Abstract
Main conclusion
Reduced GDP-l-galactose phosphorylase expression and deficiency of ascorbic acid content lead to decreased fruit set and yield in tomato plants.
Abstract
Reduced GDP-l-galactose phosphorylase expression and deficiency of ascorbic acid content lead to decreased fruit set and yield in tomato plants. GDP-l-galactose phosphorylase (GGP) catalyzes the first step committed to ascorbic acid synthesis. The participation of GDP-l-galactose phosphorylase and ascorbate in tomato fruit production and quality was studied in this work using two SlGGP1 deficient EMS Micro-Tom mutants. The SlGGP1 mutants display decreased concentrations of ascorbate in roots, leaves, flowers, and fruit. The initiation of anthesis is delayed in ggp1 plants but the number of flowers is similar to wild type. The number of fruits is reduced in ggp1 mutants with an increased individual weight. However, the whole fruit biomass accumulation is reduced in both mutant lines. Fruits of the ggp1 plants produce more ethylene and show higher firmness and soluble solids content than the wild type after the breaker stage. Leaf CO2 uptake decreases about 50% in both ggp1 mutants at saturating light conditions; however, O2 production in an enriched CO2 atmosphere is only 19% higher in wild type leaves. Leaf conductance that is largely reduced in both mutants may be the main limitation for photosynthesis. Sink-source assays and hormone concentration were measured to determine restrictions to fruit yield. Manipulation of leaf area/fruit number relationship demonstrates that the number of fruits and not the provision of photoassimilates from the source restricts biomass accumulation in the ggp1 lines. The lower gibberellins concentration measured in the flowers would contribute to the lower fruit set, thus impacting in tomato yield. Taken as a whole these results demonstrate that ascorbate biosynthetic pathway critically participates in tomato development and fruit production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascorbate is one of the most abundant compounds in plants and there is great interest in its multiple functions as an antioxidant and enzyme cofactor (Foyer and Noctor 2011; Smirnoff 2018). It is synthesized via GDP-mannose and GDP-l-galactose and the first enzyme in this pathway considered to be specific to ascorbate synthesis is GDP-l-galactose phosphorylase (Dowdle et al. 2007; Laing et al. 2007; Linster et al. 2007). It is encoded by paralogues in various species, including arabidopsis (VTC2 and VTC5). Double vtc2 vtc5 mutants which are unable to make ascorbate are not viable but can be rescued by ascorbate supplementation (Dowdle et al. 2007; Lim et al. 2016). A range of other vtc mutants and transgenic plants in different parts of the ascorbate biosynthesis pathway with 10–20% of wild type ascorbate concentrations grow relatively normally but exhibit various subtle developmental changes, increased sensitivity to environmental stresses and increased basal resistance to pathogens (Pavet et al. 2005; Barth et al. 2006; Senn et al. 2016; Caviglia et al. 2018; Plumb et al. 2018). Therefore, it is apparent that relatively severe decreases in ascorbate still enable its essential functions while higher concentrations must be assumed to be beneficial. Notably, high light intensity increases ascorbate concentration in leaves, associated with its role in removal of hydrogen peroxide and in photoprotection (Asada 1999; Bartoli et al. 2006). GDP-l-galactose phosphorylase expression is strongly controlled by light and repressed by high ascorbate in part via a conserved upstream open reading frame (uORF) in the 5′-UTR. This, along with over-expression experiments, strongly supports its role in controlling ascorbate biosynthesis (Dowdle et al. 2007; Gao et al. 2011; Yoshimura et al. 2014; Laing et al. 2015; Macknight et al. 2017; Li et al. 2018).
Tomatoes are of interest as a source of ascorbate in the diet and control of its synthesis and functions have been investigated by altering expression of various biosynthesis genes (Alhagdow et al. 2007; Gilbert et al. 2009, 2016). The availability of tomato plants with GDP-l-galactose phosphorylase deficiency enables the role of this enzyme and ascorbate in fruit production and quality (Baldet et al. 2013). Two GGP genes encode GDP-l-galactose phosphorylase in tomato with complementary function, and SlGGP1 is about thousand time more expressed than SlGGP2 (Massot et al. 2012). The Slggp1 mutant, although expressing GGP2, had low ascorbate concentration in its leaves. When this Slggp1 mutant was submitted to high irradiance conditions chlorophyll bleaching was observed (Baldet et al. 2013). In addition, transformed tomato with decreased GDP-l-galactose phosphorylase expression display increased damage when exposed to chilling (Wang et al. 2013; Yang et al. 2017). These results provide evidence for the increased susceptibility of GDP-l-galactose phosphorylase deficient tomato plants to stress. However, studies focused in modifications at the level of the fruit have not been done yet. The work focuses on the effects of GDP-l-galactose phosphorylase expression and associated ascorbate deficiency on tomato fruit yield and quality.
Material and methods
The experiments were carried out with Solanum lycopersicum L cv Micro-Tom plants with two lines deficient in expression of the GGP1 gene encoding GDP-l-galactose phosphorylase. The two EMS mutant Micro-Tom lines used here, GGP-5261 and GGP-49C12 were, respectively, from the NBRP-Tomato population (Tsukuba-Japan) and TILLING-Tomato collection (Bordeaux-France). They are truncation and splice junction mutants, respectively (Baldet et al. 2013). Plants were grown hydroponically in an air-conditioned greenhouse during spring and summer seasons under an irradiance of 700 µmol photons m−2 s−1 at midday and temperatures average of 25 ± 2 and 20 ± 3 °C during the day and night, respectively. Each experiment included 10 plants of each genotype and the harvest was taken when half mutant plants were bearing the first fully red ripe fruit.
Exogenous treatment of ascorbic acid was initiated when seedlings were twenty days-old and finished after the development of the fourth inflorescence. Each plant received 1 mL of 20 mM ascorbic acid solution (including 0.01% tween 20 as a surfactant) wetting all above ground organs (mainly in the adaxial side of the leaves). The treatment was repeated four times a week.
Sink-source experiment
A sink-source experiment was performed leaving only 4 inflorescences on each plant. The treatments consisted of plants with one (1F) or two (2F) tomatoes per inflorescence, two levels of leaf pruning ( − L and = L, for 50 and 75% of leaf area removal, respectively) and a control without organ removal (leaving only 4 inflorescences). Three independent experiments were carried out including at least five plants per treatment for each genotype (i.e., 75 plants for each experiment). Measurements were made when half mutant plants got at least one red fruit.
Ascorbate determination
Ascorbate was determined with a HPLC system (Shimadzu LC-10Atvp solvent delivery module and Shimadzu UV–Vis SPD-10Avp detector) as previously described (Bartoli et al. 2006). Root, leaf, flower, and fruit tissues were ground in 6% (v/v) trifluoracetic acid, centrifuged at 13000×g for 5 min and supernatants used for the measurements. Total AA was determined after the treatment of an aliquot with 5 mM dithiothreitol (DTT). Oxidized AA was calculated as the difference between samples with or without DTT.
Photosynthesis measurements
CO2 assimilation was measured in fully developed leaves with an infrared gas analyzer (PLC 6, Cirus-2 PPSystems) at saturating irradiance (1200 µmol photon m−2 s−1, Amax). In addition, photosynthesis was measured as O2 evolution under saturating irradiance and CO2 concentration (Ppot). Leaf discs were placed in a gas tight chamber equipped with a Clark type electrode (Hansatech, UK). Saturating CO2 atmosphere was generated including a mate imbibed with 1 M NaHCO3 (Walker 1987). Photosynthetic electron transport rate was determined with a modulated chlorophyll fluorescence system (FMS-2, Hansatech Instruments Ltd., Norfolk, UK) and calculated according to Genty et al. (1989). Chlorophyll fluorescence quenching analysis was carried out with a CF Imager (Technologica Ltd., Colchester, UK) as described by Lim et al. (2016) and the chlorophyll fluorescence parameters calculated according to Baker (2008). Leaf temperature was measured with a thermographic camera (FLUKE Ti 400) with an emissivity of 0.95. Measurements were taken to well watered plants exposed at 700 µmol photons m−2 s−1 during midday inside the greenhouse.
Ripening parameters
Fruit ethylene production was measured with a gas chromatograph system (Konik, KNK-3000-HGRC) including an alumina column and a flame ionization detector as previously reported (Bartoli et al. 1996). Firmness was measured in detached tomato fruit with a texture analyzer (TA.XT.PLUS, Micro Systems TM Goldalming, Surrey, UK) using a 2.5 mm diameter flat probe. The measurements were obtained by fruit deformation for a distance of 0.5 mm at 0.25 mm s−1 and 5.9 g trigger force. The maximum force was recorded and results expressed in force g. Total soluble solids were measured as previously described (Gergoff et al. 2016).
Hormone determination
Plant hormones were measured in whole flower tissues sampled at anthesis and collected from several plants of each independent experiment. About 100 mg of lyophilized tissues were added with 1% (v/v) AcH (40 mg ml−1) and 2H5-indoleacetic acid (IAA), 2H2-gibberellin (GA), 2H2-GA4, 2H2-GA8, 2H5-zeatin and 2H6-ABA (OlChemIm Ltd., Olomouc, Czech Republic) as internal standards. The aqueous solution was partitioned 3 times with ethyl acetate at pH 3. Organic fractions were combined, evaporated and then resuspended in methanol for hormone determination by liquid chromatography-mass spectrometry with electrospray ionization (Waters Corp., New York, NY, USA). An Alliance 2695 (Separation Module, Waters, USA) quaternary pump equipped with a Restek C18 column (2.1 × 100 mm) (Restek, USA) was used to analyze the samples. A binary solvent system used for elution consisted of 0.2% (v/v) acetic acid in H2O and methanol. MS/MS assays were done with a Micromass Quatro Ultima TM mass spectrometer (Micromass, Manchester City, UK) as described by Masciarelli et al. (2014).
Results
Growth, development, and photosynthesis in GDP-l-galactose phosphorylase mutants.
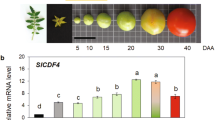
The two GDP-l-galactose phosphorylase mutants had 34–50% of wild type ascorbate in their leaves, roots, flowers, and fruit (Fig. 1a–d). Ascorbate concentration was lower in roots and the proportion of DHA was much higher than in leaves, flowers, and fruit. Exogenous ascorbate increased its concentration in leaves of the mutants by 66–79% but not in wild type plants (Table 1).
Ascorbate concentrations in leaves (a), roots (b), flowers (c) and fruit (d) of ggp1 mutants and wild type plants grown under greenhouse conditions. The values were obtained from at least three independent experiments and expressed as the means ± S.D. (ANOVA, P < 0.05). Lower and upper case letters indicate statistical differences between genotypes and stages, respectively
The vegetative biomass and leaf area at harvest were similar in all genotypes (Table 2). However, the mutants both allocated less biomass to roots (15% compared to 23% in wild type) and more to stems (20% compared to 13% in wild type). Therefore, the mutants were visibly different since they had larger internodes (Supplementary Fig. S1). The fruit fresh weight per plant was decreased in both mutants but the individual fruit weight was increased (Table 2). The mutants had a similar number of flowers but decreased fruit setting, and consequently, lower number of tomatoes than those of the wild type. Exogenous ascorbate supplementation increased fruit setting in both mutants but not in wild type plants (Table 1). In addition, anthesis was delayed in both mutants. Impairment of fruit setting may be the consequence of altered hormone concentrations. Consequently, the concentrations of various hormones in flowers at anthesis were measured (Table 3). All three gibberellins measured were decreased in the mutants but indole acetic acid (IAA), zeatin, and ABA showed no differences.
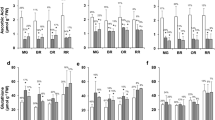
The CO2 assimilation and electron transport rates (ETR) of the mutants were 50 and 90%, of wild type, respectively (Fig. 2a, b). In addition, photosynthetic O2 production under a saturating CO2 atmosphere, was 19% higher in wild type than in the mutants (Fig. 2c). Consistent with the small effect on ETR and O2 assimilation under saturating CO2, there was no significant effect on photochemical quenching (qP), PSII maximum operating quantum efficiency (Fv’/Fm’) and PSII operating efficiency (Fq’/Fm’; φPSII) (Fig. 3). Non photochemical quenching (NPQ), which is impaired in ascorbate deficient arabidopsis mutants (Müller-Moule et al. 2002) was not affected in the mutants. Stomatal conductance was 60% higher in wild type than in the mutants (Fig. 2d). Consequently, leaf temperature measured by infrared thermal imaging was 1.8–2.5 °C higher in the mutants (Supplementary Fig. S2).
Leaf gas exchange measurements in ggp1 mutants and wild type plants as CO2 uptake (a), ETR (b), O2 production at saturating CO2 (c) and stomatal conductance (d) grown under greenhouse conditions. The values were obtained from at least three independent experiments and expressed as the means ± SD. (ANOVA P < 0.05). Lower and upper case letters indicate statistical differences between genotypes
Photosynthetic characteristics of ggp1 mutants and wild type plants measured by chlorophyll fluorescence imaging. The plants were grown in 16 h day, 21C day (150 µmol m−2 s−1)/19C dark, 55 RH day/50 RH dark for 4 weeks before transferring to an irradiance of 500 µmol m−2 s−1 for 4 days. NPQ non-photochemical quenching (a), qP photosystem II efficiency factor (b), Fq’/Fm’, PSII operating efficiency (c), Fv’/Fm’, PSII maximum efficiency (d). The values were obtained from three independent experiments and expressed as the means ± SD
The effect of GDP-l-galactose phosphorylase mutation on fruit quality
Fruit firmness decreased along ripening but both GDP-l-galactose phosphorylase mutants kept higher firmness than wild type at the red stage (Fig. 4a). Ethylene production was higher in the mutant lines than wild type fruit at the breaker and red stages (Fig. 4b). Soluble solids content (Brix) increased during fruit ripening and was also higher in mutants compared with wild type at breaker stage (Fig. 4c). Other ripening parameters such as pH and titratable acidity show no differences between genotypes (Data not shown).

taken from ggp1 mutants and wild type plants grown under greenhouse conditions at green mature, breaker and red stages. The values were obtained from at least three independent experiments and expressed as the means ± SD (ANOVA P < 0.05). Lower and upper case letters indicate statistical differences between genotypes and stages, respectively
Firmness (a), ethylene production (b) and soluble solids content (c) in fruit
The effect of source-sink manipulation on fruit yield characteristics
To investigate the relative importance of source or sink limitations on fruit yield various fruit or leaf pruning treatments were performed (Table 4). Fruit production was not modified in wild type or mutant plants after leaf removal. However, when plants were limited to two tomatoes per inflorescence (i.e., eight tomatoes per plant) the mutants kept their fruit weight per plant as in control treatment but it drastically decreased in all genotypes when only one fruit per inflorescence is maintained. For an easier interpretation of these results a principal component analysis is shown in Fig. 5. It shows a contrasting relationship between different traits such as fruit number and yield versus individual fruit fresh weight. Component 1 distinguishes treatments independently of the genotypes and component 2 the wild type from mutants (more markedly for fruit removal treatment).
Bi-plot of the first and second principal components for tomato development traits. Each parameter is represented by dark continuous lines and genotype-treatment combination by symbols (unfilled diamond). The treatments consisted in control plants that were cultivated leaving only 4 trusses (C); limited number of fruit where each plant kept 4 or 8 fruits (1F and 2F, respectively); and two levels of defoliation ( − L and = L)
Discussion
Arabidopsis vtc2 mutants, which are knockouts of one of the two genes encoding GDP-l-galactose phosphorylase, have ~ 20% of wild type ascorbate (Dowdle et al. 2007; Barth et al. 2010) and have small decreases in rosette leaf area and biomass, the extent most likely being sensitive to growth conditions (Lim et al. 2016; Caviglia et al. 2018; Plumb et al. 2018). Here, the tomato GDP-l-galactose phosphorylase mutants, which have 34–50% of wild type ascorbate in all organs, are similarly unaffected in total vegetative biomass but do have reduced allocation to roots. The reason for the effect on root growth is not evident but could be associated with re-allocation to shoots to compensate for decreased CO2 assimilation. Effects of the GDP-l-galactose phosphorylase mutations are more evident in reproductive development, the mutants being slower to anthesis and having markedly decreased fruit set and number. However, early flowering has been reported in various ascorbate deficient Arabidopsis mutants (Kotchoni et al. 2009). Whatever the delaying or accelerating effect, these results together suggest that the plant ascorbic acid concentration is linked to the regulation of time to flowering. This effect may be a species- but also an environmental-dependent phenomenon. The decrease in fruit number is associated with increased fruit size in the mutants. Larger fruit size is most easily explained by decreased competition between fruits for assimilate, since the fruit removal experiment increased individual fruit weight in all genotypes. Critically, ascorbate supplementation increased fruit set in the mutants, suggesting that ascorbate is important for this process. This observation also suggests that assimilate limitation (Ruan et al. 2012) may be only partially responsible for reduced fruit set in this case. To further investigate the cause of reduced fruit set, the effect of GDP-l-galactose phosphorylase mutations on flower hormones was investigated. GA1, GA4, and GA8 were decreased in the mutants. The chemical inhibition of gibberellin synthesis in tomato reduces fruit setting and this effect is reversed by exogenous application of the hormone (Serrani et al. 2007). Therefore, the low GA concentration in the flowers of the mutants could contribute to low fruit setting. Ethylene treatment decreases fruit set in tomato, possibly by inhibiting GA synthesis (Shinozaki et al. 2015). Arabidopsis vtc2 mutants have increased ethylene production (Caviglia et al. 2018). Therefore, if ascorbate deficiency also increases ethylene in tomato, it is possible that this could be the cause of decreased GA and fruit setting.
The large decrease in CO2 assimilation in the mutants was not matched by a large decrease in biomass, although overall fruit yield was significantly less (~ 72% of wild type). As noted above this could be partly attributed to lower fruit set. Measuring photosynthesis under high CO2 and by chlorophyll fluorescence showed that the mutations do not limit photosynthetic capacity, but the limitation is most likely caused by partial stomatal closure. Since the stomatal conductance was measured around midday, it is possible that over the course of the light period, stomata are more open in morning/evening, thus minimizing an overall effect on assimilation. Furthermore, decreasing GDP-l-galactose phosphorylase expression in tomato in another study also decreased ascorbate content by 50% while having no effect on CO2 assimilation under non-stressed conditions (Wang et al. 2013). Similarly, Arabidopsis vtc2-1 has a similar CO2 assimilation rate to wild type (Senn et al. 2016). Therefore, studies to date show that ascorbate deficiency (at least to 20% of wild type), has minimal effect on photosynthesis and vegetative biomass.
The source-sink experiment demonstrates that the number of fruit is crucial to determine fruit yield in tomato and that the higher abortion of fruit observed in ggp1 mutants does not seem to be the consequence of an insufficient provision of photoassimilates by the leaves. Similarly, Tanaka and Fujita (1974) demonstrate in assays partially removing fruit and leaves that source is not limiting yield in tomato and also that fruit size has a limited flexibility. These authors conclude that unraveling the processes establishing fruit number is an important task. In this context, the present work, using GDP-l-galactose phosphorylase deficient mutants and especially exogenous ascorbic acid supplementation, demonstrates that ascorbic acid concentration is an important factor in establishing fruit number and consequently for the improvement of tomato yield.
Ascorbic acid constitutes an important nutritional attribute of fruit (Lee and Kader 2000). However, the impact of decreased ascorbate concentration on other fruit characteristics has scarcely been addressed. Previous reports on tomato mutants in enzymes earlier in the mannose/l-galactose biosynthetic pathway (GDP-mannose pyrophosphorylase and GDP-mannose-3′,5-epimerase) show effects on fruit development and quality (Gilbert et al. 2009, 2016; Zhang et al. 2013) but these may be caused by effects on cell wall composition as well as ascorbate deficiency itself. Decreased GDP-l-galactose phosphorylase activity in the current experiments is likely to affect ascorbate synthesis more specifically. Among effects observed here in the fruit of GDP-l-galactose phosphorylase mutants is an increase in ethylene production. Vtc2 arabidopsis plants also produce more ethylene (Caviglia et al. 2018). Ethylene production is associated with a stimulation of cell wall degrading enzymes during fruit ripening (Osorio et al. 2011). However, the results observed in GDP-l-galactose phosphorylase mutants suggest that other processes prevail, leading to increased fruit firmness. For example, changes in cuticles may also delay fruit softening (Saladié et al. 2007). The modifications observed in GDP-l-galactose phosphorylase tomato lines (including increased soluble solids) suggest that ascorbate influences some aspects of fruit quality.
Conclusions
Tomato mutants in the ascorbate biosynthesis the GGP1 isoform of GDP-l-galactose phosphorylase contain 34–50% of wild type ascorbate. The results suggest that this decrease in GDP-l-galactose phosphorylase expression and ascorbate concentration influence tomato fruit set, possibly via decreased GA concentration, final fruit size and some aspects of quality. A higher flower abortion is directly caused by ascorbate deficiency. Fruit size increases in compensation, but not sufficiently to prevent a decrease in total fruit biomass. Supply of assimilates for biomass and fruit production is unlikely to be a major limiting factor.
Author contribution statement
MA, MCS, GEGG, PB, DJ, OY, EH carried out the experiments and made the statistical analysis of the data. PB, CR, NS and CGB wrote de manuscript. All authors read and approved the manuscrip
References
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Asada K (1999) The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Phys 50:601–639
Baker NR (2008) Chlorophyll fluorescence: a probe for photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baldet P, Bres C, Okabe Y, Mauxion JP, Just D, Bournonville C, Ferrand C, Mori K, Ezure H, Rothan C (2013) Investigating the role of vitamin C in tomato through TILLING identification of ascorbate-deficient tomato mutants. Plant Biotechnol 30:308–314
Barth C, De Tullio M, Conklin PL (2006) The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot 57:1657–1665
Barth C, Gouzd ZA, Steele HP, Imperio RM (2010) A mutation in GDP-mannose pyrophosphorylase causes condition hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered metabolism, and hormone homeostasis. J Exp Bot 61:379–394
Bartoli CG, Simontachi M, Montaldi E, Puntarulo S (1996) Oxidative stress, antioxidant capacity and ethylene production during ageing of cut carnation (Dianthus caryophyllus) petals. J Exp Bot 47:595–601
Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57:1621–1631
Caviglia M, Mazorra Morales LM, Concellón A, Gergoff Grozeff GE, Wilson M, Foyer CH, Bartoli CG (2018) Ethylene signaling triggered by low concentrations of ascorbic acid regulates biomass accumulation in Arabidopsis thaliana. Free Radic Bio Med 122:130–136
Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52:673–689
Foyer CH, Noctor CH (2011) Ascorbate and glutathione: The heart of the redox hub. Plant Physiol 155:2–18
Gao Y, Badejo AA, Shibata H, Sawa Y, Maruta T, Shigeoka S, Page M, Smirnoff N, Ishikawa T (2011) Expression analysis of the VTC2 and VTC5 genes encoding GDP-l-Galactose Phosphorylase, an enzyme involved in ascorbate biosynthesis, in Arabidopsis thaliana. Biosci Biotechnol Biochem 75:1783–1788
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gergoff Grozeff GE, Senn ME, Alegre M, Chaves AR, Bartoli CG (2016) Nocturnal low irradiance pulses improve fruit yield and lycopene concentration in tomato. Sci Hortic 203:47–52
Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B, Faurobert M, Gouble B, Page D, Garcia V, Petit J, Stevens R, Causse M, Fernie AR, Lahaye M, Rothan C, Baldet P (2009) GDP-D-mannose epimerase (GME) plays a key role at the intersection of ascorbate and non cellulosic cell wall biosynthesis in tomato. Plant J 60:499–508
Gilbert L, Dumont M, Ferrand C, Bournonville C, Monier A, Jorly J, Lemaire-Chamley M, Mori K, Atienza I, Hernould M, Stevens R, Lehner A, Mollet JC, Rothan C, Lerouge P, Baldet P (2016) Two tomato GDP-D-mannose epimerase isoforms involved in ascorbate biosynthesis play specifc roles in cell wall biosynthesis and development. J Exp Bot 67:4767–4777
Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C (2009) Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiol 149:803–815
Laing WA, Wright MA, Cooney J, Bulley SM (2007) The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. P Natl Acad Sci USA 104:9534–9539
Laing WA, Martinez-Sanchez M, Wright MA, Bulley SM, Brewster D, Dare AP, Rassam M, Wang D, Storey R, Macknight RC, Hellens RP (2015) An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in arabidopsis. Plant Cell 27:772–786
Lee SK, Kader AA (2000) Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Tech 20:207–220
Li T, Yang X, Yu Y, Si X, Zhai X, Zhang H, Dong W, Gao C, Xu C (2018) Domestication of wild tomato is accelerated by genome editing. Nat Biotechnol 36:1160–1163
Lim B, Smirnoff N, Cobbett CS, Golz JF (2016) Ascorbate-deficient vtc2 mutants in Arabidopsis do not exhibit decreased growth. Front Plant Sci 7:1025–1030
Linster C, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG (2007) Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway o ascorbic acid in plants. J Biol Chem 282:18879–18885
Macknight RC, Laing WA, Bulley SM, Broad RC, Johnson AAT, Hellens RP (2017) Increasing ascorbate levels in crops to enhance human nutrition and plant abiotic stress tolerance. Curr Opin Biotech 44:153–160
Masciarelli O, Llanes A, Luna V (2014) A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol Res 169:609–615
Massot C, Stevens R, Génard M, Longuenesse JJ, Gautier H (2012) Light affects ascorbate content and ascorbate-related gene expression in tomato leaves more than in fruits. Planta 235:153–163
Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128:970–977
Osorio S, Alba R, Damasceno CMB, Lopez-Casado G, Lohse M, Zanor MI, Tohge T, Usadel B, Rose JKC, Fei Z, Giovannoni JJ, Fernie AR (2011) Systems biology of tomato fruit development: Combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol 157:405–425
Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH (2005) Ascorbic acid deficiency activates cell death and disease resistance responses in arabidopsis. Plant Physiol 139:1291–1303
Plumb W, Townsend AJ, Rasool B, Alomrani S, Razak N, Karpinska B, Ruban AV, Foyer CH (2018) Ascorbate-mediated regulation of growth, photoprotection, and photoinhibition in Arabidopsis thaliana. J Exp Bot 69:2823–2835
Ruan Y-L, Patrick JW, Bouzayen M, Osorio S, Fernie AR (2012) Molecular regulation of seed and fruit set. Trends Plant Sci 17:656–665
Saladié M, Matas AJ, Isaacson T, Jenks MA, Goodwin SM, Niklas KJ, Xiaolin R, Labavitch JM, Shackel KA, Fernie AR, Lytovchenko A, O’Neill MA, Watkins CB, Rose JKC (2007) A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol 144:1012–1028
Senn ME, Gergoff Grozeff GE, Alegre ML, Barrile F, De Tullio MC, Bartoli CG (2016) Effect of mitochondrial ascorbic acid synthesis on photosynthesis. Plant Physiol Biochem 104:29–35
Serrani JC, Sanjuán R, Ruiz-Rivero O, Fos M, García-Martínez JL (2007) Gibberellin regulation of fruit set and growth in tomato. Plant Physiol 145:246–257
Shinozaki Y, Hao S, Kojima M, Sakakibara H, Ozeki-Iida Y, Zheng Y, Fei Z, Zhong S, Giovannoni JJ, Rose JKC, Okabe Y, Heta Y, Ezura H, Ariizumi T (2015) Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J 83:237–251
Smirnoff N (2018) Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic Bio Med 122:116–129
Tanaka A, Fujita K (1974) Nutrio-physiological studies on the tomato plant IV. Source-sink relationship and structure of the source-sink unit. Soil Sci Plant Nutr 20:305–315
Walker D (1987) The use of the oxygen electrode and fluorescence probes in simple measurements of photosynthesis. Oxygraphics Limited, University of Sheffield Print Unit, Sheffield, UK, p 203
Wang L-Y, Li D, Deng Y-S, Lv W, Meng Q-W (2013) Antisense-mediated depletion of tomato GDP-L-galactosephophorylase increases susceptibility to chilling stress. J Plant Physiol 170:303–314
Yang D-Y, Li M, Ma N-N, Yang X-H, Meng Q-W (2017) Tomato SlGGP-LIKE gene participates in plant responses to chilling stress and pathogenic infection. Plant Physiol Biochem 112:218–226
Yoshimura K, Nakane T, Kume S, Shiomi Y, Maruta T, Ishikawa T, Shigeoka S (2014) Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci Biotech Bioch 78:60–66
Zhang C, Ouyang B, Yang C, Zhang X, Liu H, ZhangY ZJ, Li H, Ye Z (2013) Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-D-Mannose pyrophosphorylase gene in tomato plant. PLoS ONE 8(4):e61987
Acknowledgements
We are really grateful to Dr Eduardo Tambussi for his valuable discussion of the results, Dr Gabriela Cano for her technical assistance and Dr Diego Fanello for the statistical analysis. GEGG and CGB are researchers at the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). MA and CS are fellows at CONICET and Agencia Nacional de Promoción Científica y Técnica (ANPCyT, Argentina), respectively. This work was supported by grants PICT 2015–0103 (ANPCyT) and A258 and A322 (Universidad Nacional de La Plata, Argentina).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alegre, M.L., Steelheart, C., Baldet, P. et al. Deficiency of GDP-l-galactose phosphorylase, an enzyme required for ascorbic acid synthesis, reduces tomato fruit yield. Planta 251, 54 (2020). https://doi.org/10.1007/s00425-020-03345-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03345-x