following stages of development. ImG immature green-15 mm diameter (25 DAF), Mg mature green (38 DAF), Br breaker (44 DAF), RR red ripe (52 DAF). The RNA was reverse transcribed to cDNA and subjected to qPCR. Relative mRNA level was calculated with respect to the level of UBQ transcript. Bars represent means (+SE) of three biological replications. Different letters indicates significant differences
MDHAR expression
Cytosolic MDHAR1 showed a different expression pattern in seeded and parthenocarpic fruits (Fig. 4). In seeded fruits MDHAR1 transcripts progressively increased during fruit development and ripening, whereas in parthenocarpic fruit a peak in MDHAR1 gene expression was found at stage Mg (just prior to the climacteric) but expression was low at all other stages (ImG, Br and RR). Apart from stage ImG, expression of MDHAR1 was significantly higher in seeded fruit than in the corresponding parthenocarpic fruit. Plastidial MDHAR2 expression in parthenocarpic and seeded fruit was similar at stages ImG and Br, whereas at stages Mg and RR the expression of MDHAR2 was higher in seeded fruits. The expression of MDHAR2, similar to that of MDHAR1 was higher at full ripeness in seeded fruit than in parthenocarpic fruit, but not at other stages of maturation and ripening (Fig. 4).
Accumulation of AsA recycle enzymes transcripts in developing seeded and parthenocarpic cherry tomato fruits of conchita hybrid. Different isoforms are noted on the figure. Total RNA was isolated from tomato fruits of the following stages of development, ImG immature green-15 mm diameter (25 DAF), Mg mature green (38 DAF), Br breaker (44 DAF), RR red ripe (52 DAF). The RNA was reverse transcribed to cDNA and subjected to qPCR. Relative mRNA level was calculated with respect to the level of UBQ transcript. Bars represent means (+SE) of three biological replications. Different letters indicates significant differences
DHAR expression
Cytosolic DHAR followed a similar pattern in both seeded and parthenocarpic fruits, namely a progressive decrease in expression from stage ImG to stage RR (Fig. 4). The accumulation of DHAR1 transcripts was higher in seeded fruit than in parthenocarpic fruit prior to the climacteric (stages ImG and Mg), but in the subsequent stages of ripening (Br and RR) gene expression did not differ between the two types of fruit. Overall, therefore, the expression of cytosolic DHAR1 was similar to that of cytosolic APX1, 2 and 4. The plastidial DHAR2 transcript accumulation was significantly lower than that of cytosolic DHAR1. In seeded fruit DHAR2 expression did not change during maturation and ripening, whereas in parthenocarpic fruit, there was a decrease in DHAR2 expression just prior to and after the climacteric (stages Mg and Br).
GR expression
Cytosolic GR1 gene expression followed similar patterns in seeded and parthenocarpic fruit: GR1 expression was very low before fruit maturation (stage ImG) but increased by over tenfold just prior to the climacteric (stage Mg). Following the climacteric, GR1 expression progressively decreased to a very low level in parthenocarpic fruit, whereas in seeded fruit expression fluctuated, but remained relatively high. For example, at stage RR, GR1 expression was sevenfold higher in seeded fruit than in parthenocarpic fruit (Fig. 4). Plastidial GR2 expression in seeded fruit was very low at the immature green stage (ImG) but subsequently increased (stage Mg) and, despite a decrease at stage Br, remained high to full ripeness (stage RR). In parthenocarpic fruit, the level of GR2 expression did not change between stages ImG and Br, but decreased at full ripeness (stage RR). From stage Br to full ripeness (stage RR) GR2 expression was significantly higher in seeded fruit than in parthenocarpic fruit, although overall the expression of GR genes was relatively low in comparison with the other genes implicated in the Foyer–Halliwell–Asada cycle.
Enzyme activities
APX activity
The APX activity profile was different in seeded and parthenocarpic fruit. In seeded fruit APX activity was highest prior to the climacteric (stages ImG and Mg), but progressively decreased thereafter. In contrast, in parthenocarpic fruit APX activity increased during fruit maturation (from stage ImG to stage Mg) and then remained constant. Overall, APX activity ranged from 48 to 72 μM/g FW*min in all fruits that were examined (Fig. 5).
DHAR activity
Dehydroascorbate reductase activity followed similar patterns in developing seeded and parthenocarpic fruit (Fig. 5) and was about fourfold higher at stage ImG than at the other stages of maturation and ripening. After a sharp decrease between stages ImG and Mg, DHAR activity fluctuated a little but remained relatively low. Overall, DHAR activity ranged between 9 and 62 μM/g FW*min.
Immunolocalization of APX protein in tomato fruits
The precise cellular distribution of the cytosolic APX proteins in seeded and parthenocarpic cherry tomato fruit tissues was examined by immunolocalization (Fig. 6). The APX proteins were ubiquitous in all the tissues examined, while the highest levels of immunolocalization signal were detected in the young seeded and parthenocarpic fruits. In seeded fruit, the signal was consistently more intense in the seeds throughout fruit maturation and ripening.
Immunolocalization of APX in developing cherry tomato fruits. The proteins were visualized as a darker staining. C embryonic cotyledon, ES endosperm, LP locular parenchyma, Ov ovule, P pericarp, R embryonic root, VB vascular bundle, 1 immature green-15 mm diameter, 2 mature green, 3 breaker; 4 red ripe, 5 red ripe seeds. a Seeded fruits (left column), b parthenocarpic fruits (right column). Inc 1–4 fruits in the before mentioned stages of development incubated with pre-immune serum and post-stained with saffranin
Discussion
The aim of this study was to examine one of the most important antioxidant systems of plants, the Foyer–Halliwell–Asada cycle, in tomato fruits during maturation and ripening of parthenocarpic and seeded fruits.
DHA and tAsA content of fruits during development and ripening
AsA, a low-molecular weight antioxidant, is present at millimolar concentrations in a variety of plant cells (Ishikawa and Shigeoka 2008) and participates in a range of biochemical reactions. Due to the multiple physiological and biochemical roles of AsA, its concentration varies, among others, with plant age (Zhang et al. 2009), sampling time and light intensity (Bartoli et al. 2006). In the leaves of higher plants, concentrations of 2–20 μmol/g FW have been reported (Gest et al. 2013).
tAsA levels in cherry tomato fruits ranged from 1.16 to 1.78 μmol/g FW (Fig. 2). Similar levels (1.6–2 μmol/g FW) have been reported for seeded large sized tomatoes (Alhagdow et al. 2007; Marin et al. 2004). Apart from a slight decrease just prior to the climacteric (Fig. 2 stage Mg), the tAsA concentration in seeded fruit remained constant. A similar pattern of DHA concentration has also been reported for pepper (Marin et al. 2004) and kiwi (Li et al. 2010b).
The fact that tAsA was rather constant during fruit development suggests that there is a pool of ascorbate in fruits in which reduced and oxidized AsA interconvert. So, the high levels of DHA in ImG fruits could point to the greater use of reduced AsA in the early stages of fruit development. (Raffo et al. 2002).
The elevated levels of AsA in parthenocarpic fruits during maturation could possibly be ascribed to the different proportions of pericarp and locular parenchyma (Stevens et al. 2007; de Jong et al. 2009). Indeed results of our group (data will be presented in a forthcoming paper) show higher tASA concentration in the pericarp of both parthenocarpic and seeded fruits. Different AsA levels in apple fruit parts have been also recorded (Li et al. 2008).
Ascorbate peroxidase activity and localization. Activity of DHAR
Ascorbate peroxidase activity was relatively stable during fruit development and maturation (Fig. 5), which could be a result of the activity of overlapped APX isoforms at each developmental stage. Similar data have been reported for large-sized tomato fruits during ripening (Jimenez et al. 2002).
The immunolocalization of APX (Fig. 6) confirmed the presence of the cytosolic enzyme during fruit maturation and ripening. The sole difference found between seeded and parthenocarpic fruits was the presence of the enzyme in the seed at all stages of its development. De Pinto and de Gara (2004) proposed that a correlation exists between cell division and APX presence and activity; our results suggest that a similar kind of connection also exists in the developing seeds of cherry tomato. However the strong presence of the APX protein in seeds did not contribute to the total APX activity, probably because of the redox state of AsA within the seeds and the need of the APX enzyme for reduced AsA in order to be functional (Tommasi et al. 1999; Shigeoka et al. 2002).
According to our results, the DHA content of cherry tomatoes is directly connected to DHAR activity regardless of fruit maturation and ripening (Figs. 3, 5). Thus, both seeded and parthenocarpic fruits exhibited the highest levels of DHA, the highest DHAR activity and the highest expression of DHAR1 at the ImG stage, and all three of these parameters decreased in parallel during stages Mg, Br and RR. High levels of DHA probably induce gene expression and enzyme activity.
Ascorbate peroxidase expression
The APX gene family in higher plants consists in most cases of multiple isoforms. Seven genes coding for APX have been reported in tomato plants, five of which are localized in the cytoplasm and two in the plastids (Aoki et al. 2010). The localization of APX isoforms results in distinct roles against different targets of antioxidant potential within each cell compartment and is likely to be expressed by different regulatory mechanisms (Ishikawa and Shigeoka 2008).
We attempted for the first time to study simultaneously the expression of all characterized APX genes within parthenocarpic and seeded tomato fruit during development. In general, the cytosolic isoforms APX1, APX2, APX3 and APX4 (Fig. 3). exhibited the same pattern of expression as fruit maturation and ripening proceeded in both types of fruit. APX5 expression levels were significantly lower than those of APX 1, APX2 and APX4 and its transcripts were almost tenfold higher in seeded fruits. This could be due to the seed development (Arrigoni et al. 1992) It has been suggested that cytosolic APX along with the plastidial isoforms protects chloroplast proteins from oxidation (Davletova et al. 2005). Since photosynthesis is active up to the mature green stage in fruits, it could be hypothesized that the higher abundance of transcripts found in immature green fruits may relate to their protection from oxidation (Forti and Elli 1995). APX3 expression exhibited a pattern that was the reverse of that in the cytosolic APX1, APX2 and APX4. A similar pattern of APX3 expression has been observed during fruit development of strawberry (Kim and Chung 1998).
A slightly different pattern of a cytosolic APX expression was reported by Jimenez et al. (2002) in larger-sized tomatoes but the gene studied was not identified.
A different pattern of expression was observed in the plastidial APX genes. The thylakoid APX6 is mainly expressed during the ImG and Mg stage. Possibly when the chloroplast is functional, higher APX activity is needed to protect chloroplast proteins. In Caspicum annuum a higher APX increase was found during the chloroplast to chromoplast transition, whereas only a slight difference was detected in total fruit extracts (Schantz et al. 1995). Finally, expression of the APX7 isoform gradually increased from the ImG to the RR stage in seeded tomato fruits, while in parthenocarpic fruits expression did not significantly change. The difference in the APX expression between seeded and parthenocarpic fruits could possibly be correlated with seed development and subsequent drying.
Expression of the AsA recycle genes
The two MDHAR genes exhibited different patterns of expression (Fig. 4) and showed significant differences among parthenocarpic and seeded fruits. The expression of the cytosolic MDHAR1 gene progressively increased in seeded fruit, while its expression in parthenocarpic fruit showed a peak just prior to the climacteric (stage Mg). On the contrary, expression of the plastidial MDHAR2 gene fluctuated during fruit maturation and ripening, and was significantly higher in seeded fruit than in parthenocarpic fruit at stages Mg and RR. Our results concerning cytosolic MDHAR1 in seeded fruits are in general agreement with those of Eltelib et al. (2012) and Bulley et al. (2009), who reported higher MDHAR expression in mature fruits.
The MDHAR1 isoform is likely to be present in both the cytosol and peroxisomes. Such dual targeting is also observed for another AsA antioxidant cycle enzyme, GR, in Arabidopsis (Kataya and Reumann 2010).
Monodehydroascorbate reductase activity (about 0.5 μM/g FW*min) was detected only at the ImG stage in both seeded and parthenocarpic fruits (data not shown). It is probable that under normal growing conditions fruit MDHAR activity is low compared to DHAR. Haroldsen et al. (2011) reported a four-fold higher activity of DHAR than MDHAR in tomato leaves under non-stress conditions; this ratio may be even higher in fruits.
The plastidial DHAR2 gene appeared to have a similar housekeeping expression to the plastidial MDHAR2. However, the cytosolic DHAR1 gene revealed the opposite trend to that of its equivalent cytosolic MDHAR1 gene, indicating a different regulation of cytosolic and plastidial isoforms. A gradual decrease in DHAR1 expression levels was recorded in both seeded and parthenocarpic fruits during maturation, but a corresponding increase in MDHAR1 expression occurred only in seeded fruit. Eltelib et al. (2012) reported a pattern of expression of a cytosolic DHAR in acerola that was similar to the pattern observed here.
Another interesting assumption may be derived from the comparison of the activities of DHAR and APX. DHAR seemed to have the capacity to reduce much of the DHA produced by the activity of APX at the ImG stage. This underlines the importance of DHAR in AsA metabolism during fruit development under normal conditions. The role of DHAR in maintaining the AsA pool has been proved in tobacco and maize. Chen et al. (2003) produced tobacco and maize kernels with elevated AsA levels (two- to four-fold increase) by overexpressing a DHAR from wheat and demonstrating that AsA levels could be enhanced through AsA recycling. From a comparison of enzyme activities, it appears that after the ImG stage, DHAR activity could only reduce a portion of the DHA produced. Due to the complexity of the regulation of AsA metabolism, some other factor alone or in combination, like in situ biosynthesis, long distance transport or pectin breakdown, could have contributed to the elevated levels of the reduced antioxidant in ripening fruits (Ishikawa et al. 2006; Badejo et al. 2012). Furthermore DHA is promptly metabolized to various other products, therefore keeping the tAsA levels in fruits constant (Badejo et al. 2012).
By comparing seeded and parthenocarpic fruits, a possible effect of seed development can be detected in plastidial GR2 expression. Interestingly, Ioannidi et al. (2009) reported results on the expression pattern of plastidial GR2 that were similar to those of this experiment, but studying the expression of GR in comparison to other factors showed no clear pattern. This inconsistency in GR expression suggested that while the enzyme is considered a part of the Foyer–Halliwell–Asada cycle, probably because of the multifunctional role of glutathione in plant cells (Noctor et al. 2002), GR gene expression seems to be mostly unaffected by the redox status of ascorbic acid.
Conclusion
Our results indicated differences between seeded and parthenocarpic fruits in transcription level, tAsA levels and APX activity during fruit development. Parthenocarpy down regulated the expression and enzyme activity of APX5, APX6, MDHAR1 and GR2.
Abbreviations
- AsA:
-
l-Ascorbic acid
- DHA:
-
Dehydroascorbic acid
- tAsA:
-
Total ascorbate (reduced AsA + DHA)
- ImG:
-
Immature green
- Mg:
-
Mature green
- Br:
-
Breaker
- RR:
-
Red ripe
- APX:
-
Ascorbate peroxidase
- MDHAR:
-
Monodehydroascorbate reductase
- DHAR:
-
Dehydroascorbate reductase
- GR:
-
Glutathione reductase
- ROS:
-
Reactive oxygen species
References
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Aoki K, Yano K, Suzuki A, Kawamura S, Sakurai N, Suda K, Kurabayashi A, Suzuki T, Tsugane T, Watanabe M, Ooga K, Torii M, Narita T, Shin IT, Kohara Y, Yamamoto N, Takahashi H, Watanabe Y, Egusa M, Kodama M, Ichinose Y, Kikuchi M, Fukushima S, Okabe A, Arie T, Sato Y, Yazawa K, Satoh S, Omura T, Ezura H, Shibata D (2010) Large-scale analysis of full-length cDNAs from the tomato (Solanum lycopersicum) cultivar Micro-Tom, a reference system for the Solanaceae genomics. BMC Genomics 11:210–215
Arrigoni O, De Gara L, Tommasi F, Liso R (1992) Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol 99:235–238
Badejo AA, Wada K, Gao Y, Maruta T, Sawa Y, Shigeoka S, Ishikawa T (2012) Translocation and the alternative d-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the d-mannose/l-galactose pathway. J Exp Bot 63:229–239
Bartoli CG, Yu J, Gomez F, Fernandez L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57:1621–1631
Bulley SM, Rassam M, Hoser D, Otto W, Schunemann N, Wright M, MacRae E, Gleave A, Laing W (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-l-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot 60:765–778
Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142:775–787
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. PNAS 100:3525–3530
Davey M (2003) Rocket-powered high-performance liquid chromatographic analysis of plant ascorbate and glutathione. Anal Biochem 316:74–81
Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17:268–281
de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH (2009) The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J 57:160–170
de Pinto MC, de Gara L (2004) Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J Exp Bot 55:2559–2569
Delis C, Krokida A, Georgiou S, Pena-Rodriguez LM, Kavroulakis N, Ioannou E, Roussis V, Osbourn AE, Papadopoulou KK (2011) Role of lupeol synthase in Lotus japonicus nodule formation. New Phytol 189:335–346
di Matteo A, Sacco A, Anacleria M, Pezzotti M, Delledonne M, Ferrarini A, Frusciante L, Barone A (2010) The ascorbic acid content of tomato fruits is associated with the expression of genes involved in pectin degradation. BMC Plant Biol 10:163–173
Eltelib HA, Fujikawa Y, Esaka M (2012) Overexpression of the acerola (Malpighia glabra) monodehydroascorbate reductase gene in transgenic tobacco plants results in increased ascorbate levels and enhanced tolerance to salt stress. S Afr J Bot 78:295–301
Forti G, Elli G (1995) The function of ascorbic acid in photosynthetic phosphorylation. Plant Physiol 109:1207–1211
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Gest N, Gautier H, Steevens R (2013) Ascorbate as seen through plant evolution: the rise of a successful molecule? J Exp Bot 64:33–53
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Grantz AA, Brummell DA, Bennett AB (1995) Ascorbate free radical reductase mRNA levels are induced by wounding. Plant Physiol 108:411–418
Haroldsen VM, Chi-Ham CL, Kulkarni S, Lorence A, Bennett AB (2011) Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol Biochem 49:1244–1249
Hazra P, Dutta AK (2010) Inheritance of parthenocarpy in tomato (Solanum lycopersicum) and its association with two marker characters. Int J Plant Sci 1:144–149
Henmi K, Demura T, Tsuboi S, Fukuda H, Iwabuchi M, Ogawa K (2005) Change in the redox state of glutathione regulates differentiation of tracheary elements in Zinnia cells and Arabidopsis roots. Plant Cell Physiol 46:1757–1765
Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK (2009) Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot 60:663–678
Ishikawa T, Shigeoka S (2008) Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci Biotech Biochem 72:1143–1154
Ishikawa T, Dowdle J, Smirnoff N (2006) Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plant 126:343–355
Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux P (2002) Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 214:751–758
Kataya AR, Reumann S (2010) Arabidopsis glutathione reductase 1 is dually targeted to peroxisomes and the cytosol. Plant Signal Behav 5:171–175
Kim IJ, Chung WI (1998) Molecular characterization of a cytosolic ascorbate peroxidase in strawberry fruit. Plant Sci 133:69–77
Li MJ, Ma FW, Zhang M, Pu F (2008) Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica Borkh cv. Gala). Plant Sci 174:606–612
Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW (2010a) Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol Plant 139:421–434
Li M, Ma F, Liu J, Li J (2010b) Shading the whole vines during young fruit development decreases ascorbate accumulation in kiwi. Physiol Plant 140:225–237
Marin A, Ferreres F, Tomás-Barberán F, Gil MI (2004) Characterization and quantification of antioxidant constituents of sweet pepper. J Agric Food Chem 52:3861–3869
Najami N, Janda T, Barriah W, Kayam G, Tal M, Guy M, Volokita M (2008) Ascorbate peroxidase gene family in tomato: its identification and characterization. Mol Genet Genomics 279:171–182
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Panchuk II, Volkov RA, Schoffl F (2002) Heat stress and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol 129:838–853
Pandolfini T, Rotino GL, Camerini S, Defez R, Spena A (2002) Optimization of transgene action at the post-transcriptional level: high quality parthenocarpic fruits in industrial tomatoes. BMC Biotech 2:1–11
Raffo A, Leonardi C, Fogliano V, Ambrosino P, Salucci M, Gennaro L, Bugianesi R, Giuffrida F, Quaglia G (2002) Nutritional value of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1) harvested at different ripening stages. J Agric Food Chem 50:6550–6556
Schantz ML, Schreiber H, Guillemaut P, Schantz R (1995) Changes in ascorbate peroxidase activities during fruit ripening in Capsicum annuum. FEBS Lett 23:149–152
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Shu DF, Wang LW, Duan M, Deng YS, Meng QW (2011) Antisense-mediated depletion of tomato chloroplast glutathione reductase enhances susceptibility to chilling stress. Plant Physiol Biochem 49:1228–1237
Smirnoff N (2011) Vitamin C: the metabolism and functions of ascorbic acid in plants. Adv Bot Res 9:109–177. doi:10.1016/B978-0-12-385853-5.00003-9
Stevens R, Buret M, Duffé P, Garchery C, Baldet P, Rothan C, Causse M (2007) Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol 1431:943–953
Tommasi F, Paciolla C, Arrigoni O (1999) The ascorbate system in recalcitrant and orthodox seeds. Physiol Plant 105:193–198
Tsaniklidis G, Delis C, Liakopoulos G, Karapanos I, Katinakis P, Passam HC, Aivalakis G (2012) Induced parthenocarpic cherry tomato fruits did not shown significant differences in l-ascorbate content but showed different pattern in GalLDH and GME expression. Plant Growth Regul 68:493–502
Zhang W, Lorence A, Gruszewski HA, Chevone BI, Nessler CL (2009) AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol 150:942–950
Zou L, Li H, Ouyang B, Zhang J, Ye Z (2006) Cloning and mapping of genes involved in tomato ascorbic acid biosynthesis and metabolism. Plant Sci 170:120–127
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsaniklidis, G., Delis, C., Nikoloudakis, N. et al. l-Ascorbic acid metabolism in parthenocarpic and seeded cherry tomatoes. Plant Growth Regul 72, 141–153 (2014). https://doi.org/10.1007/s10725-013-9845-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9845-0