Abstract
The role of exogenous methyl jasmonate (MeJA) application in moderating in vitro water stress on banana growth was investigated. Shoot tips were treated using various levels (0, 5, 10, 20, 40, 80, 100, 120, 140 and 160 μM) of MeJA before and during the imposition of 30 g L−1 PEG induced water stress in the media. Proliferation rate significantly improved with increasing doses of MeJA up to 80 μM whether the shoot tips were stressed by PEG or unstressed. The same trend was observed in terms of enhanced fresh weight increase and shoot vigour rate under water stress. PEG significantly reduced the relative water content (%) of shoot tips by about 35 % as compared with those of unstressed conditions, while MeJA application up to 40 μM reduced the inhibitory effect of PEG on leaf water loss (%). Shoot tips under water stressed conditions, responded positively to MeJA by exhibiting a significant increase in proline, although increasing levels to 100 μM and higher had no effect. MeJA alleviated the effect of PEG—induced loss of chlorophyll, although it had no additional benefit by altering the dosages. Under water stress, MeJA application up to 40 μM was also effective in reducing oxidative injury as indicated by significant reduction in H2O2 and MDA contents of shoot tips; higher dosages exhibited no further advantageous effect. These results suggested the participation of MeJA in improving drought tolerance of banana and moderating oxidative stress leading to enhanced plant performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water stress is one of the important factors that, restricts worldwide productivity of crop plants. Bananas are produced as a commercial crop in the tropical and subtropical regions of the world. The plants are very sensitive to water stress, which negatively affects their growth and productivity and is often associated with oxidative injury (Ismail et al. 2004; Chai et al. 2005; Turner et al. 2007; Farooq et al. 2009).
Methyl jasmonate (MeJA) and jasomonic acid (JA), communally referred to as jasmonates, occur in crop plants as plant growth regulators (PGR) and affect a variety of morphological, physiological and biochemical processes, especially those related to stress tolerance. Jasmonates also activate plant defense mechanisms in response to various pathogens and environmental stresses such as heavy metals and elemental toxicity, drought, low temperature and salinity (Parthier 1991; Wang and Buta 1994; Wasternack and Parthier 1997; Meir et al. 1998; Cheong and Choi 2003; Huguet-Robert et al. 2003; Gonzalez-Aguilar et al. 2004; Rohwer and Erwin 2008; Keramat et al. 2009; Aftab et al. 2010; Anjum et al. 2011). In rose, MeJA protects against botrytis rot by inducing resistance mechanisms in the treated cut flowers without damaging flower quality (Meir et al. 1998). Exogenous application of MeJA leads to modification of physiological processes in plants which may be useful in protecting them prior to transfer or subculture into saline or water stressed conditions in the soil or in tissue culture media (Rohwer and Erwin 2008). Exogenous application of MeJA may also induce the expression of many defence genes in crop plants (Ding et al. 2002).
Environmental stresses such as elemental toxicity, drought, salinity, heat or coldness and other antagonistic conditions may also trigger oxidative stress in plants, which results in generating the formation of reactive oxygen species (ROS). ROS are partly reduced or activated conjugates of oxygen, leading to enhanced lipid peroxidation and cellular damage (Farooq et al. 2009; Jaleel et al. 2009; Aftab et al. 2010; Anjum et al. 2011). Wang (1999) asserted that MeJA treatment of strawberry under water stressed conditions, noticeably reduced membrane—lipid peroxidation as expressed by malondialdehyde (MDA) accumulation. Keramat et al. (2009) and Aftab et al. (2010) reported that MeJA treatment of soybean plants subjected to cadmium as a heavy metal and Artemisia annua against boron toxicity, respectively alleviated damage of the stress and reduced oxidative stress by decreasing MDA and H2O2 contents.
Studying the role of MeJA in alleviating NaCl—induced salt stress on soybean, Yoon et al. (2009) asserted that MeJA application significantly relieved the negative effects of NaCl on plant growth, chlorophyll and proline content. Treatment of zucchini squash with MeJA also delayed the beginning of chilling injury (Wang and Buta 1994). Chilling injury of guava was also reported to be decreased when treated with MeJA (Gonzalez-Aguilar et al. 2004). MeJA was shown to regulate stomatal closure (Pospisilova 2003; Rohwer and Erwin 2008). Nonetheless, Kim et al. (2009) reported that plants produced MeJA throughout drought stress which consequently prompted the production of ABA leading to a loss of grain yield. Likewise, Fang and Kao (2001) reported that MeJA promoted senescence of rice leaves and postulated that increase of lipid peroxidation (indicated by MDA accumulation) and H2O2 content during leaf senescence could be attributed to MeJA. The current study is aimed at evaluating the role of exogenously applied MeJA on the performance of stressed and unstressed banana shoot tips. It was hypothesized that MeJA could ameliorate water stress tolerance in banana by regulating the growth, proliferation rate, proline accumulation, chlorophyll levels, tissue water status, oxidative stress and membrane lipid peroxidation.
Materials and methods
Preparing plant material, shoot tips and media, applying MeJA and treating with osmotic stress
Shoot tips were separated from micropropagated cultures of banana (Musa acuminata cv. ‘Berangan’, AAA) and prepared according to the procedures of Strosse et al. (2008) by cutting off sheathing leaves (namely, pseudo stem tissue) at about 6 mm above the apical meristems. This was followed by successive removal of sheathing leaves. Explants consisted of apical meristems, which were enveloped by three to five sheathing leaves and 2–3 mm of corm tissue (true stem, straight below the apical meristem). MeJA treatment of shoot tips comprised two steps. In the first step, a stock solution of MeJA at 500 μM in ethanol was prepared and kept at 4 °C. This solution was diluted with sterile deionized water using filter—sterilization under aseptic conditions to obtain working solutions (0, 5, 10, 20, 40, 80, 100, 120, 140 and 160 μM) of MeJA. MeJA was applied by soaking explants of uniform size in 100—ml Erlenmeyer flasks, which contained 30 ml of MeJA at the above mentioned concentrations on a rotary shaker (at 80 rpm) for 48 h. All the treatments were carried out at 25 °C under room temperature. In the second step, micropropagation medium used consisted of MS (Murashige and Skoog 1962)—based tissue culture medium, which contained 3 % sucrose and 22.2 μM benzyl aminopurine (BAP) solidified by 2.8 g L−1 gelrite with and without 30 g L−1 polyethylene glycol (PEG M.W. 6000). After adjusting the pH to 5.6, media were autoclaved at 121 °C for 20 min. Subsequently, MeJA was applied while filter—sterilized MeJA solutions containing the above mentioned concentrations were concurrently added when the reference media were just above the solidification temperature (approximately 50 °C). After that, MeJA treated explants (shoot tips) with sizes almost similar to those of the first step treatments were taken for the next experiments with MeJA, they were transferred in bottles provided with 50 ml micropropagation medium containing similar MeJA treatments at 0, 5, 10, 20, 40, 80, 100, 120, 140 and 160 μM. Five such explants were inoculated onto reference medium with their respected MeJA concentration treatments. In vitro culture responses of MeJA—treated shoot tips from the control set including the MS medium supplemented with 22.2 μM BAP and similar concentrations of MeJA, were also measured in the absence of PEG in order to separately determine the effects of PEG and MeJA. Cultures were maintained in growth chambers at 28 ± 2 °C, under 16 h photoperiod provided by fluorescent light of 1,500 Lux for 2 months.
Osmotic potential of water stress agent
The osmotic potential of PEG was determined by its concentration and by temperature according to the equation given by Michel and Kaufmann (1973). Thus at 25 °C, 30 g L−1 PEG should exert an osmotic potential of −0.0241 MPa in the micropropagation medium.
Morphological (tissue cultural) and physiological measurements
Proliferation rate was determined by counting all the shoots regenerated per explant after 2 months of MeJA—treated shoot tips inoculation onto various MeJA doses which contained micropropagation media under PEG stressed and non PEG stressed (normal) conditions. Shoot vigour was also evaluated by being rated on a scale of 1–5, in which 1 = no growth, 2 = below average, 3 = average, 4 = above average and 5 = excellent. Since the fresh weight of the initial explants used for the experiment was required to be as steady as possible, the mean fresh weight of 5 shoot tips for each replication was considered around 0.8 ± 0.1 g at the starting point, then the fresh weight of the proliferated shoots after 2 months of culture with MeJA treatments under both PEG induced water stressed and unstressed conditions minus shoot tips fresh weight at the starting point was served as increase of fresh weight.
Plant water status (relative water contents and leaf water loss)
The relative water contents (RWC %) of shoot tips was determined according to Mata and Lamattina (2001). Five leaf samples were weighed (as fresh weight, FW) immediately after being detached from in vitro developing shoots. The same tissues were then submerged in the test tubes containing distilled water for 2 h at 25 °C and then their turgid weights (TW) were measured. The samples were then dried in an oven at 80 °C for at least 48 h in order for their dry weights (DW) to be obtained and the relative water content (RWC %) was calculated using the following relation:
Leaf water loss (LWL %) was evaluated according to the method of Xing et al. (2004). Five leaf samples were weighed (as fresh weight, W1) immediately after being detached from in vitro developing shoots, then the leaves were left to evaporate in room conditions for 2 h and were reweighed (W2). LWL % was calculated using the following formula:
Proline assay and chlorophyll measurement
The proline content was measured according to the method described in Bates et al. (1973). The standard curve for proline was prepared by dissolving proline in distilled water with covering the concentration range of 1–10 μg/ml.
Three hundred mg of fresh leaves was detached from in vitro regenerating shoots, placed in a mortar and thoroughly ground with a pestle. Then, 5 ml of 80 % acetone was added to 15 ml falcon tubes containing the grinded leaves. The tubes were centrifuged at 4 °C for 15 min at 3,000 rpm and then the absorbance of chlorophyll content was measured at 663 and 645 nm wavelengths using spectrophotometry. 80 % acetone was used as a blank control. The chlorophyll concentrations were calculated using the formula given by Porra (2002).
Oxidative stress indices (ROS production and MDA formation)
The concentration of malondialdehyde (MDA) which is a product of lipid peroxidation was assessed by the thiobarbituric acid (TBA) according to Wang et al. (2009), as 1 g fresh leaves was detached from in vitro regenerating shoots, placed in a mortar containing 5 ml 0.6 % TBA in 10 % trichloroacetic acid (TCA) and ground with a pestle. The mixture was heated at 100 °C for 15 min. These samples were cooled on ice for 5 min; subsequently, the mixtures were centrifuged at 5,000 rpm for 10 min. The absorbances of the supernatant at 450, 532 and 600 nm wavelengths were recorded and MDA content was calculated on a fresh weight by (nmol MDA g−1 FW) = 6.45 (OD532 – OD600)–0.56 (OD450) × 1,000. Hydrogen peroxide (H2O2) was assessed spectrophotometrically after the reaction with potassium iodide (KI), according to the method presented in Velikova and Loreto (2005). Leaf tissues (1 g) were ground and homogenized in a mortar containing 10 ml 0.1 % trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000×g for 15 min. Afterwards, 0.5 ml of the supernatant was added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml reagent (1 M KI in fresh double distilled water) and then the absorbance of the supernatant was read at 390 nm. The blank probe was prepared using 0.1 % TCA in the absence of leaf extract. The content of H2O2 was calculated applying a standard curve prepared by identified concentrations of hydrogen peroxide.
Statistical analysis
The experiments were conducted in a completely randomized design (RCD) with two factors (two condition treatments including stressed and unstressed media × 10 levels of MeJA) with three replications (n = 3) using five explants per replication. The data of morphological and physiological characteristics were subjected to an analysis of variance (ANOVA) using an SAS statistical program. Then, significant differences among the mean values of treatments were compared using least significant difference (LSD) test method at p ≤ 0.05 in the MSTAT-C computer program.
Results
Growth and morphological characteristics
Bananas (Musa acuminata cv. ‘Berangan’, AAA) were found to be sensitive to water stress since 30 g L−1 PEG application through the media showed an injurious effect on various growth parameters including morphological and physiological attributes of shoot tips. The shoot proliferation responses of cultivar ‘Berangan’ to various MeJA concentrations under both stressed and unstressed conditions are shown in Fig. 1. Application of 40 μM MeJA evoked the best performance of shoot proliferation when the media free from PEG were applied (Fig. 1b) but under water stress condition, 80 μM MeJA caused the best results by the appearance of several elongated shoots (Fig. 1f). In vitro water stress using polyethylene glycol (PEG 6000) at 30 g L−1 significantly reduced proliferation rate of shoot tips (Figs. 1h, 2). Application of MeJA in the media enhanced the number of shoots in the explants under both water stressed and non water stressed conditions (Fig. 2). Higher proliferation rates were observed with the non PEG—stressed shoot tips and the best results for regeneration capacity was obtained for the shoot tips treated with 40 μM MeJA under the normal condition (Figs. 1, 2). However, application of MeJA through the PEG—stressed and unstressed media higher than 80 μM significantly decreased the proliferation rate of shoot tips (Fig. 2). Under water stress condition, shoot tip cultures indicated a noticeable decline in the fresh weight increase as compared with those under unstressed conditions, however, this decline was significantly alleviated (Fig. 3), with increasing the dosage of MeJA up to 80 μM while raising the concentration of MeJA higher than 80 μM resulted in no beneficial effects or even inhibition. However, under non water stressed (normal) condition, the inhibitory effect of MeJA on fresh weight increase at the applied concentrations was more pronounced at lower (above 40 μM) levels (Fig. 3). Figure 4 shows the shoot vigour rate responses of shoot tips to MeJA under stressed and unstressed conditions. It is obvious that banana shoot tips showed a significant reduction in the rate of shoot vigour when subjected to water stress caused by PEG as compared with those of non water stressed condition. However, MeJA treatments significantly alleviated this reduction up to 80 μM while the application of higher concentrations (100, 120, 140 and 160 μM) indicated no significant effect (Fig. 4).
Shoot proliferation responses of banana cultivar ‘Berangan’, 2 months after inoculation of shoot tips on multiplication medium (MS + 22.2 μM BAP) modified with various MeJA concentrations under PEG—induced water stressed and unstressed conditions: (a) and (b) show non MeJA treated as well as 40 μM MeJA supplemented shoot tips respectively, grown on multiplication medium without PEG. c indicates proliferating shoot tip treated with the highest (160 μM) concentration of MeJA, grown under control conditions (multiplication medium without PEG). d, e, f, g and h indicate 100, 80, 40, 5 and 0 μM treated shoot tips respectively, grown under 30 g L−1 PEG. (Bar = 8 mm in a–h)
Influence of methyl jasmonate (MeJA) application on proliferation rate in shoot tips of banana (Musa acuminata cv. ‘Berangan’) on the media with the addition of 30 g L−1 PEG induced water stress (stressed) and without the addition of PEG to the media (unstressed) for a period of 2 months. Bars showing the same latter are not significantly different at p ≤ 0.05 as determined by LSD test. Each value represents the mean ± SD (n = 3)
Influence of varying concentrations of methyl jasmonate (MeJA) application on fresh weight increase (g) in proliferating shoot tips of banana (Musa acuminata cv. ‘Berangan’) on the media with the addition of 30 g L−1 PEG induced water stress (stressed) and without the addition of PEG to the media (unstressed) for a period of 2 months. Bars showing the various letters are significantly different at p ≤ 0.05 according to LSD test. Each value represents the mean ± SD (n = 3)
Influence of methyl jasmonate (MeJA) application on shoot vigour rate in regenerated shoots of banana (Musa acuminata cv. ‘Berangan’) on the media with the addition of 30 g L−1 PEG induced water stress (stressed) and without the addition of PEG to the media (unstressed) for a period of 2 months. Bars showing the various letters are significantly different at p ≤ 0.05 according to LSD test. Each value represents the mean ± SD (n = 3)
Relative water contents, leaf water loss, proline accumulation and chlorophyll levels
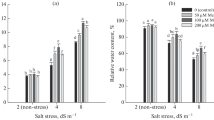
Application of PEG in the medium significantly reduced the relative water content of shoot tips, by about 35 %, as compared with the non PEG—stressed explants (Fig. 5). MeJA—treated shoot tips indicated no significant change in the percentage of relative water content when the media free from PEG were applied (Fig. 5). In contrast, when PEG—induced water stress was implemented through the media, MeJA caused a significant increase in RWC % of shoot tips compared with those of non MeJA—treated although there was no significant difference among varying MeJA levels in terms of increased RWC % (Fig. 5). Nevertheless, the mean comparison for relative water content (RWC %) of shoot tips demonstrated a non significant increase with the increasing levels of MeJA up to 80 μM under the water stressed condition (Fig. 5). When shoot tip cultures were stressed with PEG, leaf water loss (LWL %) indicated a significant increase compared with those of the non stressed (controls) condition (Fig. 6). The shoot tips treated with MeJA maintained water content better than those of the non MeJA—treated under stressed conditions, as raising the dosage of MeJA up to 40 μM significantly reduced the leaf water loss (LWL %) and the doses higher than this showed no further advantageous effect (Fig. 6). The mean proline content of non MeJA—treated shoot tips caused by 30 g L−1 PEG indicated a significant increase when compared with those obtained from the media without PEG addition (Fig. 7). However, under water stress induced by PEG, 80 and 100 μM MeJA significantly caused the highest amounts of proline (9.86 ± 0.46 and 9.82 ± 0.86 μmols g−1 FW, respectively) and the proline accumulation was less for the treatment of MeJA at high concentrations (Fig. 7). MeJA concentrations up to 10 μM did not significantly change the content of proline in shoot tips under unstressed conditions although, in such a condition, 120 μM MeJA caused a higher level (4.75 ± 0.8) of proline accumulation as compared with the one obtained by the other concentrations (Fig. 7). Table 1 shows total chlorophyll content for both non MeJA—treated shoot tips (controls) and MeJA—treated shoot tips under water stressed and unstressed conditions. Significant reduction in the chlorophyll content was detected in shoot tips grown on the media supplemented with 30 g L−1 PEG induced water stress compared with those in the unstressed condition (Table 1). When the media were subjected to PEG induced water stress, the shoot tips supplemented with MeJA caused a significant reduction in PEG—induced loss of chlorophyll content (mg g−1 FW) compared with non MeJA—treated explants (20.61 ± 0.26), as 140 μM MeJA caused the highest chlorophyll concentration (27.2 ± 0.86 mg g−1 FW) of shoot tips although varying dosages of MeJA indicated no significant difference in terms of enhanced chlorophyll levels (Table 1).
Influence of methyl jasmonate (MeJA) application on percentage of relative water content (RWC %) of regenerated shoots of banana (Musa acuminata cv. ‘Berangan’) on the media with the addition of 30 g L−1 PEG induced water stress (stressed) and without the addition of PEG to the media (unstressed) for a period of 2 months. Bars showing the various letters are significantly different at p ≤ 0.05 according to LSD test. Each value represents the mean ± SD (n = 3)
Influence of methyl jasmonate (MeJA) application on percentage of leaf water loss (LWL %) of banana (Musa acuminata cv. ‘Berangan’) in regenerated shoots on the media with the addition of 30 g L−1 PEG induced water stress (stressed) and without the addition of PEG to the media (unstressed) for a period of 2 months. Bars showing the same latter are not significantly different at p ≤ 0.05 as determined by LSD test. Each value represents the mean ± SD (n = 3)
Influence of methyl jasmonate (MeJA) application on proline accumulation in leaves of banana (Musa acuminata cv. ‘Berangan’) derived from regenerated shoots on the media with the addition of 30 g L−1 PEG induced water stress (stressed) and without the addition of PEG to the media (unstressed) for a period of 2 months. Bars showing the various letters are significantly different at p ≤ 0.05 according to LSD test. Each value represents the mean ± SD (n = 3)
Oxidative stress and lipid peroxidation
Changes in hydrogen peroxide (H2O2) and malondialdehyde (MDA) are also presented in Table 1. Upon the exposure of banana shoot tip cultures to PEG induced water stress, H2O2 and consequently MDA levels increased in detached leaves while under normal (the media without addition of PEG induced water stress) condition, the shoot tips indicated significant reduction in the contents of H2O2 and MDA (Table 1). Application of MeJA was effective in reducing the amounts of H2O2 and MDA when shoot tips were subjected to PEG induced water stress, the amounts of H2O2 and MDA indicated significant reduction with increase in the dosage of MeJA up to 40 μM and the higher doses exhibited no significant effect although the lowest levels of H2O2 (27.63 ± 1.28 μmol g−1 FW) and MDA (41.78 ± 2.77 nmol g−1 FW) were recorded to be 120 μM MeJA as compared with control plants (non MeJA—treated shoot tips) in which, H2O2 and MDA significantly recorded the highest amounts (47.04 ± 5.01 μmol g−1 FW and 80.44 ± 8.31 nmol g−1 FW, respectively) (Table 1). However, without PEG—induced water stress into the media (unstressed conditions), applying various levels of MeJA had no significant effects on the H2O2 content of shoot tips in comparison with those of non MeJA—treated ones although, under such conditions, MDA accumulation of MeJA—treated explants showed significant reduction compared with those of the controls without any significant difference among its varying concentrations (Table 1).
Discussion
Water stress caused by 30 g L−1 PEG (−0.0241 MPa) applied to the medium inhibited the growth of Musa acuminata cv. ‘Berangan’ as evidenced by the reduced proliferation rate, shoot vigour and fresh weight. PEG induced water stress adversely affected physiological attributes as well. However, the stress generated by PEG was ameliorated when the shoot tips were supplemented with the exogenous MeJA application, directly followed by the implementation in the medium, although various levels of MeJA in some physiological attributes of banana cultivar ‘Berangan’ indicated no significant effects.
Plant growth is affected negatively by abiotic stresses and exogenous PGR application is of great significance in relieving the hostile effects of these stresses (Jaleel et al. 2009). Similarly, Yoon et al. (2009) and Anjum et al. (2011) reported the suppressing effects of stresses caused by NaCl and water deficiency, respectively on soybean growth. They asserted that MeJA application considerably lessened the hostile effects of NaCl and water stress on plant growth, chlorophyll content and proline accumulation. Earlier reports also suggested that PEG treatment significantly decreased explant survival, shoot multiplication and growth rate of Egyptian banana cultivars (Ebrahim et al. 2006) and resulted in enhanced oxidative injury expressed as lipid peroxidation in banana cultivar ‘Berangan’ (Chai et al. 2005). The present study confirmed earlier reports that the application of MeJA in water stressed plants reduced water loss of leaves (Wang 1999; Anjum et al. 2011). Reduced percentage of leaf water loss in the shoot tips exposed to water stress in response to MeJA treatments could be attributed to the possible regulatory effects of MeJA on stomatal closure and lead to the enhanced ability of tissues for water maintenance (Pospisilova 2003; Rohwer and Erwin 2008). The results of this study showed that the proline content was higher in water stressed shoot tips and it was the highest in the stressed shoot tips supplemented with MeJA. Similarly, Anjum et al. (2011) asserted that MeJA application further enhanced the proline contents and also helped to maintain relative water content in water stressed soybean plants as compared with the respective controls. MeJA significantly enhanced Proline accumulation in the shoot tip cultures subjected to PEG—induced water stress, thereby, suggesting that MeJA may lessen the adverse effect of water stress on bananas. Accumulation of proline under stress conditions in plants, correlated with stress tolerance (Jyothsnakumari and Sudhakar 2003; Ali et al. 2007) and osmoregulatory role of proline for overcoming water stress conditions has been previously shown in canola leaf discs, apple and sweet cherry shoot tip cultures (Huguet-Robert et al. 2003; Sotiropoulos 2007; Sivritepe et al. 2008). However, applying cadmium as a heavy metal stress, Kovacik et al. (2011) reported that proline content of Scenedesmus quadricauda was not affected after 24 h of exposure to MeJA treatments. Crop plants react to water deficiency leading to tolerance against drought stress by changing some physiological and biochemical attributes (Farooq et al. 2009), therefore, treatment of banana shoot tips with MeJA caused an increase in proline accumulation as a physiological change which might be associated with the strategies which match the plant in order to withstand water stress conditions. Chlorophyll contents of shoot tip cultures were also adversely affected by PEG as the reduction in chlorophyll levels due to water stress was reported by Kirnak et al. (2001). Yoon et al. (2009) reported that MeJA significantly increased the chlorophyll content under stress caused by NaCl which further supported the present results in banana shoot tip cultures under water stress although varying dosages of MeJA indicated no significant difference in terms of enhanced chlorophyll levels of water stressed explants. Cadmium application as heavy metal stress reduced total chlorophyll contents of Scenedesmus quadricauda, while MeJA alleviated the negative effect of the heavy metal stress (Kovacik et al. 2011).
Lipid peroxidation was examined by estimating MDA formation in the shoot tip cultures of banana. The water stress is known to induce oxidative stress by increasing the level of H2O2 in the stressed tissues. The overproduction of ROS caused by water stress resulted in enhanced level of MDA, which was considered a marker for membrane lipid peroxidation (Jaleel et al. 2009; Anjum et al. 2011) hence, the current results demonstrated that the PEG—treated shoot tips of banana exhibited a high amount of H2O2 associated with the enhanced rate of lipid peroxidation. The stimulation of MDA formation in water stressed shoot tip cultures of banana was also consistent with other reports in which plants tended to increase MDA in response to a variety of stresses (Wang 1999; Chai et al. 2005; Keramat et al. 2009; Aftab et al. 2010; Anjum et al. 2011). However, MeJA—treated shoot tips of banana accumulated less H2O2 and MDA contents as compared with non MeJA—treated explants under water stressed conditions, suggesting that MeJA may also play an important role in inducing tolerance to oxidative stress conditions in plants. This result is supported the findings of Wang (1999) and Anjum et al. (2011), which mentioned that MeJA treatments of strawberry and soybean under water stressed conditions resulted in reduced amounts of MDA. The inhibitory effect of MeJA on oxidative stress damages caused by boron toxicity in Artemisia was also reported in Aftab et al. (2010). Similarly, Norastehnia and Nojavan-Asghari (2006) treated maize seedlings with various concentrations (0, 5, 10, 20, 50 and 100 μM) of MeJA after paraquat imposed oxidative stress. They reported that the 50 and 100 μM of MeJA were the most efficient concentrations in reducing the lipid peroxidation. As reviewed by Rohwer and Erwin (2008), exogenous MeJA may have a part not only in the defense mechanism against wounding and pathogen stresses, but also in relieving the injurious effect of drought stress. Nonetheless, the efficiency of MeJA in relieving the hostile effects of different kinds of stresses was more depended on its application mode in each crop plant. In this regard, the current results suggested that the shoot tips of banana supplemented with MeJA during the imposition of water stress in the tissue culture media, certainly contributed to the higher tolerance to water stress by enhancing some morphological and physiological attributes of this crop, furthermore, the acclimation process to water stress initiated by MeJA treatment could be attributed to pretreatment of explants with MeJA before the imposition of water stress. However, the efficiency of MeJA in alleviating the negative effects of water stress was relied on the concentration of the applied MeJA. A previous report (Wang 1999) also demonstrated that MeJA appeared to modify the metabolism of strawberry so that they can better tolerate water, stress which is in accordance with the present results in the case of banana. The capability of this procedure, however, should be further attempted by the known performance for physiological responses identified by drought tolerance under field conditions as final confirmatory tests.
Abbreviations
- BAP:
-
Benzyl aminopurine
- DW:
-
Dry weights
- FW:
-
Fresh weight
- JA:
-
Jasomonic acid
- LSD:
-
Least significant difference
- LWL:
-
Leaf water loss
- MeJA:
-
Methyl jasmonate
- MW:
-
Molecular weight
- MDA:
-
Malondialdehyde
- PEG (M.W. 6000):
-
Polyethylene glycol
- PGR:
-
Plant growth regulators
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water contents
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- TW:
-
Turgid weights
References
Aftab T, khan MMA, Idress M, Naeem M, Moinuddin, Hashmi N (2010) Methyl jasmonate counteracts boron toxicity by preventing oxidative stress and regulating antioxidant enzyme activities and artemisinin biosynthesis in Artemisia annua L. Protoplasma 248(3):601–612. doi:10.1007/s00709-010-0218-5
Ali MB, Hahn EJ, Paek KY (2007) Methyl Jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12:607–621
Anjum SA, Wang L, Farooq M, Khan I, Xue L (2011) Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J Agron Crop Sci 197(4):296–301. doi:10.1111/j.1439-037X.2011.00468.x
Bates LS, Waldern RP, Teare ID (1973) Rapid determination of free proline from water stress studies. Plant Soil 39:205–207
Chai TT, Fadzillah NM, Kusnan M, Mahmood M (2005) Water stress—induced oxidative damage and antioxidant responses in micropropagated banana plantlets. Biol Plant 49(1):153–156. doi:10.1007/s00000-005-3156-9
Cheong JJ, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19(7):409–413. doi:10.1016/S0168-9525(03)00138-0
Ding CK, Wang CY, Gross KC, Smith DL (2002) Jasmonate and salicylate induce the expression of pathogenesis-related-proteins genes and increase resistance to chilling injury in tomato fruit. Planta 214:895–901
Ebrahim MKH, Ibrahim IA, Emara HA, Komor E (2006) Impact of polyethylene glycol—induced water stress on growth and development of shoot tip cultures from different banana (Musa spp.) cultivars. J Appl Hort 8(1):53–57
Fang WC, Kao CH (2001) Inhibition of methyl jasmonate—promoted senescence in rice leaves by a metal chelator, 2, 2′—bipyridine. J Plant Growth Regul 33:87–93. doi:10.1023/A:1017539513518
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29(1):185–212. doi:10.1051/agro:2008021
Gonzalez-Aguilar GA, Tiznado-Hernandez ME, Zavaleta-Gatica R, Martines-Tellez MA (2004) Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem Biophys Res Commun 313(3):694–701
Huguet-Robert V, Sulpice R, Lefort C, Maerskalck V, Emery N, Larher FR (2003) The suppression of osmoinduced proline response of Brassica napus L. var Oleifera discs by polyunsaturated fatty acids and methyl—jasmonate. Plant Sci 164:119–127. doi:10.1016/S0168-9452(02)00343-6
Ismail MR, Yusoff MK, Maziah M (2004) Growth, water relations, stomatal conductance and proline concentration in water stressed banana (Musa spp.) plants. Asian J Plant Sci 3(6):709–713
Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436. doi:10.1007/s11738-009-0275-6
Jyothsnakumari G, Sudhakar C (2003) Effects of jasmonic acid on groundnut during early seedling growth. Biol Plant 47:453–456. doi:10.1023/B:BIOP.0000023894.72554.b2
Keramat B, Kalantari KM, Arvin MJ (2009) Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.). Afr J Biotech 3(5):240–244
Kim EH, Park SH, Kim JK (2009) Methyl jasmonate triggers loss of grain yield under drought stress. Plant Signal Behav 4(4):348–349
Kirnak H, Kaya C, Tas I, Higgs D (2001) The influence of water deficit on vegetative growth, physiology, fruit yield and quality in eggplants. Bull J Plant Physiol 27(3–4):34–46
Kovacik J, Klejdus B, Stork F, Hedbavny J, Backor M (2011) Comparison of methyl jasmonate and cadmium effect on selected physiological parameters in Scenedesmus quadricauda (chlorophyta, chlorophyceae). J Phycol 47(5):1044–1049. doi:10.1111/j.1529-8817.2011.01027.x
Mata CG, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204. doi:10.1104/pp.126.3.1196
Meir S, Droby S, Davidson H, Alsevia S, Cohen L, Horev B, Philosoph-Hadas S (1998) Suppression of Botrytis rot in cut rose flowers by postharvest application of methyl jasmonate. Postharvest Biol Technol 13:235–243
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51:914–916
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Norastehnia A, Nojavan-Asghari M (2006) Effects of methyl jasmonate on the enzymatic antioxidant defense system in maize seedling subjected to paraquat. Asian J Plant Sci 5(1):17–23
Parthier B (1991) Jasmonates, new regulators of plant growth and development: many facts and few hypotheses on their actions. Bot Acta 104:446–454
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Pospisilova J (2003) Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol Plant 46:491–506
Rohwer CL, Erwin JE (2008) Horticultural applications of jasmonates: a review. J Hort Sci Biotechnol 83(3):283–304
Sivritepe N, Erturk U, Yerlikaya C, Turkan I, Bor M, Ozdemir F (2008) Response of the cherry rootstock to water stress induced in vitro. Biol Plant 52(3):573–576. doi:10.1007/s10535-008-0114-4
Sotiropoulos TE (2007) Effect of NaCl and CaCl2 on growth and contents of minerals, chlorophyll, proline and sugars in the apple rootstock M4 cultured in vitro. Biol Plant 51:177–180. doi:10.1007/s10535-008-0114-4
Strosse H, Andre E, Sagi L, Swennen R, Panis B (2008) Adventitious shoot formation is not inherent to micropropagation of banana as it is in maize. Plant Cell Tiss Org Cult 95(3):321–332. doi:10.1007/s11240-008-9446-1
Turner DW, Fortescue JA, Thomas DS (2007) Environmental physiology of the bananas (Musa spp.). Braz J Plant Physiol 19(4):463–484
Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermo tolerance in Phragmites ausrralis leaves exposed to high temperatures and during the recovery from a heat stress. Plant, Cell Environ 28:318–327
Wang SY (1999) Methyl jasmonate reduces water stress in strawberry. J Plant Growth Regul 18:127–134. doi:10.1007/PL00007060
Wang CY, Buta JG (1994) Methyl jasmonate reduces chilling injury in Cucurbita pepo through its regulation of abscisic acid and polyamine levels. Environ Exp Bot 34(4):427–432 http://dx.doi.org/10.1016/0098-8472 (94)90025-6
Wang F, Zeng B, Sun Z, Zhu C (2009) Relationship between proline and Hg+2—induced oxidative stress in tolerant rice mutant. Arch Environ Contam Toxicol 56(4):723–731. doi:10.1007/s00244-008-9226-2
Wasternack C, Parthier B (1997) Jasmonate—signaled plant gene expression. Trends Plant Sci 2:302–307
Xing H, Tan L, An L, Zhao Z, Wang S, Zhang C (2004) Evidence for the involvement of nitric oxide and reactive oxygen species in osmotic stress tolerance of wheat seedlings: inverse correlation between leaf abscisic acid accumulation and leaf water loss. Plant Growth Reg 42:61–68. doi:10.1023/B:GROW.0000014894.48683.1b
Yoon JY, Hamayun M, Lee SK, Lee IJ (2009) Methyl jasmonate alleviating salinity stress in soybean. J Crop Sci Biotech 12(2):63–68. doi:10.1007/s12892-009-0060-5
Acknowledgments
The authors acknowledge the financial support of Research University Grant Scheme (RUGS)—UPM, University Putra Malaysia for the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmood, M., Bidabadi, S.S., Ghobadi, C. et al. Effect of methyl jasmonate treatments on alleviation of polyethylene glycol -mediated water stress in banana (Musa acuminata cv. ‘Berangan’, AAA) shoot tip cultures. Plant Growth Regul 68, 161–169 (2012). https://doi.org/10.1007/s10725-012-9702-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9702-6