Abstract
This study was performed to determine the response of Impatiens walleriana, an important horticultural plant, to exogenous application of methyl jasmonate (MeJA) during in vitro PEG8000−induced water deficit. Shoots were pretreated with graded MeJA concentrations (0, 5, 50, and 100 µM) in Murashige and Skoog (MS) medium for 7 days. After pretreatment, shoots were exposed to different treatments for 20 days: MS with polyethylene glycol (PEG8000 − 0 or 3%), MS with PEG8000 (3%) + MeJA (5, 50 or 100 µM), and MS with MeJA (5, 50 or 100 µM) only. MeJA pretreatment had positive effects on I. walleriana growth (fresh weight, plant height, number of leaves per plant and proliferation rate) in non-stressed shoots, especially after application of the lowest MeJA concentration (5 µM). In the water deficit conditions caused by PEG8000, pretreatment with 5 µM MeJA increased all analyzed growth parameters, and caused decreased photosynthetic pigment, proline and total amino acid content, total polyphenol and DPPH (1,1′-diphenyl-2-picrylhydrazyl) activity, hydrogen peroxide and malondialdehyde content. Additionally, changes in the activity of antioxidant enzymes (superoxide dismutase, peroxidase and catalase) were induced. Pretreatment with higher MeJA concentrations (50 and 100 µM) mainly suppressed the I. walleriana shoots growth under water deficit, combination treatments and treatment only with MeJA. These results indicate the potential of MeJA’s in enhancing I. walleriana ability to withstand the water stress in vitro. I. walleriana improved water stress resistance by promoting defense-related metabolism, which was mainly induced by pretreatment with the lowest applied MeJA concentration (5 µM).
Key message
Methyl jasmonate application (5 µM) improved I. walleriana growth, physiological and biochemical response under water deficit caused by PEG8000 in vitro.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Jasmonate are a group of lipid-derived compounds with diverse roles in regulating various plant developmental processes, and responses to many biotic and abiotic stresses (reviewed in Wasternack and Hause 2013; Dar et al. 2015; Kaminska 2021). Jasmonate members such as jasmonic acid (JA), methyl ester - methyl jasmonate (MeJA) and jasmonoyl-L-isoleucine (JA-Ile) are widely distributed in plants and act as natural growth regulators. Jasmonates regulate the cell cycle and proliferation, organogenesis, somatic embryogenesis, seed germination, shoot and root growth and development, tuber formation, leaf movement and senescence, and reproductive organ development in plants (Wasternack and Hause 2013; Kaminska 2021). Furthemore, jasmonates are crucial in plant responses to various biotic (herbivores, mechanical wounding, necrotrophic pathogens, nematodes and other microorganisms) and abiotic stress factors (UV-stress, osmotic stress, salt stress, water deficit, cold stress, temperature stress, heavy metal stress, ozone stress) (Dar et al. 2015; Kaminska 2021). In this regard, jasmonate are very important components in plant defense signaling pathways, acting synergistically and/or antagonistically in signaling crosstalk to alleviate adverse effects of stress.
In different in vitro culture study, jasmonate are commonly used to increase the production of secondary metabolites in plants (Dowom et al. 2017; Jeong et al. 2018; Ali et al. 2019). The role of jasmonate during abiotic stress conditions in vitro is still very poor and limited, compared with observations made and achieved in greenhouse and field experiments with foliar application of jasmonate. Pretreatment of wheat seedlings with 0.1 µM MeJA in vitro reduced the level of mannitol drought-induced growth retardation, membrane structure lesions and enhanced accumulation of the dehydrin transcripts and dehydrin proteins (Allagulova et al. 2020). Methyl jasmonate improved drought tolerance of banana shoots grown in vitro by moderating oxidative stress (Mahmood et al. 2012). In strawberry grown in vitro under PEG-induced water stress, the application of JA has been presented as an effective way to alleviate the harmful effects of dehydration (Yosefi and Javadi 2020). Mitigating abiotic stress effects of jasmonate application in vitro has also described in wheat (Ma et al. 2015), Malus×domestica (Wang et al. 2019), and Solanum tuberosum (Efimova et al. 2019).
Among the abiotic stresses, water deficit is the most harmful that restricts plants productivity worldwide. The water deficit can severely affect a plant’s growth and development and is often associated with an oxidative burst–the production of reactive oxygen species (ROS). The increased ROS production causes the damage to plant cells through oxidative modification of proteins and nucleic acids, as well as membrane lipid peroxidation (Hasanuzzaman et al. 2020). Plants respond to water stress complexly on a molecular, biochemical, physiological, and morphological levels. Water deficit tolerance is the ability of plants to resist stress through various physiological changes such as the accumulation of osmoprotectants, components of antioxidant defense, proteins with a protective role, and various products of secondary metabolism (reviewed in Ilyas et al. 2021). To simulate water deficit in vitro, a high molecular weight polyethylene glycol (PEG6000 or above) was commonly used. Polyethylene glycol is a neutral polymer that is highly soluble in water. Polyethylene glycol lowers the medium water potential which reduces the amount of water available to the plant root system, and has been utilized in many in vitro studies (Kacem et al. 2017). The effects of different molecular weights of PEG in plants studied by Lawlor (1970), suggested that larger polymers (20 000) can form viscous solutions and froth when aerated, while smaller polymers (200) can penetrate plants and cause damage over long periods.
The current study evaluated the effects of exogenously applied MeJA at different concentrations on the performance of in vitro grown water-stressed and non-stressed I. walleriana shoots. I. walleriana is a popular ornamental plant cultivated worldwide, but it can also be used as a hyperaccumulator (Wei et al. 2012) and source of secondary metabolites with biological activities (Haider and Ullah 2019; Hanachi et al. 2020). Generally, plants are very sensitive to water stress, which has a negative effect on their growth and development. A previous report explained the effects of exogenous salicylic acid on the performance of I. walleriana under PEG8000-induced water deficit in vitro (Antonić et al. 2016). Salicylic acid has a powerful water deficit-ameliorating potential on I. walleriana shoots grown in vitro, with only beneficial and not growth-retarding or other negative effects on this species, according to the authors. Another growth regulator, MeJA, was hypothesized to improve I. walleriana’s ability to withstand the water deficit caused by PEG8000 by controlling growth, proliferation rate, pigment content, proline accumulation, polyphenol accumulation, oxidative stress and membrane lipid peroxidation, as well as enzymatic antioxidant defense.
Materials and methods
Plant material and culture conditions
I. walleriana shoots were grown in vitro on MS (Murashige and Skoog 1962) basal medium containing 30 g/l sucrose, 7 g/l agar, 100 mg/l myo-inositol, and mineral elements. Explants with shoot apical meristem (2.5 cm long), for each pretreatment, were excised from the micropropagated plants and transferred to MS medium supplemented with graded MeJA concentrations (0, 5, 50, and 100 µM). The shoot cultures were grown under long day (16/8 h photoperiod) conditions, at irradiance of 47 µmol m− 2 s− 1 and temperature of 25 ± 2 °C. After 7 days long pretreatment, the explants were further transferred to different treatment media: MS with PEG8000 (0 or 3%), MS with PEG8000 (3%) and MeJA (5, 50 or 100 µM) and MS with only MeJA (5, 50 or 100 µM). A schematic representation of the experimental setup is shown in Fig. 1. All media tested were autoclaved at 121 °C for 20 min after adjusting the pH to 5.6. The stock solution of MeJA (1 M) (Sigma Aldrich) was prepared in 96% ethanol, and then filter sterilized. This solution was diluted with sterile deionized water under aseptic conditions to obtain working solutions, which were further added after media sterilization. After 20 days of different treatments, growth parameters such as plant height, fresh shoot weight (FW), the average number of shoots (proliferation rate, PR) and leaves per plant, were determined. For all physiological and biochemical analyses, leaves with petioles were collected, frozen in liquid nitrogen and stored at the − 80 °C before analysis.
Determination of photosynthetic pigments content
Extraction of photosynthetic pigments from 20 mg leaf tissue was performed using 2 ml of 96% ethanol. Samples were incubated in a water bath (Univeba JP Selecta) at 70 °C for 10 min and absorbance of the extracts was measured using a UV-visible spectrophotometer (Shimadzu UV-160, Kyoto, Japan) at 470, 648, and 664 nm. The total chlorophyll and carotenoid content were calculated using the formulas proposed by Lichtenthaler (1987).
Proline and total amino acid determination
Free amino acids were extracted from 250 mg of leaf tissue, and the ninhydrin reaction determined the free proline as earlier reported method (Antonić et al. 2016). For proline and total amino acid content, the absorbance of the obtained yellow reaction product was measured at 350 and 570 nm using an ELISA Micro Plate Reader (LKB 5060–006, Winooski, Vermont, SAD). Since many other substances absorb at 350 and 570 nm, as well as a mixture of proline and ninhydrin, the absorbance of samples lacking ninhydrin is determined simultaneously. Total amino acid and proline contents were calculated using glycine and proline standard curves.
Total polyphenol content and DPPH (1,1′-diphenyl-2-picrylhydrazyl) activity determination
The methods for spectrophotometrically determination of total polyphenol content and antioxidant activity based on the DPPH radical concentration were previously described by Đurić et al. (2020).
Oxidative stress indicators – hydrogen peroxide (H2O2) and malondialdehyde (MDA) determination
The extraction for both assays was accomplished by adding 1.5 ml of 0.1% trichloroacetic acid to 150 mg of ground tissue. The H2O2 content was determined spectrophotometrically using the method described by Velikova et al. (2000), while the MDA content was determined using the Health and Packer (1968) method. The ELISA Micro Plate Reader (LKB 5060-006, Winooski, Vermont, SAD) was used to perform both assays.
Antioxidant enzyme activity assays
Total soluble proteins were extracted according to the method described by Milošević et al. (2012), while the protein content was determined using the Bradford method (Bradford 1976). Superoxide-dismutase activity was determined according to Beyer and Fridovich (1987), with modifications described by Antonić et al. (2016). Peroxidase activity was determined according to the method of Vuleta et al. (2010), by monitoring the increase in absorbance at 430 nm over 2 minutes. The catalase activity was estimated by monitoring the consumption of hydrogen peroxide at 240 nm, where the decrease in absorbance is proportional to the CAT activity (Claiborne, 1985). The method slightly contained modifications. To 1 mL of a reaction mixture containing 0.05 M K-phosphate buffer (pH 7) and 30% H2O2 was added 10 µl of enzymatic extract and measured the decrease in absorbance at 240 nm during two minutes. Total CAT activity was expressed as mmol H2O2 min− 1 mg− 1-soluble protein. All enzyme activities were measured using a UV-visible spectrophotometer (Shimadzu UV-160, Kyoto, Japan).
Statistical analysis
All in vitro culture experiments were repeated 3 times with seven shoot explants for each treatment (n = 21), and the results are presented as the mean ± standard error. The STATISTICA software version 8 was used to assess statistical differences between experimental treatments using standard two-factor analysis of variance (ANOVA). The mean differences are compared by the least significant difference (LSD) test with statistical significance of p ≤ 0.05. Graphical representation of results was performed using the Microsoft Office Excel program (2010).
Results
The effect of exogenous application of MeJA on I. walleriana shoots growth under water deficit conditions caused by PEG8000
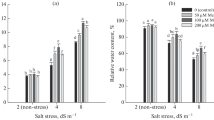
Shoots transferred from MS pretreatment medium without MeJA on medium containing PEG8000 (3%) showed a 11.35 and 14.29% reduced FW and proliferation rate compared with the shoots constantly grown on MS medium (Fig. 2a and d). However, pretreatment with 5 µM MeJA had beneficial effects on all growth parameters of non-stressed shoots grown on MS treatment medium. Pretreatment with 50 µM MeJA increased FW, shoot height and the average mean number of leaves per no-stressed shoots, while pretreatment with 100 µM MeJA increased shoot height and proliferation rate of no-stressed shoots grown on MS treatment medium. Shoots pretreated with 5 µM MeJA and then transferred to PEG8000 treatment medium, had higher FW (by 19.89%), shoot height (by 2.37%), number of leaves per shoot (by 40.10%) and proliferation rate (by 53.12%) compared with shoots grown on MS with PEG8000, without MeJA pretreatment. All analyzed growth parameters were increased and, better than control shoots constantly grown on MS medium. Pretreatment with 50 and 100 µM MeJA did not improve shoot growth under PEG8000 induced water deficit conditions. Additionally, all analyzed parameters were below values from shoots treated with PEG8000 after MS pretreatment, and also bellow values of control shoots grown on MS medium throughout the whole experimental period. Pretreatment with all applied MeJA concentrations had adverse effects on shoots grown on medium supplemented with PEG8000 and MeJA, while the best growth (FW and plant height) was noted after treatment with 5 µM MeJA only (Fig. 2a and b).
The effect of exogenous MeJA on fresh shoots weight (a), height (b), average number of leaves per shoot (c), and proliferation rate (d) of I. walleriana under PEG8000-induced water deficit in vitro. Results are presented as mean ± standard error, with significant differences between treatments based on LSD test (p ≤ 0.05)
Morphological differences between I. walleriana from different treatments are presented in Fig. 3. Compared with the shoots continuously grown on MS medium (Fig. 3a), water-stressed shoots without MeJA pretreatment were smaller (Fig. 3b). Shoots pretreated with 5 µM MeJA grew better in water deficit than plants treated with PEG8000 alone (Fig. 3d), while the best morphological performance was observed in shoots grown on a medium with only 5µM MeJA (Fig. 3f). After pretreatment with 50 and 100 µM MeJA, shoots growth was slow down in the water deficit caused by PEG8000 (Fig. 3 h and l), while in combination treatment media (Fig. 3i and m) and media with only MeJA (Fig. 3j and n), the growth was significantly suppressed. Moreover, shoots pretreated with 50 µM MeJA had no observed the proliferation rate in water deficit and combination treatment medium (Fig. 3 h and i). Additionally, proliferation rate was not observed in combination treatment medium with PEG8000 and 100 µM MeJA, and medium with only 100 µM MeJA (Fig. 3 m and n).
The effect of exogenous MeJA on I. walleriana shoots growth and morphology under PEG8000-induced water deficit in vitro. Treatment after pretreatment on MS medium: a MS; b MS + PEG8000 3%. Treatment after pretreatment with 5 µM MeJA: c MS; d MS + PEG8000 3%; e MS + PEG8000 3% + 5 µM MeJA; f MS + 5 µM MeJA. Treatment after pretreatment with 50 µM MeJA: g MS; h MS + PEG8000 3%; i MS + PEG8000 3% + 50 µM MeJA; j MS + 50 µM MeJA. Treatment after pretreatment with 100 µM MeJA: k MS; l – MS + PEG8000 3%; m MS + PEG8000 3% + 100 µM MeJA; n MS + 100 µM MeJA
Changes in photosynthetic pigment content in I. walleriana leaves under PEG8000-induced water deficit and exogenous MeJA application in vitro
Exposure of shoots to water deficit treatment caused by PEG8000 induced an increment of photosynthetic pigments in I. walleriana leaves (Fig. 4). Total chlorophyll and total carotenoids both increased by 23.73 and 22.57%, respectively. Pretreatment with MeJA (5, 50 and 100 µM) increased total chlorophyll (by 25.00, 19.34 and 19.06%, respectively) and carotenoid content (by 29.23, 20.06 and 19.41%, respectively) in shoots grown on MS treatment medium, compared with shoots continuously grown on MS medium.
During water deficit caused by PEG8000, pretreatment with 5 µM MeJA reduced total chlorophyll and carotenoid content by 14.34 and 16.25%, respectively, compared with shoots grown on MS treatment medium. Pretreatment with 5 µM MeJA increased total chlorophyll and carotenoids in PEG8000 + MeJA combination medium, compared with shoots grown on medium containing only PEG8000. However, the lowest level of photosynthetic pigments was recorded in shoots grown on medium containing only 5 µM MeJA.
Pretreatment with 50 µM MeJA caused the highest increase in total chlorophyll and carotenoid content (by 22.66 and 9.24%, respectively) in shoots grown on MS + PEG8000 treatment medium compared with shoots grown on MS treatment medium. However, the concentration of photosynthetic pigments was significantly lower in shoots grown on medium supplemented with PEG8000 and MeJA as well as on medium with only 50 µM MeJA.
The most pronounced reduction in total chlorophyll and carotenoid content was observed in shoots grown on PEG8000 + MeJA combination medium with, after pretreatment with 100 MeJA. Total chlorophyll and carotenoid content was reduced by 30.54 and 26.82% compared with shoots grown on MS treatment medium. In shoots grown on MS + PEG8000 treatment medium after pretreatment, slightly reduced photosynthetic content was recorded compared with shoots grown on MS treatment medium, while the photosynthetic content in shoots grown on MS + MeJA treatment medium was lower than in shoots grown on MS and MS + PEG8000 medium, but still higher than in MS combination treatment medium containing PEG8000 + MeJA.
Proline and total amino acid content in I. walleriana leaves under PEG8000-induced water deficit and exogenous MeJA application in vitro
The water deficit induced by PEG8000 increased proline and total amino acid content in I. walleriana leaves by 50.23 and 24.81% compared with control shoots grown on MS medium during the entire experimental period (Fig. 5). Compared with control shoots grown on MS treatment medium without MeJA pretreatment, pretreatment with MeJA (5, 50 and 100 µM) increased proline content by 38.25, 33.15 and 68.91%, and total amino acids by 12.16, 18.95 and 30.42%, respectively, in non-non-stressed shoots grown on MS treatment medium. Pretreatment with 5 µM MeJA slightly increased proline and total amino acid content in PEG8000 -induced water deficit conditions compared with shoots pretreated with the same MeJA concentration and grown on the MS treatment medium.
However, compared with shoots grown under water deficit conditions without MeJA pretreatment, proline content was slightly lower while amino acids did not change statistically significant. After pretreatment with 5 µM MeJA, proline and amino acid level were the highest in the combination medium containing PEG8000 + MeJA. It was interesting to note that on medium supplemented only with 5 µM MeJA in I. walleriana shoots proline and amino acid content decreased and was detected at lower levels than in the corresponding treatment control.
Pretreatment with 50 and 100 µM MeJA increased proline (by 29.37 and 9.75%) and total amino acid content (by 23.66 and 23.12%) under water deficit conditions compared with shoots pretreated with the same MeJA concentration and grown on the MS treatment medium. Also, these values are higher than those in shoots grown under water deficit conditions after MS pretreatment. Pretreatment with 100 MeJA slightly increased proline content in combination medium with PEG8000 + MeJA in relation to proline content in shoots grown on medium containing only PEG8000, while the proline content was decreased bellow control values in the treatment medium containing only MeJA in 5, 50 and 100 µM concentration. The total amino acid content was the highest in treatment medium containing only MeJA after pretreatment with 100 µM MeJA.
Total polyphenol content and DPPH activity in I. walleriana leaves under PEG8000-induced water deficit and exogenous MeJA application in vitro
According to the results, all treatments showed a similar trend in total polyphenol and DPPH activity (Fig. 6). Compared with control shoots grown constantly on MS medium, polyethylene glycol induced water deficit caused an increases in total polyphenol content and DPPH activity by 55.30 and 39.19%, respectively. Pretreatment with 5 and 50 µM MeJA reduced total polyphenol content and DPPH activity in non-stressed shoots, whereas pretreatment with 100 µM MeJA did not induce a statistically significant difference in relation to control shoots grown constantly on MS medium. Pretreatment with 5, 50 and 100 µM MeJA increased total polyphenol content (by 11.61, 46.72 and 55.04%) and DPPH activity (by 31.91, 48.15 and 63.80%) in water deficit conditions caused by PEG8000, compared with shoots pretreated with the same MeJA concentration and grown on the MS treatment medium.
However, total polyphenol content and DPPH activity were lower in water deficit conditions after MeJA pretreatment (5 and 50 µM) than in shoots water-stressed without MeJA pretreatment. In contrast to pretreatment with 100 µM MeJA, pretreatments with 5 and 50 µM MeJA increased total polyphenol content (by 53.68 and 30.16%) and DPPH activity (by 58.15 and 39%) in shoots grown on combination medium supplemented with PEG8000 and MeJA, compared with shoots grown on medium containing only PEG8000. Total polyphenol content and DPPH activity were decreased after the treatment with 5 and 50 µM MeJA, but slightly increased in shoots grown on medium supplemented only with 100 µM MeJA.
Hydrogen peroxide and malondialdehyde content in I. walleriana leaves under PEG8000-induced water deficit and exogenous MeJA application in vitro
During the entire experimental period, shoots grown on treatment medium containing PEG8000 increased their H2O2 and MDA contents in I. walleriana leaves by 38 and 65.58%, respectively, compared with shoots grown on MS medium (Fig. 7a and b). Pretreatment with MeJA (5, 50 and 100 µM) did not increase of H2O2 and MDA content in non-stressed shoots. Furthermore, pretreatment with 5 µM MeJA slightly decreased both H2O2 and MDA content in shoots grown on MS treatment medium. After pretreatment with 5, 50 and 100 µM MeJA, H2O2 increased by 21.79, 28.94 and 19.00% in water deficit conditions, while MDA increased by 56.35, 159.00 and 64.51% compared with non-stressed shoots. However, values of H2O2 were still lower than in shoots exposed to water deficit without MeJA pretreatment. Alternatively, MDA content was lower only in shoots pretreated with 5 µM MeJA compared with shoots exposed to water deficit without MeJA pretreatment. Pretreatment with 5 and 50 µM MeJA concentrations increased H2O2 content (by 7.32 and 22.37%) in shoots grown on combination medium supplemented with PEG8000 and MeJA, compared with shoots grown on medium containing only PEG8000. H2O2 content was lower in shoots grown on treatment medium containing only MeJA with 5 and 50 µM compared with this combination medium. There were no statistically significant differences in H2O2 content between shoots pretreated with 100 µM MeJA and grown on PEG8000, PEG8000 + MeJA, or only MeJA containing medium. MDA content increased by 47.00% in shoots grown on combination medium after pretreatment with 5 µM MeJA compared with shoots grown on medium containing only PEG8000. Pretreatment with 50 µM MeJA reduced MDA content by 20.17% in shoots grown on combination medium, whereas pretreatment with 100 µM MeJA increased MDA content slightly compared with shoots grown on medium containing only PEG8000. When compared with the combined medium, MDA content was decreased in all treatment media supplemented only with MeJA.
Antioxidant enzyme activities in I. walleriana leaves under PEG8000-induced water deficit and exogenous MeJA application in vitro
Changes in antioxidant enzyme activities are presented in Fig. 8. Polyethylene glycol -induced water deficit decreased SOD (by 18.77%) and CAT (by 18.45%) activities, while increasing POX activity by 15.35%, compared with shoots grown on MS medium throughout experiment.
During the entire experiment, pretreatment with 100 µM MeJA slightly increased SOD activity of non-stressed shoots compared with shoots grown on MS medium (Fig. 8a). Compared with shoots grown on MS treatment medium, pretreatment with all applied MeJA concentration (5, 50 and 100 µM) resulted in decreased SOD activity in water deficit conditions (by 10.54, 34.26 and 28.00%, respectively). SOD activity was also lower in shoots pretreated with 50 and 100 µM MeJA and further grown on combination medium supplemented with PEG8000 and MeJA, than in shoots grown on MS medium, but slightly higher than in shoots grown on medium supplemented only with PEG8000. The highest SOD activity was detected in shoots pretreated with 100 MeJA and then grown on medium supplemented with MeJA only.
Pretreatment with higher MeJA concentrations (50 and 100 µM) decreased POX activity of non-stressed shoots (by 49.00 and 16.72%, respectively) compared with shoots grown on MS medium during the entire experimental period (Fig. 8b). Peroxidase activity was the highest in shoots pretreated with MeJA (5, 50 or 100 µM) and grown on treatment medium containing PEG8000. Increased POX activity was determined in water deficit conditions after the pretreatments with 5, 50 or 100 µM MeJA (24.66, 173.00 and 22.05%, respectively) compared with shoots grown on MS medium after the same MeJA pretreatments. Peroxidase activity was lower in shoots grown on a combination medium containing PEG8000 and MeJA than in shoots grown on medium with PEG8000 only, while the lowest POX activity was observed in shoots grown on a medium only with MeJA.
Pretreatment with MeJA decreased CAT activity in non-stressed shoots compared with shoots grown on MS medium during the entire experimental period, with the highest effect of 100 µM MeJA (Fig. 8c). When compared with non-stressed shoots treated with the same MeJA concentration, shoots pretreated with 5 and 50 µM MeJA, increased CAT activity in water deficit conditions by 27.90 and 7.56%, respectively. Shoots pretreated with 100 µM MeJA, on the other hand, reduced CAT activity in water deficit conditions by 50.39%. The catalase activity was lower in shoots pretreated with 5 and 50 µM MeJA, and grown on a combination treatment medium with PEG8000 and MeJA, than in shoots grown on a medium only with PEG8000. CAT activity was higher in shoots pretreated with 100 µM MeJA and grown on a combination treatment medium, than in shoots grown on medium with PEG8000 only. Pretreatments with 5 and 100 µM MeJA decreased CAT activity in shoots grown on treatment medium supplemented with 5 and 100 µM MeJA only compared with shoots grown on a medium with PEG8000 and MeJA, whereas pretreatment with 50 µM caused a slight increase in CAT activity in shoots grown on treatment medium supplemented with 50 µM MeJA only.
Discussion
Growth retardation is the first plant response to water stress and has been observed in many plant species (Allagulova et al. 2020; Yosefi and Javadi 2020). This study clearly indicated that the I. walleriana fresh shoots weight, as well as the proliferation rate, were reduced by PEG8000-induced water deficit. Water stress caused by PEG8000 also affected on some physiological and biochemical parameters in I. walleriana. It was observed an increase in photosynthetic pigments, proline and total amino acid content, total polyphenol content and DPPH activity, MDA and H2O2 content, and altered antioxidant enzyme activities in I. walleriana leaves. Under PEG8000 induced stress conditions a, significant increase in total chlorophyll content in I. walleriana shoots could be explained by the loss of turgor or reduction in leaf growth, resulting in higher chlorophyll content. However, this water deficit-induced increase in total chlorophyll content could also be explained as plants ability to cope with water deficit to reduce photooxidation of pigments and degradation of chlorophyll. Increased chlorophyll content has also been observed in potatoes during drought (Ramirez et al. 2014), and this result is explained as a physiological trade-off between growth and investment in secondary metabolism, widely studied processes in plants at molecular and physiological level (Figueroa-Macías et al. 2021). The higher content of carotenoids impairs the involvement in antioxidant defense during water deficit, as described in Helianthus annuus (Ghobadi et al. 2013), and wheat (Naderi et al. 2020). The higher proline content may improve tolerance due to ROS scavenging and the alleviation of cell damage through osmoprotection during water deficit (Soni and Abdin 2017; Naderi et al. 2020). Also, increased total amino acid level can also be the result of the expression of new stress proteins during water deficit. Oxidative stress is usually manifested by increased H2O2 and MDA contents (Rahimi et al. 2018; Sarker and Oba 2018; Shafiq et al. 2019), while increased total polyphenols and DPPH activity of secondary metabolites as antioxidants act to prevent damage of macromolecules by ROS (Quan et al. 2016; Shafiq et al. 2019; Islam et al. 2020). Positively correlations between elevated MDA, H2O2, compatible solutes (proline) and non-enzymatic antioxidants (carotenoids, total polyphenol content) indicated that all these compounds play an important role in the ROS detoxification in I. walleriana during PEG-induced water deficit in vitro. Also, increased POX activity during water deficit, probably contributed to the elimination of produced H2O2 in I. walleriana.
Pretreatment with MeJA had beneficial effects on the growth parameters of I. walleriana non-stressed plants, which depended on the applied concentration of MeJA in pretreatment. It can be concluded that MeJA enhanced division and elongation of plant cells resulting in improved plant growth. However, only pretretament with 5 µM MeJA increased all measured parameters - fresh weight, plant height, number of leaves per plant and proliferation rate of non-stressed plants. Since the jasmonate regulate various plant developmental processes, it is not surprising that MeJA, at certain concentrations, can promote plant growth under normal conditions and protect against stress-related damage. Accordingly, the stress generated by PEG8000 was reduced when I. walleriana shoots were pretreated with MeJA by direct implementation in growth medium. Most importantly, pretreatment with the lowest MeJA concentration (5 µM) induced an increase in all analyzed growth parameters in shoots grown on medium containing PEG8000, and an increase in fresh weight and plant height in shoots grown on medium containing only MeJA in treatment. These results showed that the MeJA effect was concentration dependent, and that pretreatment with a higher MeJA concentration (50 and 100 µM MeJA) had mainly inhibitory effects on the growth of I. walleriana growth, particularly in combination treatment medium and in medium supplemented with MeJA alone. Recent research reported that jasmonate application improved strawberry growth attributes under both stress and non-stress conditions in vitro (Yosefi and Javadi 2020). Similar results are also described for banana and wheat (Mahmood et al. 2012; Allagulova et al. 2020). However, in Verbascum nudicuale and V. sinuatum, MeJA negatively affected on the growth parameters under water deficit induced by PEG in vitro (Ghasemlou et al. 2019; Karamian et al. 2020). Regarding to MeJA (5 µM) effects on improving I. walleriana growth in vitro, these effects could be connected to certain molecular mechanisms of acting. However, literature data indicate there are no many works on the molecular mechanisms underlying the action of MeJA in improving plant stress tolerance to water stress. There are few studies conducted ex vitro, indicating that plant stress tolerance to water stress could be associated with MeJA induction of different genes. In Pennisetum glaucum, foliar application of MeJA induced gene expression for heme binding, oxidation–reduction process, as well as response to oxidative stress in drought-sensitive genotype, while in drought-tolerant genotype, gene expression was mainly related to terpene synthase activity, lyase activity, magnesium ion binding, and thylakoid (Ndiaye et al. 2022). Additionally, in wheat exogenously applied MeJA altered expression of microRNAs, which on the other hand could regulate the expression of target genes associated with water stress tolerance (Ma et al. 2019). Regarding the studies in vitro, it has been shown that MeJA pretreatment increased dehydrin transcript in wheat during mannitol-induced water stress, and authors indicated that dehydrins may be involved in MeJA protective effect on wheat (Allagulova et al. 2020). Namely, exogenous MeJA treatment accelerated wheat seedlings development in the normal conditions and reduced drought-induced growth inhibition, as well as membrane structures lesions. Altered gene expression by MeJA during plants exposure to other abiotic stresses such as cold are also described, and indicated on increased expression of key regulatory transcription factors in the activation of jasmonate response in banana (Zhao et al. 2013), and cold-regulated (COR) genes in wheat (Repkina et al. 2021).
Similar to the effect on growth parameters, MeJA pretreatment increased total chlorophyll and carotenoid content in non-stressed I. walleriana shoots, with the highest effect after application of 5 µM MeJA in pretreatment. These results can be explained as an MeJA contribution to the acceleration of photosynthesis and plant productivity. Under water deficit conditions, however, the lowest content of total chlorophyll and carotenoid in the leaves, as well as proline and total amino acid content, were determined in shoots pretreated with 5 µM MeJA. Additionally, total polyphenol content and DPPH activity was the lowest in shoots pretreated with 5 µM MeJA and subjected further to water stress, indicating the changes in oxidative status in plant tissues. From this, it can be concluded that the lowest activity of secondary metabolites could be related to the lowest level of oxidative stress in I. walleriana shoots. In fact, MDA and H2O2 contents were also very low, which can be explained as MeJA contribution to ROS elimination and damage control under stress conditions. Similar results have been previously reported for banana grown in vitro (Mahmood et al. 2012). Namely, MeJA application up to 40 µM was effective in reducing oxidative damage, as indicated by a significant reduction in H2O2 and MDA content in banana shoot tips. Plants have evolved various non-enzymatic and enzymatic antioxidant defense mechanisms to counteract the adverse effects of ROS. Increased enzymatic antioxidant activities, especially CAT and POX, could also contribute to ROS elimination and reduce the negative effects of oxidative stress in I. walleriana during water deficit after pretreatment with the lowest MeJA concentration.
Pretreatments with higher MeJA concentrations (50 and 100 µM) suppressed the growth of I. walleriana under conditions of water deficit, combination treatment medium and medium supplemented only MeJA, which was accompanied by a change in the analyzed physiological and biochemical parameters with these treatments. Pretreatment with 50 µM MeJA, induced the highest increase in total chlorophyll and carotenoid content in water deficit conditions caused by PEG8000, as well as the highest H2O2 content in I. walleriana shoots. The MDA content was also very high, similar to shoots grown under water deficit conditions without MeJA pretreatment and shoot grown under water stress after pretreatment with 100 µM MeJA. The application of graded MeJA concentration in the pretreatment, increased proline content, total amino acid, as well as total polyphenol content and DPPH activity under water deficit conditions, indicated a higher level of oxidative stress and, a potential synergistically effect of MeJA on PEG8000-induced water stress.
In this regard, the results suggest that the tolerance to water stress could be attributed to the pretreatment of I. walleriana shoots with MeJA before the imposition of water stress. However, the effectiveness of MeJA’s in reducing the adverse consequences of water stress, depends on the applied MeJA concentration.
Conclusion
Exogenous application of MeJA affects on the growth, physiology, and biochemistry of I. walleriana shoots grown in vitro, depending on MeJA concentration. Indeed, the application of 5 µM MeJA had a growth-stimulating and protective effect on I. walleriana shoots exposed to PEG8000-induced water deficit in vitro, and promoted the growth of non-stressed shoots. Pretreatment with 5 µM MeJA significantly reduced the negative effect of water stress on the growth rate of I. walleriana and maintained a low level of oxidative stress. From this, it can be concluded that MeJA could be used as a potential growth promoters of I. walleriana shoots under water stress, and therefore, our study recommends the use of 5 µM MeJA to alleviate water stress in vitro in this plant species.
Data availability
All data generated or analyzed during this study are included in the article.
References
Ali M, Mujib A, Gulzar B, Zafar N (2019) Essential oil yield estimation by gas chromatography–mass spectrometry (GC–MS) after methyl jasmonate (MeJA) elicitation in in vitro cultivated tissues of Coriandrum sativum L. 3 Biotech 9:1–16. https://doi.org/10.1007/s13205-019-1936-9
Allagulova C, Avalbaev A, Fedorova K, Shakirova F (2020) Methyl jasmonate alleviates water stress-induced damages by promoting dehydrins accumulation in wheat plants. Plant Physiol Biochem 155:676–682. https://doi.org/10.1016/j.plaphy.2020.07.012
Antonić D, Milošević S, Cingel A, Lojić M, Trifunović-Momčilov M, Petrić M, Simonović A (2016) Effects of exogenous salicylic acid on Impatiens walleriana L. grown in vitro under polyethylene glycol-imposed drought. S Afr J Bot 105:226–233. https://doi.org/10.1016/j.sajb.2016.04.002
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Claiborne A (1985) “Catalase activity. ” in Handbook of methods for Oxygen Radical Research, Editor R. A. Greenwald. CRC Press, Boca Raton, FL, pp 283–284
Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H (2015) Jasmonates counter plant stress: a review. Environ Exp Bot 115:49–57. https://doi.org/10.1016/j.envexpbot.2015.02.010
Dowom SA, Abrishamchi P, Radjabian T, Salami SA (2017) Enhanced phenolic acids production in regenerated shoot cultures of Salvia virgata Jacq. After elicitation with ag + ions, methyl jasmonate and yeast extract. Ind Crops Prod 103:81–88. https://doi.org/10.1016/j.indcrop.2017.03.043
Đurić M, Subotić A, Prokić L, Trifunović-Momčilov M, Cingel A, Vujičić M, Milošević S (2020) Morpho-physiological and molecular evaluation of drought and recovery in Impatiens walleriana grown ex vitro. Plants 9:1559. https://doi.org/10.3390/plants9111559
Efimova MV, Mukhamatdinova EA, Kovtun IS, Kabil FF, Medvedeva YV, Kuznetsov VV (2019) September Jasmonic acid enhances the potato plant resistance to the salt stress in vitro. In: Doklady Biological Sciences, vol. 488, No. 1. Pleiades Publishing, pp 149–152. https://doi.org/10.1134/S0012496619050077
Figueroa-Macías JP, García YC, Núñez M, Díaz K, Olea AF, Espinoza L (2021) Plant growth-defense trade-offs: molecular processes leading to physiological changes. IJMS 22:693. https://doi.org/10.3390/ijms22020693
Ghasemlou F, Amiri H, Karamian R, Mirzaie-asl A (2019) Alleviation of the effects of on drought stress Verbascum nudicuale by methyl jasmonate and titanium dioxide nanoparticles. Iran J Plant Physiol 9:2911–2920. https://doi.org/10.22034/IJPP.2019.668857
Ghobadi M, Taherabadi S, Ghobadi ME, Mohammadi GR, Jalali-Honarmand S (2013) Antioxidant capacity, photosynthetic characteristics and water relations of sunflower (Helianthus annuus L.) cultivars in response to drought stress. Ind Crops Prod 50:29–38. https://doi.org/10.1016/j.indcrop.2013.07.009
Haider F, Ullah N (2019) Antioxidant and antimicrobial activity of Impatiens walleriana local to Malaysia. Mor J Chem. https://doi.org/10.48317/IMIST.PRSM/morjchem-v7i3.12261
Hanachi P, Salehizadeh S, Ramezani R, Zarringhalami R (2020) Comparison of antioxidant and anti-bacterial activities of Ocimum basilicum and Impatiens walleriana and their anticancer properties on SKOV-3 cancer cell line. J Food Sci Technol 17:95–107
Hasanuzzaman M, Bhuyan MHM, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Ilyas M, Nisar M, Khan N, Hazrat A, Khan AH, Hayat K, Ullah A (2021) Drought tolerance strategies in plants: a mechanistic approach. J Plant Growth Regul 40:926–944. https://doi.org/10.1007/s00344-020-10174-5
Islam MJ, Kim JW, Begum MK, Sohel MAT, Lim YS (2020) Physiological and biochemical changes in sugar beet seedlings to confer stress adaptability under drought condition. Plants 9:1511. https://doi.org/10.3390/plants9111511
Jeong YJ, An CH, Park SC, Pyun JW, Lee J, Kim SW, Kim CY (2018) Methyl jasmonate increases isoflavone production in soybean cell cultures by activating structural genes involved in isoflavonoid biosynthesis. J Agric Food Chem 66:4099–4105. https://doi.org/10.1021/acs.jafc.8b00350
Kacem NS, Delporte F, Muhovski Y, Djekoun A, Watillon B (2017) In vitro screening of durum wheat against water-stress mediated through polyethylene glycol. JGEB 15:239–247. https://doi.org/10.1016/j.jgeb.2017.04.004
Kamińska M (2021) Role and activity of jasmonates in plants under in vitro conditions. PCTOC 146:425–447. https://doi.org/10.1007/s11240-021-02091-6
Karamian R, Ghasemlou F, Amiri H (2020) Physiological evaluation of drought stress tolerance and recovery in Verbascum sinuatum plants treated with methyl jasmonate, salicylic acid and titanium dioxide nanoparticles. Plant Biosyst 154:277–287. https://doi.org/10.1080/11263504.2019.1591535
Lawlor DW (1970) Absorption of polyethylene glycols by plants and their effects on plant growth. New Phytol 69:501–513. https://doi.org/10.1111/j.1469-8137.1970.tb02446.x
Lichtenthaler HK (1987) Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J Plant Physiol 131:101–110. https://doi.org/10.1016/S0176-1617(87)80271-7
Ma C, Wang Z, Sun M, Zhang L, Kong B, Lin T (2015) Hydrogen peroxide acts as a signaling molecule for the methyl jasmonate-induced antioxidant defense in wheat callus to promote enhanced drought tolerance. J Agric Sci 7:99. https://doi.org/10.5539/jas.v7n11p99
Ma C, Zhang J, Yuan J, Guo J, Xiong Y, Feng Y (2019) Differential expression of microRNAs are responsive to drought stress and exogenous methyl jasmonate in wheat (Triticum aestivum). IJAB 22:475–486
Mahmood M, Bidabadi SS, Ghobadi C, Gray DJ (2012) Effect of methyl jasmonate treatments on alleviation of polyethylene glycol-mediated water stress in banana (Musa acuminata cv.‘Berangan’, AAA) shoot tip cultures. Plant Growth Regul 68:161–169. https://doi.org/10.1007/s10725-012-9702-6
Milošević S, Simonović A, Cingel A, Jevremović S, Todorović S, Filipović B, Subotić A (2012) Response of antioxidative enzymes to long-term Tomato spotted wilt virus infection and virus elimination by meristem-tip culture in two Impatiens species. Physiol Mol Plant Pathol 79:79–88. https://doi.org/10.1016/j.pmpp.2012.05.003
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naderi S, Fakheri BA, Maali-Amiri R, Mahdinezhad N (2020) Tolerance responses in wheat landrace Bolani are related to enhanced metabolic adjustments under drought stress. Plant Physio Biochem 150:244–253. https://doi.org/10.1016/j.plaphy.2020.03.002
Ndiaye A, Diallo AO, Fall NC, Diouf RD, Diouf D, Kane NA (2022) Transcriptomic analysis of methyl jasmonate treatment reveals gene networks involved in drought tolerance in pearl millet. Sci Rep 12:1–13. https://doi.org/10.1038/s41598-022-09152-6
Quan NT, Anh LH, Khang DT, Tuyen PT, Toan NP, Minh TN, Xuan TD (2016) Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 6:23. https://doi.org/10.3390/agriculture6020023
Rahimi Y, Taleei A, Ranjbar M (2018) Long-term water deficit modulates antioxidant capacity of peppermint (Mentha piperita L.). Sci Hortic 237:36–43. https://doi.org/10.1016/j.scienta.2018.04.004
Ramírez DA, Yactayo W, Gutiérrez R, Mares V, De Mendiburu F, Posadas A, Quiroz R (2014) Chlorophyll concentration in leaves is an indicator of potato tuber yield in water-shortage conditions. Sci Hortic 168:202–209. https://doi.org/10.1016/j.scienta.2014.01.036
Repkina N, Ignatenko A, Holoptseva E, MiszalskI Z, Kaszycki P, Talanova V (2021) Exogenous methyl jasmonate improves cold tolerance with parallel induction of two cold-regulated (COR) genes expression in Triticum aestivum L. Plants. https://doi.org/10.3390/plants10071421
Sarker U, Oba S (2018) Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl Biochem Biotechnol 186:999–1016. https://doi.org/10.1007/s12010-018-2784-5
Shafiq S, Akram NA, Ashraf M (2019) Assessment of physio-biochemical indicators for drought tolerance in different cultivars of maize (Zea mays L.). Pak J Bot 51:1241–1247. https://doi.org/10.30848/PJB2019-4(21
Soni P, Abdin MZ (2017) Water deficit-induced oxidative stress affects artemisinin content and expression of proline metabolic genes in Artemisia annua L. FEBS Open Bio 7:367–381. https://doi.org/10.1002/2211-5463.12184
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vuleta A, Manitašević Jovanović S, Šešlija D, Tucić B (2010) Seasonal dynamics of foliar antioxidative enzymes and total anthocyanins in natural populations of Iris pumila L. J Plant Ecol 3:59–69. https://doi.org/10.1093/jpe/rtp019
Wang Y, Xu H, Liu W, Wang N, Qu C, Jiang S, Chen X (2019) Methyl jasmonate enhances apple’cold tolerance through the JAZ–MYC2 pathway. PCTOC 136:75–84. https://doi.org/10.1007/s11240-018-1493-7
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058. https://doi.org/10.1093/aob/mct067
Wei JL, Lai HY, Chen ZS (2012) Chelator effects on bioconcentration and translocation of cadmium by hyperaccumulators, Tagetes patula and Impatiens walleriana. Ecotoxicol Environ Saf 84:173–178. https://doi.org/10.1016/j.ecoenv.2012.07.004
Yosefi A, Javadi T (2020) Jasmonic acid improved in vitro strawberry (Fragaria× ananassa Duch.) Resistance to PEG-induced water stress. PCTOC 142:549–558. https://doi.org/10.1007/s11240-020-01880-9
Zhao ML, Wang JN, Shan W, Fan JG, Kuang JF, Wu KQ, Lu WJ (2013) Induction of jasmonate signalling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit. Plant Cell Environ 36:30–51. https://doi.org/10.1111/j.1365-3040.2012.02551.x
Acknowledgements
This research was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia through contract number 451-03-68/2022-14/200007.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MĐ was involved in all experimental work and writing the first draft of the manuscript; AS contributed to study conception and design and supervised whole experimental work; MTM and SM were involved in all physiological and biochemical analysis. All authors contributed to the manuscript realization, read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by K X Tang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Đurić, M., Subotić, A., Trifunović-Momčilov, M. et al. Improvement of water deficit stress tolerance of Impatiens walleriana shoots grown in vitro by methyl jasmonate. Plant Cell Tiss Organ Cult 154, 351–365 (2023). https://doi.org/10.1007/s11240-022-02432-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02432-z