Abstract
Parthenocarpy in tomato is often induced by auxins to overcome fertilization problems due to low temperatures. To estimate the effect of this agronomical practice on the physiology and dietary value of cherry tomato fruits we determined l-ascorbic acid, the expression and immunolocalization of galactono 1,4 lactone dehydrogenase and the expression of GDP-mannose 3′,5′-epimerase, key genes in l-ascorbic acid biosynthesis. The levels of l-ascorbic acid did not differ between seeded and parthenocarpic fruits while the relative expression of galactono 1,4 lactone dehydrogenase and GDP-mannose 3′,5′-epimerase gene transcripts showed some significant differences between seeded and parthenocarpic fruits. The galactono 1,4 lactone dehydrogenase immunohistolocalization signal was stronger in the ovules and mature embryos of seed-containing fruits. Our data suggest that although there were differences in the expression of the studied genes and in enzyme localization, these did not cause differences in the l-ascorbic acid content of parthenocarpic fruits produced by auxin application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Out of season production of tomatoes aims at meeting the increasing consumer demand for year-round fresh, high quality fruits and vegetables, especially in Northern Europe and America (van der Vorst et al. 2011).
In many European countries, especially within the Mediterranean basin, tomatoes in winter are produced in unheated greenhouses and tunnels in which temperatures are unfavorable for pollination and fertilization due to the sensitivity of pollen development and anther dehiscence to cold (de Jong et al. 2009). Because the provision of heating is expensive, many tomato growers resort to auxin application to achieve fruit set, resulting in the production of parthenocarpic fruit (De Jong et al. 2009; Mazzucato et al. 1998).
In recent years, cherry tomatoes have become increasingly favoured for fresh consumption thanks to their exceptional organoleptic and qualitative characteristics; for example, sweeter flavor, higher antioxidant, free amino acid and l-ascorbic acid (vitamin C, l-AsA) levels, longer shelf life and better marketability than the more commonly known, larger-fruiting varieties. Consequently, the commercial and economic importance of cherry tomato is increasing (Martinelli et al. 2009; Raffo et al. 2002). An important attribute of tomato fruit quality is its l-AsA content. It is reported that cultivation of tomato seedlings in a medium supplemented with auxin increased the l-AsA levels in roots (Tyburski et al. 2008). However, international bibliography lacks information about the effect of auxin induced parthenocarpy on the l-AsA content of developing and mature fruits. So we studied the l-AsA content in seeded and parthenocarpic cherry tomato fruits, as well as the expression of the l-galactono-1,4-lactone dehydrogenase (GalLDH, EC 1.3.2.3) and GDP-d-mannose-3′,5′-epimerase (GME, EC 5.1.3.18) genes, along with enzyme GalLDH immunolocalization. In various plant species, plant lines that over-express GalLDH have shown considerably increased levels of l-AsA, indicating that GalLDH plays a pivotal role in l-AsA biosynthesis (Imai et al. 2009; Liu et al. 2011). Moreover, GalLDH-silenced transgenic tomato plant lines exhibited a strong reduction in growth, thus underlining the importance of GalLDH and l-AsA biosynthesis for plant growth (Alhagdow et al. 2007).
GME is a key enzyme in l-AsA biosynthesis, contributing to both l-galactono-1,4-lactone and l-gulono-1,4-lactone pathways (Fig. 1). In Arabidopsis thaliana it has been reported that GME catalyses the epimerization of both the 3′ and 5′ positions of GDP-α-d-mannose to yield GDP-β-l-galactose or the 5′ epimerization to GDP-β-l-gulose (Major et al. 2005). Transgenic tomato lines that were RNAi-silenced for GME exhibited sharp drop in l-AsA levels along with reduced cell expansion and plant growth (Gilbert et al. 2009).
l-AsA biosynthetic pathways. Broken arrows show more than one enzymatic reaction step (Badejo et al. 2012)
Due to an evolutionary paradox, humans lost the ability to synthesize vitamin C, making its intake from their diet mandatory. Vitamin C, found mainly in fresh fruit and vegetables, is a highly effective antioxidant and is essential for the maintenance of human health through its contribution to immune system enhancement, collagen synthesis and for defense against ageing. Moreover, thanks to its antioxidant properties, vitamin C has been purported to reduce the incidence of human cancer and ischemic vascular disease (Davey et al. 2000; Poiroux-Gonord et al. 2010).
In the present study, we compared the l-AsA content, the expression of the gene and the immunolocalization of GalLDH and the expression GME, of seeded and parthenocarpic cherry tomatoes, in order to detect possible differences in the physiology and dietary value of the former as a result of auxin application.
Materials and methods
Plant materials and growth conditions
Plants of cherry tomato (Solanum lycopersicum L. var. cerasiforme cv. Conchita F1 and cv. Cherellino F1, de Ruiter seeds, Melbourne Australia), two productive hybrids with a long shelf life, were cultivated in a glasshouse of the Agricultural University of Athens, Greece between December and May. Mean minimum and maximum temperatures in the greenhouse were 15.7 ± 2.0 and 26.6 ± 4.3 °C, respectively, (Spring, {March–May}) and 12.9 ± 1.9 and 23.9 ± 4.4 °C (Winter, {Oct-Feb}). Solar radiation varied between 700 and 1,400 μmol m−2 s−1 PAR (spring) and 700–1,350 μmol m−2 s−1 PAR (winter). Seedless fruits were produced by the emasculation of closed flowers and subsequent spraying with Ortomone a commercial synthetic auxin preparation (Spirou, Athens Greece) containing 50 ppm 2-naphthoxyacetic acid. Individual flowers were tagged after treatment. Fruits were harvested systematically at the following stages: immature green (diameter 15 mm) (25 DAF-days after flowering), mature green (38 DAF), breaker (<10 % red colour) (44 DAF), and red ripe (52 DAF). Each harvest was carried out at 11 am and replicated three times. Samples were immediately frozen in liquid nitrogen, homogenized using a pestle and mortar and then stored at −80 °C.
qPCR analysis
Real time experiments were conducted as previously described (Delis et al. 2011). Total RNA was isolated from three replicates of each sample using a RNeasy extraction Kit (Qiagen, Hilden Germany). The total RNA was quantified spectrophotometrically by measuring the absorbance at 260 nm and the absorbance ratio of 260/280 nm in Nanodop (Thermo, Wilmington USA) and on 3 % w/v agarose gel. To eliminate genomic DNA, samples were treated with DNase I (Takara, Otsu Shiga Japan) at 37 °C for 30 min; subsequently. Ubiquitin (UBQ) primers were used on qPCR to test for complete DNA removal using S. lycopersicum genomic DNA as the positive control.
First strand cDNA was reverse transcribed with Affinity ScriptTM Multi Temperature (Stratagene, Santa Clara, USA) from 2 μg of DNase-treated total RNA at 42 °C for 5 min and 55 °C for 60 min using oligo(dT) primer according to the manufacturer’s instructions. The resulting first-strand cDNA was normalized for the expression of the house-keeping gene of UBQ. Gene specific primers for the tomato GalLDH (highly similar with tobacco GalLDH (Tabata et al. 2001)), GME (Gilbert et al. 2009) and UBQ genes were designed with Beacon designer v 7.01 software (Premier biosoft, Palo Alto, USA) (Table 1).
qPCR reactions were performed on the MX-3005P system (Stratagene, Santa Clara USA) using Kapa Probe Fast Universal 2X qPCR Master Mix (Kapa, Woburn, USA) and gene-specific primers following the manufacturer’s instructions. Annealing was performed at 60 °C for 45 s, elongation was performed at 72 °C for 11 s and denaturation was performed at 95 °C for 3 s. The primer specificity and the formation of primer-dimers were monitored by dissociation curve analysis and agarose gel electrophoresis on a 3 % (w/v) gel. In all samples a single amplicon was detected.
The expression levels of a S. lycopersicum UBQ gene were used as internal standards to normalize small differences in the amounts of cDNA template. For the relative quantification of gene expression, a modification of the comparative threshold cycle method was used. The relative abundance of all transcripts amplified was normalized to the constitutive expression level of UBQ mRNA. Relative transcript levels in different samples for the gene of interest were calculated as a ratio against the UBQ gene transcripts. Data were analyzed according to Pfaffi (2001) and PCR efficiency (E) for each amplicon was calculated employing linear regression of the log (fluorescence) per cycle number data, using LinRegPCR software (Ramakers et al. 2003). For all samples, the qPCR reaction was performed in triplicate for each gene.
Ascorbic acid determination
The l-AsA and DHA contents of tomatoes were determined according to the method of Davey (2003). Briefly, fruits were harvested, pulverized/homogenized in liquid nitrogen and total l-AsA was extracted from 500 mg homogenate in 1 ml of 6 % cold metaphosphoric acid (Panreac, Barcelona Spain), 2 mM EDTA (Sigma-Aldrich, St. Louis, USA) and 1 % insoluble PVP (MP Biochemicals, Eschwege Germany) on ice. The samples were centrifuged at 15,000g at 4 °C for 15 min. The supernatants were removed and stored on ice; 500 μl of the extraction medium was added to the precipitate and a second centrifugation followed. Extracts were pooled and used for l-AsA and DHA quantitation by HPLC.
HPLC analyses were conducted using a Prominence HPLC system (Shimadzu, Tokyo Japan) equipped with a DGU-20A5 degasser, an SPD-M20A UV–Vis photodiode array detector and a CTO-20A thermo-stated column compartment. A 20 μl aliquot of extract was injected into a Zorbax Stablebond-C18 column, 5 μm, 250 × 4.6 mm (Agilent Technologies, Palo Alto, USA). Sample analytes were separated according to Davey (2003) and quantitative determination based on a reference curve at 243 nm. For DHA quantitation, to 500 μl of fruit extract, 250 μl of 200 mM DTT in 250 μl 400 mM Tris base was added and incubated for 15 min at room temperature; the reaction was stopped with 250 μl 8 % o-H3PO4 (Carlo Erba, Rodano Italy) and the total l-AsA was quantified by HPLC. The amount of DHA was determined as the difference between the reference and the above measurement. Pure DHA (Sigma-Aldrich, St. Louis, USA) was used for the reference curve.
Immunohistolocalization of GalLDH
Polyclonal antibodies coded AS06 182 (Agrisera, Vannas Sweden) directed against GalLDH protein were used for immunodetection. Sliced fruits of the hybrid Conchita were collected at Immature green, Mature Green, Breaker and Red ripe stages of development (25, 38, 44 and 52 DAF respectively), fixed in 4 % (v/v) paraformaldehyde and 0.25 % (v/v) glutaraldehyde (Sigma-Aldrich, St. Louis USA) in 10 mM phosphate buffer (pH 7) (Merck-Darmstadt, Germany) at 4 °C, overnight. Embedding in paraffin (Gurr, Poole, UK) and sectioning was carried out as previously described (Kavroulakis et al., 2000). Sliced fruits were block-stained with 0.5 % (w/v) saffranin (Gurr, Poole, UK). Sections (12 μm for smaller fruits or 20 μm for larger fruits) were blocked for 1 h in TBS (10 mM Tris–HCl (Sigma-Aldrich, St. Louis, USA) 100 mM NaCl (Sigma-Aldrich, St. Louis, USA), pH 8) containing 3 % (w/v) BSA (Pan biotech, Aidenbach, Germany) and 0.05 % (w/v) Tween-20 (Atlas chemicals, Amman, Jordan) at room temperature, and incubated overnight with an antiserum raised against GalLDH. Pre-immune serum and immune serum were used at a dilution of 1:1,500. Alkaline phosphatase-conjugated anti-rabbit IgGs (Promega, Madison USA) diluted 1:5,000 in TBS containing 1 % (w/v) BSA and 0.05 % (v/v) Tween-20 were used as secondary antibodies. Signal detection was performed using the alkaline phosphatase substrate, 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Promega, Madison, USA). No signal over background was observed using the pre-immune serum as a control.
The sections were examined using a Zeiss Axiolab (Carl Zeiss, Jena, Germany) microscope and photographed with a Powershot A620 digital camera system (Canon, Tokyo, Japan).
Statistical analysis
Statistics were performed using Statgraphics Centurion (Statpoint Technologies, Warrenton, USA). Significant differences between treatments were determined by two-way ANOVA and post hoc comparisons by least significant difference (p < 5 %).
Results/discussion
To compare the role and contribution of GME and GalLDH genes in the physiological procedures and l-AsA biosynthesis of developing and mature seed-containing and parthenocarpic tomato fruits, GME and GalLDH transcripts accumulation, the localization of the GalLDH enzyme and the content of l-AsA were determined.
Expression profiles of GalLDH and GME
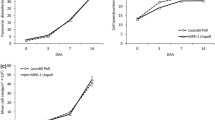
Although GalLDH and GME gene expression has been examined in tomato plants, the role of these genes in developing seed-containing and parthenocarpic fruits is less well documented (Alhagdow et al. 2007; Martinelli et al. 2009). Transcripts of GalLDH and GME were detected at all stages of fruit development irrespective of the presence or absence of seeds. The abundance of transcripts of the GalLDH gene (Fig. 2) followed a similar pattern in both seeded and parthenocarpic fruits of both hybrids, with a peak of accumulation at the breaker stage followed by a decrease at full maturity (red ripe stage) which was highly significant in the seeded fruits of both hybrids and in parhenocarpic fruit of Cherelino, but not of Conchita hybrid. The fact that the highest levels of transcripts occur at the breaker stage in both hybrids can be attributed to the concomitant climacteric rise in respiration (Alexander and Grierson 2002). The higher GalLDH expression in parthenocarpic fruits at the immature stage (15 mm) of Conchita may reflect possible differences in initial auxin concentrations within naturally pollinated and auxin-set fruits, as the later receive once a high auxin dosage in the beginning of their development, while the naturally pollinated fruits receive auxins gradually from the seeds as they develop. In Cherelino hybrid no statistical significant differences were observed at the immature green stage. In melon fruits, a peak in GalLDH transcripts was reported just prior to full maturity (Pateraki et al. 2004), as in acerola (Malpighia glabra) fruits (Badejo et al. 2009).
Recently, Ioannidi et al. (2009) reported that the highest accumulation of transcripts in seed-containing Ailsa Craig tomatoes (larger-fruiting than cherry tomato) occurred during the early stages of fruit development and negatively correlated with l-AsA levels. Our results, however, suggested a different GalLDH gene expression pattern in cherry tomato indicating the function of an active mechanism that keeps the total l-AsA pool in fruits within certain levels.
The expression of GME gene which code for an enzyme that participates in both l-galactono-1,4-lactone and l-gulono-1,4-lactone biosynthetic pathways was studied to illuminate the pivotal role of the enzyme in l-AsA biosynthesis. GME expression in Conchita followed different expression patterns comparing to GalLDH while both genes exhibiting a similar expression pattern in Cherelino (Fig. 3). The differences of gene transcripts accumulation between hybrids were most visible in breaker stage suggesting a different regulation in l-AsA biosynthesis in this developmental stage. It is worth noticing that in all tissues examined GME transcript levels were higher than GalLDH.
GME expression between seeded and parthenocarpic fruits of Conchita and Cherelino exhibited some differences in expression during fruit development, which is more noticeable in red ripe fruits.
The different GALDH and GME expression patterns in the two hybrids, could lead to hypothesis that the function of alternative l-AsA biosynthetic pathways (other than the one having as final step GalLDH) could be dependent on the genotype. Ioannidi et al. (2009) using semi-quantitative PCR, reported higher levels of GME transcripts in the early stages of fruit development in tomato, while at the breaker stage a secondary peak were also detected.
GalLDH immunolocalization
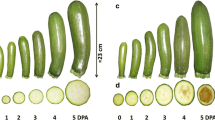
The precise cellular location of the GalLDH protein in seed-containing and parthenocarpic cherry tomato fruit tissues was examined by immunolocalization since this has not previously been reported in the literature. The GalLDH protein was localized in all tissues (Fig. 4), but the localization signal was stronger in the fast-growing tissues of both parthenocarpic and seed-containing fruits (Fig. 4-1). With the exception of the developing seed, where the signal was constantly strong, no other major differences were detected between seeded and parthenocarpic fruits. The strong localization of GalLDH in tomato seeds is unlikely to reflect a higher l-AsA concentration within the seeds, because of possible high ascorbate peroxidase activity, as was observed in mature Vicia faba embryos (Arrigoni et al. 1992). This would result in high DHA production, leaving very low levels of free l-AsA despite possible GalLDH activity inside the seeds. A decrease of l-AsA content in maturing seeds was also found in Acer platanoides and Acer pseudoplatanus (Pukacka and Ratajczak 2010), whereas significant differences in ascorbate concentration between seed-containing and transgenic parthenocarpic tomato fruits were not noted (Rotino et al. 2005).
Immunolocalization of GalLDH in developing tomato fruits of Conchita hybrid. The proteins were visualized as a darker staining. C embryonic cotyledon, ES endosperm, LP locular parenchyma, Ov ovule, P pericarp, R embryonic root, VB vascular bundle, 1 immature green-15 mm diameter, 2 mature green, 3 breaker; 4 red ripe, 5 red ripe seeds. a Seeded fruits (left column), b parthenocarpic fruits (right column). Inc 1–5 fruits in the before mentioned stages of development incubated with pre-immune serum and post-stained with saffranin
l-AsA
The levels of free and total l-AsA, were determined at all four stages of fruit development in both seed-containing and parthenocarpic fruits, which, to our knowledge, is the first time this has been done for parthenocarpic tomato fruits produced by hormone application. In contrast, however, the l-AsA content of parthenocarpic fruits from three transgenic lines has been recorded at the breaker stage (Martinelli et al. 2009). Two of those lines showed similar l-AsA concentrations with the wild type and one higher. However, there are still concerns about the cultivation of transgenic plants especially in Europe (Gomez-Galera et al. 2012).
Free l-AsA content
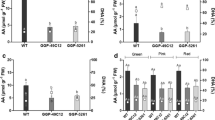
The free l-AsA content (Fig. 5) of cherry tomato fruits ranged from 0.25 to 1.4 μmol/g Fresh Weight (FW) irrespective of the presence or absence of seeds. As ripening progressed the l-AsA contents of fruits increased and at full maturity ranged from 1.36 to 1.47 μmol/g FW, values that are well within the previously reported range (Gautier et al. 2008; Ioannidi et al. 2009). The same pattern of l-AsA accumulation during fruit development and maturation was observed in both hybrids. Minor differences between seed-containing and parthenocarpic fruits occurred in the late ripening stages of Conchita. Similar results, were obtained in ripe parthenocarpic tomatoes from transgenic plants (Rotino et al. 2005).
Immature green fruits exhibited the lowest free l-AsA content (Fig. 5), but the levels of l-AsA increased as the fruits developed and matured irrespective of the presence or absence of seeds. This pattern of l-ASA accumulation during ripening was also reported by Alhagdow et al. (2007) and Gautier et al. (2008) for the fruits of two seed-containing cherry tomato cultivars and by Ioannidi et al. (2009) for large-fruiting tomato varieties.
Oxidized ascorbic acid/total l-AsA content (DHA/total l-AsA)
The percentage of DHA/total l-AsA in tomato fruits ranged from 94 to 9 % in both seed-containing and parthenocarpic fruits. As ripening progressed the percentage of DHA progressively decreased, following a similar pattern in both hybrids irrespective of whether seeds were present or not (Fig. 6).
Oxidized l-AsA (dehydroascorbic acid-DHA)/Total (free + oxidized) l-AsA ratio in developing seeded and parthenocarpic cherry tomato fruits of Conchita and Cherelino hybrids. 15 mm immature green-15 mm diameter (25 DAF), Mg mature green (38 DAF), Br breaker (44 DAF), RR red ripe (52 DAF). Bars represent means (±SE) of three biological replications
During the early stages of fruit development, l-AsA was detected mostly in its oxidized form, DHA, but during maturation the level of free l-AsA progressively increased to become the dominant form in both seed-containing and parthenocarpic fruits. Therefore the percentage ratio of DHA/total l-AsA progressively decreased as the fruits matured and ripened. These results are consistent with those of Gautier et al. (2008) who found a decrease in the DHA/total l-AsA ratio as the fruits matured. This finding can possibly be attributed to three factors.
-
a.
The high antioxidant requirement of young, actively growing fruits (Mondal et al. 2004).
-
b.
There is evidence that cell wall synthesis may be a significant sink for l-AsA (De Gara 2004).
-
c.
Catabolism of l-AsA to oxalate and the formation of calcium oxalate crystals in the idioblast. Using microautoradiography, it has been proposed that l-AsA is involved in the formation of these crystals in Pistia stratiotes L. and Medicago truncatula (Kostman et al. 2007).
Conclusion
The agricultural practice of auxin-induced fruit set allows the out of season production of tomatoes without the necessity for heat, thus lowering the cost of production, albeit in large-fruiting cultivars at the expense of fruit quality. In cherry tomato, the presence or absence of seeds has no significant effect on l-AsA content and apart from a few small differences in the physiology of seed-containing and parthenocarpic fruit, no effect of hormone application on the dietary (vitamin C) value of cherry tomatoes was detected.
References
Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53:2039–2055
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1, 4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Arrigoni O, de Gara L, Tommasi F, Liso R (1992) Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol 99:235–238
Badejo AA, Eltelib HA, Fukunaga K, Fujikawa Y, Esaka M (2009) Increase in ascorbate content of transgenic tobacco plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant Cell Physiol 50:423–428
Badejo AA, Wada K, Gao Y, Maruta T, Sawa Y, Shigeoka S, Ishikawa T (2012) Translocation and the alternative d-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the d-mannose/l-galactose pathway. J Exp Bot 63:229–239
Davey M (2003) Rocket-powered high-performance liquid chromatographic analysis of plant ascorbate and glutathione. Anal Biochem 316:74–81
Davey MD, Van Montagu M, Inze D, Sanmartin M, Kanellis AK, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J (2000) Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability, and effects of processing. J Sci Food Agric 80:825–860
de Gara L (2004) Class III peroxidases and ascorbate metabolism in plants. Phytochem Rev 3:195–205
de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH (2009) The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J 57:160–170
Delis C, Krokida A, Georgiou S, Pena-Rodriguez LM, Kavroulakis N, Ioannou E, Roussis V, Osbourn AE, Papadopoulou KK (2011) Role of lupeol synthase in Lotus japonicus nodule formation. New Phytol 189:335–346
Gautier H, Massot C, Stevens R, Serino S, Genard M (2008) Regulation of tomato fruit ascorbate content is more highly dependent on fruit irradiance than leaf irradiance. Ann Bot 103:495–504
Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B, Faurobert M, Gouble B, Page D, Garcia V, Petit J, Stevens R, Causse M, Fernie AR, Lahaye M, Rothan C, Baldet P (2009) GDP-d-mannose 3, 5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J 60:499–508
Gomez-Galera S, Twyman RM, Sparrow PA, van Droogenbroeck B, Custers R, Capell T, Christou P (2012) Field trials and tribulations-making sense of the regulations for experimental field trials of transgenic crops in Europe. Plant Biotech J. Published online doi:10.1111/j.1467-7652.2012.00681.x
Imai T, Niwa M, Ban Y, Hirai M, Ôba K, Moriguchi T (2009) Importance of the l-galactonolactone pool for enhancing the ascorbate content revealed by l-galactonolactone dehydrogenase- overexpressing tobacco plants. Plant Cell Tissue Organ Cult 96:105–112
Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK (2009) Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot 60:663–678
Kavroulakis N, Flemetakis E, Aivalakis G, Katinakis P (2000) Carbon metabolism in developing soybean root nodules: the role of carbonic anhydrase. Mol Plant Microbe Interact 13:14–22
Kostman TA, Tarlyn NM, Franceschi VR (2007) Research note: autoradiography utilising labelled ascorbic acid reveals biochemical and morphological details in diverse calcium oxalate crystal-forming species. Funct Plant Biol 34(4):339–342
Liu Y, Yu L, Wang R (2011) Level of ascorbic acid in transgenic rice for l-galactono-1, 4-lactone dehydrogenase overexpressing or suppressed is associated with plant growth and seed set. Acta Physiol Plant 33:1353–1363
Major LL, Wolucka BA, Naismith JH (2005) Structure and function of GDP-mannose-3′, 5′-epimerase: an enzyme which performs three chemical reactions at the same active site. J Am Chem Soc 127:18309–18320
Martinelli F, Uratsu SL, Reagan RL, Chen Y, Tricoli D, Fiehn O, Rocke DM, Gasser CS, Dandekar AM (2009) Gene regulation in parthenocarpic tomato fruit. J Exp Bot 60:3873–3890
Mazzucato A, Taddei AR, Soressi GP (1998) The parthenocarpic fruit (pat) mutant of tomato (Lycopersicon esculentum Mill.) sets parthenocarpic fruits and has aberrant anther and ovule development. Development 125:107–114
Mondal K, Sharma NS, Malhotra SP, Dhawan K, Singh R (2004) Antioxidant systems in ripening tomato fruits. Biol Plant 48(1):49–53
Pateraki I, Sanmartin M, Kalamaki MS, Gerasopoulos D, Kanellis AK (2004) Molecular characterization and expression studies during melon fruit development and ripening of L-galactono-1, 4-lactone dehydrogenase. J Exp Bot 55:1623–1633
Pfaffi MW (2001) A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acid Res 29:2003–2007
Poiroux-Gonord F, Bidel LP, Fanciullino AL, Gautier H, Lauri-Lopez F, Urban L (2010) Health benefits of vitamins and secondary metabolites of fruits and vegetables and prospects to increase their concentrations by agronomic approaches. J Agric Food Chem 58:12065–12082
Pukacka S, Ratajczak E (2010) Ascorbate and glutathione metabolism during development and desiccation of beech (Fagus sylvatica L.) seeds. Plant Growth Regul 62:77–83
Raffo A, Cherubino L, Fogliano V, Ambrosino P, Salucci M, Gennaro L, Bugianesi R, Giuffrida F, Qua G (2002) Nutritional value of cherry tomatoes (Lycopersicon esculentum Cv. Naomi F1) harvested at different ripening stages. J Agric Food Chem 50:6550–6556
Ramakers C, Ruijter JM, Deprez RH, Moorman AF (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339:62–66
Rotino GL, Acciarri N, Sabatini E, Mennella G, Lo Scalzo R, Maestrelli A, Molesini B, Pandolfini T, Scalzo J, Mezzetti B, Spena A (2005) Open field trial of genetically modified parthenocarpic tomato: seedlessness and fruit quality. BMC Biotech 5:32–40
Tabata K, Oba K, Suzuki K, Esaka M (2001) Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J 27(2):139–148
Tyburski J, Krzeminski L, Tretyn A (2008) Exogenous auxin affects ascorbate metabolism in roots of tomato seedlings. Plant Growth Regul 54:203–215
van der Vorst JGAJ, van Kooten O, Luning PA (2011) Towards a diagnostic instrument to identify improvement opportunities for quality controlled logistics in agrifood supply chain networks. Int J Food Syst Dyn 2:94–105
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsaniklidis, G., Delis, C., Liakopoulos, G. et al. Induced parthenocarpic cherry tomato fruits did not shown significant differences in l-ascorbate content but showed different pattern in GalLDH and GME expression. Plant Growth Regul 68, 493–502 (2012). https://doi.org/10.1007/s10725-012-9739-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9739-6